Extended Telemonitored Follow-Up After Acute Coronary Syndrome: A Healthcare Pathway That Improves Cardiovascular Prevention and Patient Experience, and Reduces Outpatient Visits †
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Design
2.3. Study Population
2.4. Clinical Data Collection and Definitions
2.5. Assessment of Lipid Parameters
2.6. Patient Experience Evaluation
2.7. Measurement of Outpatient Resource Utilisation
2.8. Description of the Telemonitored Follow-Up Programme
2.9. Statistical Analysis
3. Results
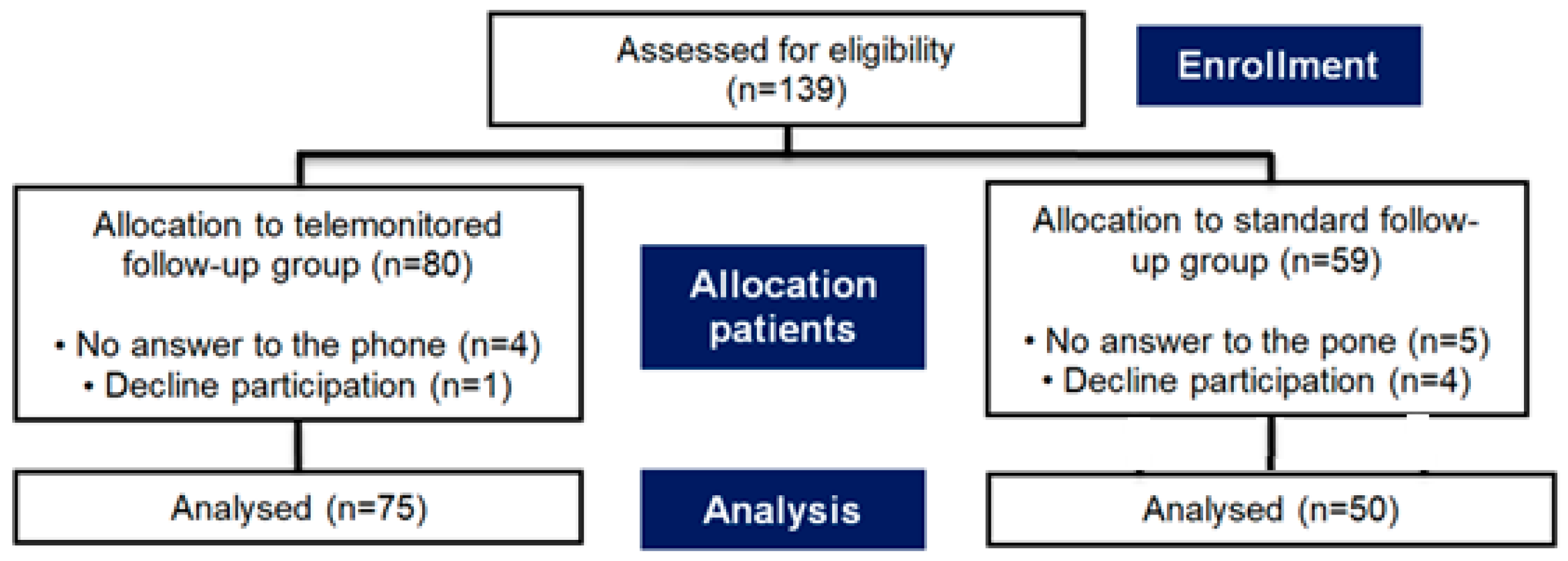
3.1. Study Cohorts
3.2. Baseline Characteristics
3.3. Lipid Parameters
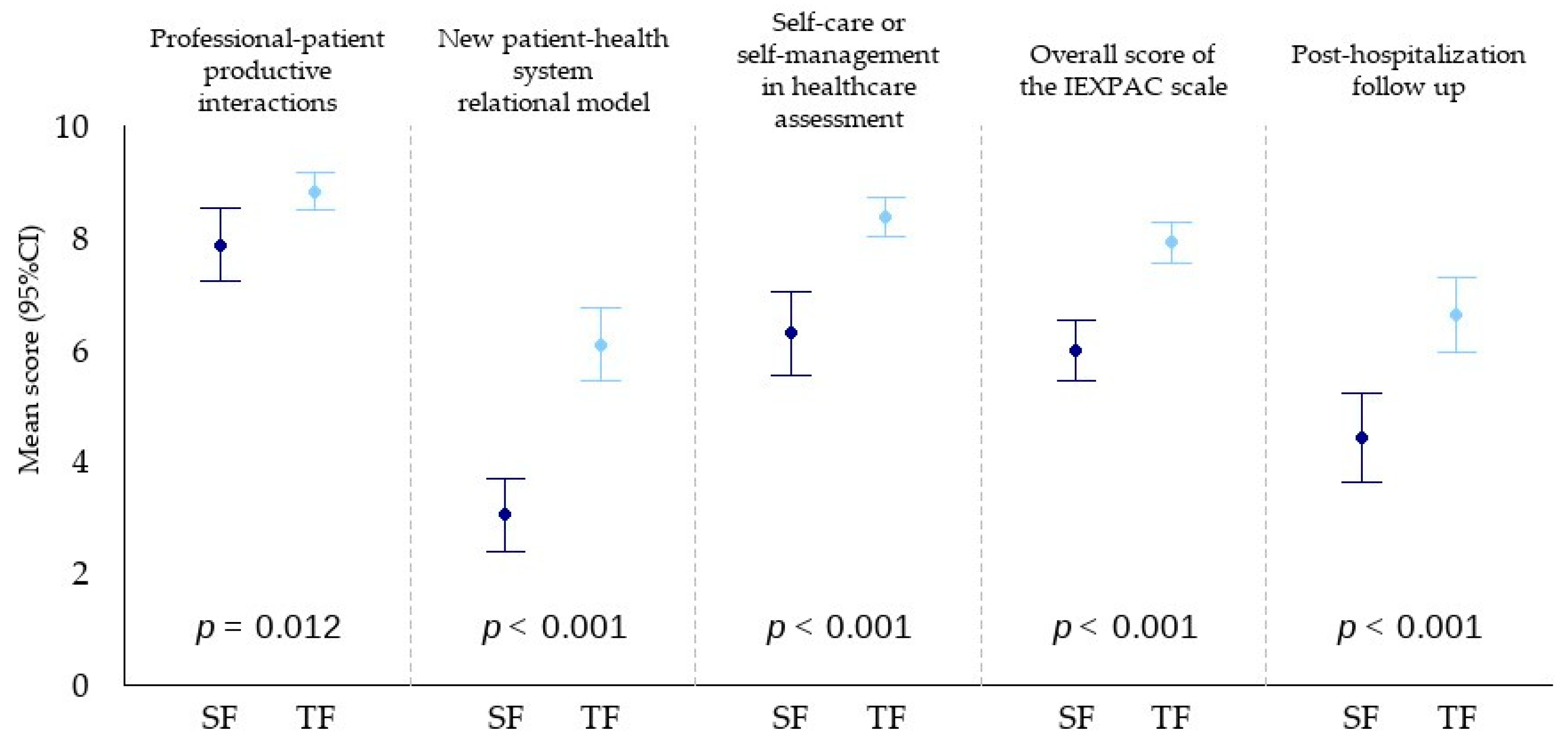
3.4. Patient Experience
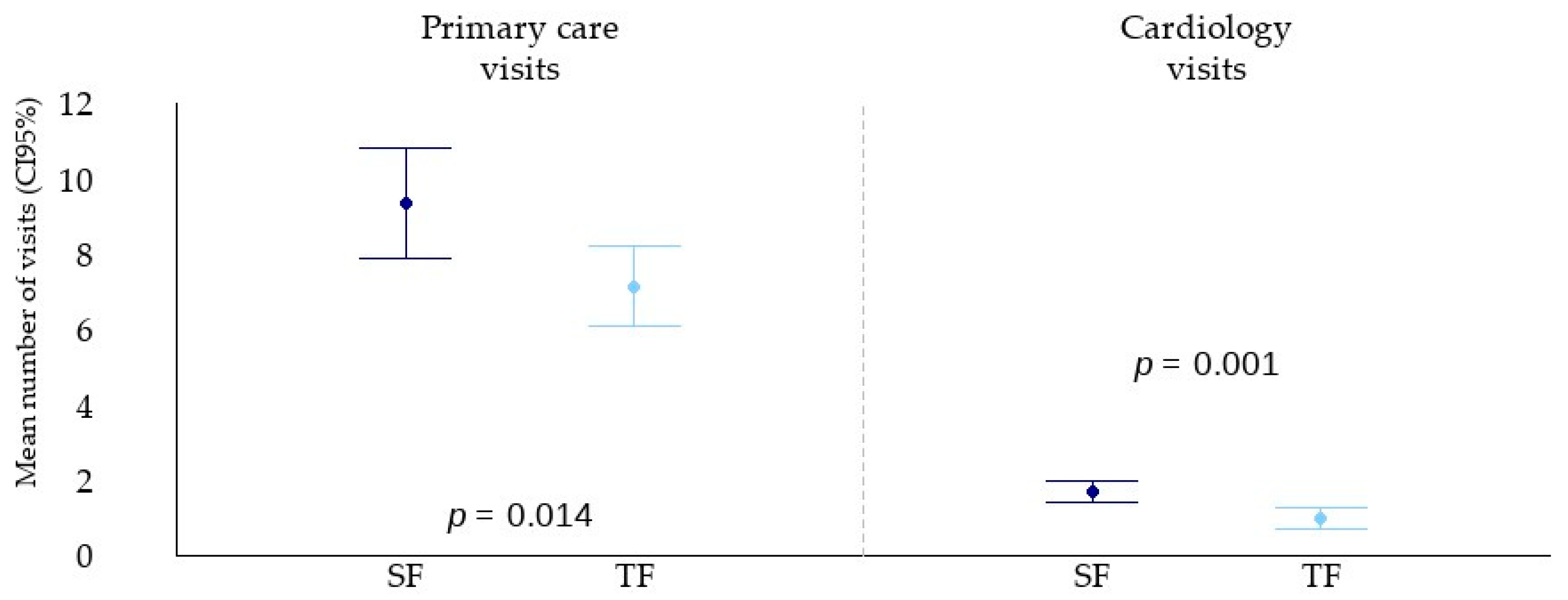
3.5. Outpatient Visits
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, A.; Dan, G.A.; Dweck, M.R.; Ibanez, B.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, A.C.; Capsula, M.; Badimon, L.; Chapman, M.J.; De Baker, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Kotseva, K.; De Baker, G.D.; De Baker, D.D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marqués-Vidal, P.; Jennings, C.; Abreu, A.; et al. risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Harris, R. The effectiveness and experience of self-management following acute coronary syndrome: A review of the literature. Int. J. Nurs. Stud. 2016, 61, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Chindhy, S.; Taub, P.R.; Lavie, C.J.; Shen, J. Current challenges in cardiac rehabilitation: Strategies to overcome social factors and attendance barriers. Expert. Rev. Cardiovasc. Ther. 2020, 18, 777–789. [Google Scholar] [CrossRef]
- Scherrenberg, M.; Falter, M.; Dendale, P. Providing comprehensive cardiac rehabilitation during and after the COVID-19 pandemic. Eur. J. Prev. Cardiol. 2021, 28, 520–521. [Google Scholar] [CrossRef]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Cathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core Components of Cardiac Rehabilitation Programs: 2024 Update: A Scientific Statement from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef]
- Kenny, E.; Byrne, M.; McEvoy, J.W.; Connolly, S.; McSharry, J. Exploring patient experiences of participating in digital cardiac rehabilitation: A qualitative study. Br. J. Health Psychol. 2024, 29, 149–164. [Google Scholar] [CrossRef]
