Prevalence and Related Factors of Post-ERCP Pancreatitis in Cholangiocarcinoma Patients: A Retrospective Study in Northeast Thailand
Abstract
1. Introduction
2. Materials and Methods