- Avlijas, T.; Squires, J.E.; Lalonde, M.; Backman, C. A concept analysis of the patient experience. Patient Exp. J. 2023, 10, 15–63. [Google Scholar] [CrossRef]
- Doyle, C.; Lennox, L.; Bell, D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013, 3, e001570. [Google Scholar] [CrossRef]
- Davy, C.; Bleasel, J.; Liu, H.; Tchan, M.; Ponniah, S.; Brown, A. Effectiveness of chronic care models: Opportunities for improving healthcare practice and health outcomes: A systematic review. BMC Health Serv. Res. 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, N.A.; Berchtold, P.; Ullman, K.; Busato, A.; Egger, M. Integrated care programmes for adults with chronic conditions: A meta-review. Int. J. Qual. Health Care 2014, 26, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Nuño, R.; Coleman, K.; Bengoa, R.; Sauto, R. Integrated care for chronic conditions: The contribution of the ICCC Framework. Health Policy. 2012, 105, 55–64. [Google Scholar] [CrossRef]
- Dalli-Peydró, E.; Sanz-Sevilla, N.; Tuzón-Segarra, M.T.; Miró-Palau, V.; Sánchez-Torrijos, J. Cosín-Sales, J. A randomized controlled clinical trial of cardiac telerehabilitation with a prolonged mobile care monitoring strategy after an acute coronary syndrome. Clin. Cardiol. 2022, 45, 31–41. [Google Scholar] [CrossRef]
- Mira, J.J.; Nuño-Solinís, R.; Guilabert-Mora, M.; Solas-Gaspar, O.; Fernández-Cano, P. Development and Validation of an Instrument for Assessing Patient Experience of Chronic Illness Care. Int. J. Integr. Care 2016, 16, 13. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Elm, E.V.; Altman, D.G.; Gøtzsche, P.; Mulrow, C.D. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Orozco-Beltrán, D.; Cinza-Sanjurjo, S.; Escribano-Serrano, J.; López-Simarro, F.; Fernandez, G.; Gómez-García, A.; Cedenilla-Horcajuelo, M.; Ferreira de Campos, K. Experience of patients with diabetes and other cardiovascular risk factors with health professionals and health care in Spain. J. Clin. Med. 2021, 10, 2831. [Google Scholar] [CrossRef]
- Sinclair, M.; O’Toole, J.; Malawaraarachchi, M.; Leder, K. Comparison of response rates and cost-effectiveness for a community- based survey: Postal, internet and telephone modes with generic or personalised recruitment approaches. BMC Med. Res. Methodol. 2012, 12, 132. [Google Scholar] [CrossRef]
- Beatty, A.L.; Beckie, T.M.; Dodson, J.; Goldstein, C.M.; Hughes, J.W.; Kraus, W.E.; Martin, S.S.; Olson, T.P.; Pack, Q.R.; Stolp, H.; et al. A New Era in Cardiac Rehabilitation Delivery: Research Gaps, Questions, Strategies, and Priorities. Circulation 2023, 147, 254–266. [Google Scholar] [CrossRef]
- Ramachandran, H.J.; Jiang, Y.; Tam, W.W.S.; Yeo, T.J.; Wang, W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 1017–1043. [Google Scholar] [CrossRef] [PubMed]
- Nkonde-Price, C.; Reynolds, K.; Najem, M.; Yang, S.J.; Batiste, C.; Cotter, T.; Lafti, D.; Gin, N.; Funahashi, T. Comparison of Home-Based vs Center-Based Cardiac Rehabilitation in Hospitalization, Medication Adherence, and Risk Factor Control Among Patients with Cardiovascular Disease. JAMA Netw. Open. 2022, 5, e2228720. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; Astin, F.; Kotseva, K.; Pogosova, N.; De Smedt, D.; De Becquer, G.; Ridén, L.; Wood, D.; Jennings, C. Poor adherence to lifestyle recommendations in patients with coronary heart disease: Results from the EUROASPIRE surveys. Eur. J. Prev. Cardiol 2022, 29, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cobo, C.; Bernal-Jimenez, M.A.; Calle, G.; Gheorge, L.L.; Gutierrez-Barrios, A.; Cañadas, D.; Tur, J.A.; Vazquez-Garcia, R.; Santi-Cano, M.J. Efficacy of a Mobile Health App (eMOTIVA) Regarding Compliance with Cardiac Rehabilitation Guidelines in Patients with Coronary Artery Disease: Randomized Controlled Clinical trial. JMIR Mhealth Uhealth 2024, 12, e55421. [Google Scholar] [CrossRef]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Redeker, N.S.; et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults. Circulation. 2010, 122, 406–441. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, R.; Cheng, H.; Xu, L.; Wang, L.; He, C.; Wei, Q. Longer-Term Effects of Cardiac Telerehabilitation on Patients with Coronary Artery Disease: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2023, 11, e46359. [Google Scholar] [CrossRef]
- Medina-Inojosa, J.R.; Grace, S.L.; Supervia, M.; Stokin, G.; Bonikowske, A.R.; Thomas, R.; Lopez-Gimenez, F. Dose of Cardiac Rehabilitation to Reduce Mortality and Morbidity: A Population-Based Study. J. Am. Heart Assoc. 2021, 10, e021356. [Google Scholar] [CrossRef]
- Sultani, R.; Tong, D.C.; Peverelle, M.; Lee, Y.S.; Baradi, A.; Wilson, A.M. Elevated Triglycerides to High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio Predicts Long-Term Mortality in High-Risk Patients. Heart Lung Circ. 2020, 29, 414–421. [Google Scholar] [CrossRef]
- Zhang, K.; Qi, X.; Zhu, F.; Dong, Q.; Gou, Z.; Wang, F.; Xiao, L.; Li, M.; Chen, L.; Wang, Y.; et al. Remnant cholesterol is associated with cardiovascular mortality. Front. Cardiovasc. Med. 2022, 9, 984711. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, S.G.; Jeon, H.S.; Lee, J.W.; Youn, Y.J.; Lee, Y.J.; Lee, S.J.; Hong, S.J.; Ahn, C.M.; Ko, Y.G.; et al. Remnant cholesterol as a residual risk in atherosclerotic cardiovascular disease patients under statin-based lipid-lowering therapy: A post hoc analysis of the RACING trial. J. Clin. Lipidol. 2024, 18, e905–e914. [Google Scholar] [CrossRef]
- Drexel, H.; Mader, A.; Larcher, B.; Festa, A.; Vonbank, A.; Fraunberger, P.; Leiherer, A.; Saely, C.H. Remnant cholesterol and long-term incidence of death in coronary artery disease patients. Aherosclerosis 2025, 401, 119048. [Google Scholar] [CrossRef]
- Bays, H.E.; Neff, D.; Tomassini, J.E.; Tershakovec, A.M. Ezetimibe: Cholesterol lowering and beyond. Expert. Rev. Cardiovasc. Ther. 2008, 6, 447–470. [Google Scholar] [CrossRef]
- Dalli-Peydró, E.; Gisbert-Criado, R.; Amigó, N.; Sanz-Sevilla, N.; Cosín-Sales, J. Cardiac telerehabilitation with long-term follow-up reduces GlycA and improves lipoprotein particle profile: A randomised controlled trial. Int. J. Cardiol. 2022, 369, 60–64. [Google Scholar] [CrossRef]
- Otvos, J.D.; Guyton, J.R.; Connelly, M.A.; Akapame, S.; Bittner, V.; Kopecky, S.L.; Lacy, M.; Marcovina, S.M.; Muhlestein, J.B.; Boden, W.E. Relations of GlycA and lipoprotein particle subspecies with cardiovascular events and mortality: A post hoc analysis of the AIM-HIGH trial. J. Clin. Lipidol. 2018, 12, 348–355. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; ESC Scientific Document Group; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 29, 3415–3537. [Google Scholar] [CrossRef]
- Abdulwahab, S.A.; Zedan, H.S. Factors Affecting Patient Perceptions and Satisfaction with Telemedicine in Outpatient Clinics. J. Patient. Exp. 2021, 8, 23743735211063780. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.C.; Maita, K.C.; Inselman, J.W.; Avila, F.R.; Torres-Guzman, R.A.; Coffey, J.D.; Christopherson, L.A.; Leuenberger, A.M.; Bell, S.J.; Pahl, D.F.; et al. Patient Satisfaction with a Multisite, Multiregional Remote Patient Monitoring Program for Acute and Chronic Condition Management: Survey-Based Analysis. J. Med. Internet Res. 2023, 25, e44528. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, N.S.; Hartley, A.; Howard, J.; Hajhosseiny, R.; Khawaja, S.; Seligman, H.; Akbari, T.; Alharbi, B.A.; Bassett, P.; Al-Lamee, R.; et al. Randomized Trial of Remote Assessment of Patients After an Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2024, 83, 2250–2259. [Google Scholar] [CrossRef]
- Dalli-Peydró, E.; Muñoz-Ramos, N.; Fresneda-Fresneda, A.; Serrats-López, R.; Herrera-Vázquez, A.; Rubio-Sánchez, A.; de la Fuente-Rivas, F.; Pellicer-Iborra, V.; Ramírez-Candela, S.; Llanos-Garaboa, A. Extended telemonitoring after acute coronary syndrome improves the patient experience and reduces outpatient visits. In Proceedings of the Annual Meeting of the Clinical Cardiology Association of the Spanish Society of Cardiology, Barcelona, Spain, April 2024. [Google Scholar]
- Dalli-Peydró, E.; Martí, M.P.; Tuzón-Segarra, M.T.; Fresneda-Fresneda, A.; Muñoz-Ramos, N.; Cosín-Sales, J. Cost-utility of cardiac telerehabilitation versus conventional hospital rehabilitation after ACS in Spain. Rev. Esp. Cardiol. 2024, 77, 99–101. [Google Scholar] [CrossRef]
| Variable | Standard Follow-Up | Telemonitored Follow-Up | p-Value | |
|---|---|---|---|---|
| Patients [n (%)] | 50 (100.0%) | 75 (100.0%) | ||
| Sex [n (%)] | Male | 46 (92.0%) | 65 (86.7%) | 0.402 |
| Female | 4 (8.0%) | 10 (13.3%) | ||
| Age (years) | Mean (SD) | 60.8 (7.9) | 58.0 (9.4) | 0.079 |
| Median (Range) | 61 (41–75) | 58 (34–75) | ||
| Body Mass Index | Mean (SD) | 28.1 (4.0) | 27.9 (4.6) | 0.864 |
| Median (Range) | 28 (21–39) | 27 (20–43) | ||
| Hypertension [n (%)] | No | 24 (48.0%) | 36 (48.0%) | 1.000 |
| Yes | 26 (52.0%) | 39 (52.0%) | ||
| Diabetes Mellitus [n (%)] | No | 40 (80.0%) | 57 (76.0%) | 0.666 |
| Yes | 10 (20.0%) | 18 (24.0%) | ||
| Dyslipidemia [n (%)] | No | 25 (50.0%) | 38 (50.7%) | 1.000 |
| Yes | 25 (50.0%) | 37 (49.3%) | ||
| Smoking [n (%)] | No | 24 (48.0%) | 37 (49.3%) | 1.000 |
| Yes | 26 (52.0%) | 38 (50.7%) | ||
| AMI [n (%)] | No | 11 (22.0%) | 17 (22.7%) | 1.000 |
| Yes | 39 (78.0%) | 58 (77.3%) | ||
| UA [n (%)] | No | 40 (80.0%) | 58 (77.3%) | 0.826 |
| Yes | 10 (20.0%) | 17 (22.7%) | ||
| Statin treatment [n (%)] | None | 6 (12.0%) | 2 (2.7%) | 0.120 |
| Atorvastatin 80 mg | 17 (34.0%) | 23 (30.7%) | ||
| Atorvastatin 40 mg | 7 (14.0%) | 19 (25.3%) | ||
| Rosuvastatin 20 mg | 20 (40.0%) | 31 (41.3%) | ||
| Ezetimibe treatment [n (%)] | No | 20 (40.0%) | 13 (17.3%) | 0.007 |
| Yes | 30 (60.0%) | 62 (82.7%) | ||
| LVEF (%) | Mean (SD) | 57.1 (7.7) | 57.3 (6.6) | 0.912 |
| Median (Range) | 60 (35–70) | 60 (40–75) |
| Lipid Parameter | Time Reference | Standard Follow-Up | Telemontored Follow-Up | p-Value | p-Value * |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) [mean (SD)] | Hospital admission | 179.4 (47.0) | 166.2 (43.3) | 0.109 | 0.072 |
| 12-month post-discharge | 138.6 (52.3) | 125.8 (30.0) | 0.087 | 0.096 | |
| Decrease during follow-up | 40.9 (57.4) | 40.4 (48.5) | 0.958 | 0.800 | |
| HDL cholesterol (mg/dL) [mean (SD)] | Hospital admission | 44.9 (12.6) | 41.1 (10.6) | 0.071 | 0.065 |
| 12-month post-discharge | 47.4 (10.0) | 46.3 (11.5) | 0.578 | 0.526 | |
| Decrease during follow-up | −2.54 (10.1) | −5.23 (8.6) | 0.112 | 0.121 | |
| LDL cholesterol (mg/dL) [mean (SD)] | Hospital admission | 106.4 (44.5) | 95.6 (38.8) | 0.155 | 0.093 |
| 12-month post-discharge | 65.0 (44.9) | 57.9 (21.9) | 0.242 | 0.212 | |
| Decrease during follow-up | 41.4 (54.6) | 37.7 (41.3) | 0.670 | 0.544 | |
| VLDL cholesterol (mg/dL) [mean (SD)] | Hospital admission | 27.3 (10.6) | 29.2 (12.0) | 0.371 | 0.311 |
| 12-month post-discharge | 25.9 (13.1) | 21.1 (11.5) | 0.034 | 0.051 | |
| Decrease during follow-up | 1.41 (10.2) | 8.11 (12.6) | 0.002 | 0.003 | |
| Remnant cholesterol (mg/dL) [mean (SD)] | Hospital admission | 28.1 (11.6) | 29.5 (12.0) | 0.536 | 0.415 |
| 12-month post-discharge | 26.1 (13.7) | 21.7 (12.9) | 0.070 | 0.140 | |
| Decrease during follow-up | 2.00 (10.6) | 7.76 (13.2) | 0.011 | 0.018 | |
| Triglycerides (mg/dL) [mean (SD)] | Hospital admission | 141.0 (57.6) | 143.7 (53.3) | 0.787 | 0.788 |
| 12-month post-discharge | 140.4 (81.0) | 105.1 (52.9) | 0.004 | 0.011 | |
| Decrease during follow-up | 0.6 (58.0) | 38.6 (50.9) | <0.001 | 0.001 | |
| Total cholesterol/HDL ratio [mean (SD)] | Hospital admission | 4.30 (1.73) | 4.23 (1.36) | 0.826 | 0.719 |
| 12-month post-discharge | 2.99 (1.04) | 2.84 (0.95) | 0.412 | 0.510 | |
| Decrease during follow-up | 1.31 (1.75) | 1.39 (1.34) | 0.752 | 0.944 | |
| Triglycerides/HDL ratio [mean (SD)] | Hospital admission | 3.49 (1.90) | 3.77 (1.74) | 0.384 | 0.397 |
| 12-month post-discharge | 3.17 (2.30) | 2.48 (1.58) | 0.051 | 0.100 | |
| Decrease during follow-up | 0.32 (1.90) | 1.29 (1.59) | 0.002 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalli-Peydró, E.; Serrano-Romero, A.; Serrats-López, R.; Minaya-Zaballos, A.S.; Herrera-Vásquez, A.; Ramírez-Candela, S.; Arias-Fresneda, A.; Llanos-Gabaroa, A.; Muñoz-Ramos, N.; Fresneda-Fresneda, A.; et al. Extended Telemonitored Follow-Up After Acute Coronary Syndrome: A Healthcare Pathway That Improves Cardiovascular Prevention and Patient Experience, and Reduces Outpatient Visits. J. Clin. Med. 2025, 14, 7283. https://doi.org/10.3390/jcm14207283
Dalli-Peydró E, Serrano-Romero A, Serrats-López R, Minaya-Zaballos AS, Herrera-Vásquez A, Ramírez-Candela S, Arias-Fresneda A, Llanos-Gabaroa A, Muñoz-Ramos N, Fresneda-Fresneda A, et al. Extended Telemonitored Follow-Up After Acute Coronary Syndrome: A Healthcare Pathway That Improves Cardiovascular Prevention and Patient Experience, and Reduces Outpatient Visits. Journal of Clinical Medicine. 2025; 14(20):7283. https://doi.org/10.3390/jcm14207283
Chicago/Turabian StyleDalli-Peydró, Ernesto, Alicia Serrano-Romero, Rocío Serrats-López, Alvaro Salvador Minaya-Zaballos, Alan Herrera-Vásquez, Sofía Ramírez-Candela, Angela Arias-Fresneda, Alejandra Llanos-Gabaroa, Nuria Muñoz-Ramos, Amparo Fresneda-Fresneda, and et al. 2025. "Extended Telemonitored Follow-Up After Acute Coronary Syndrome: A Healthcare Pathway That Improves Cardiovascular Prevention and Patient Experience, and Reduces Outpatient Visits" Journal of Clinical Medicine 14, no. 20: 7283. https://doi.org/10.3390/jcm14207283
APA StyleDalli-Peydró, E., Serrano-Romero, A., Serrats-López, R., Minaya-Zaballos, A. S., Herrera-Vásquez, A., Ramírez-Candela, S., Arias-Fresneda, A., Llanos-Gabaroa, A., Muñoz-Ramos, N., Fresneda-Fresneda, A., & Cosín-Sales, J. (2025). Extended Telemonitored Follow-Up After Acute Coronary Syndrome: A Healthcare Pathway That Improves Cardiovascular Prevention and Patient Experience, and Reduces Outpatient Visits. Journal of Clinical Medicine, 14(20), 7283. https://doi.org/10.3390/jcm14207283





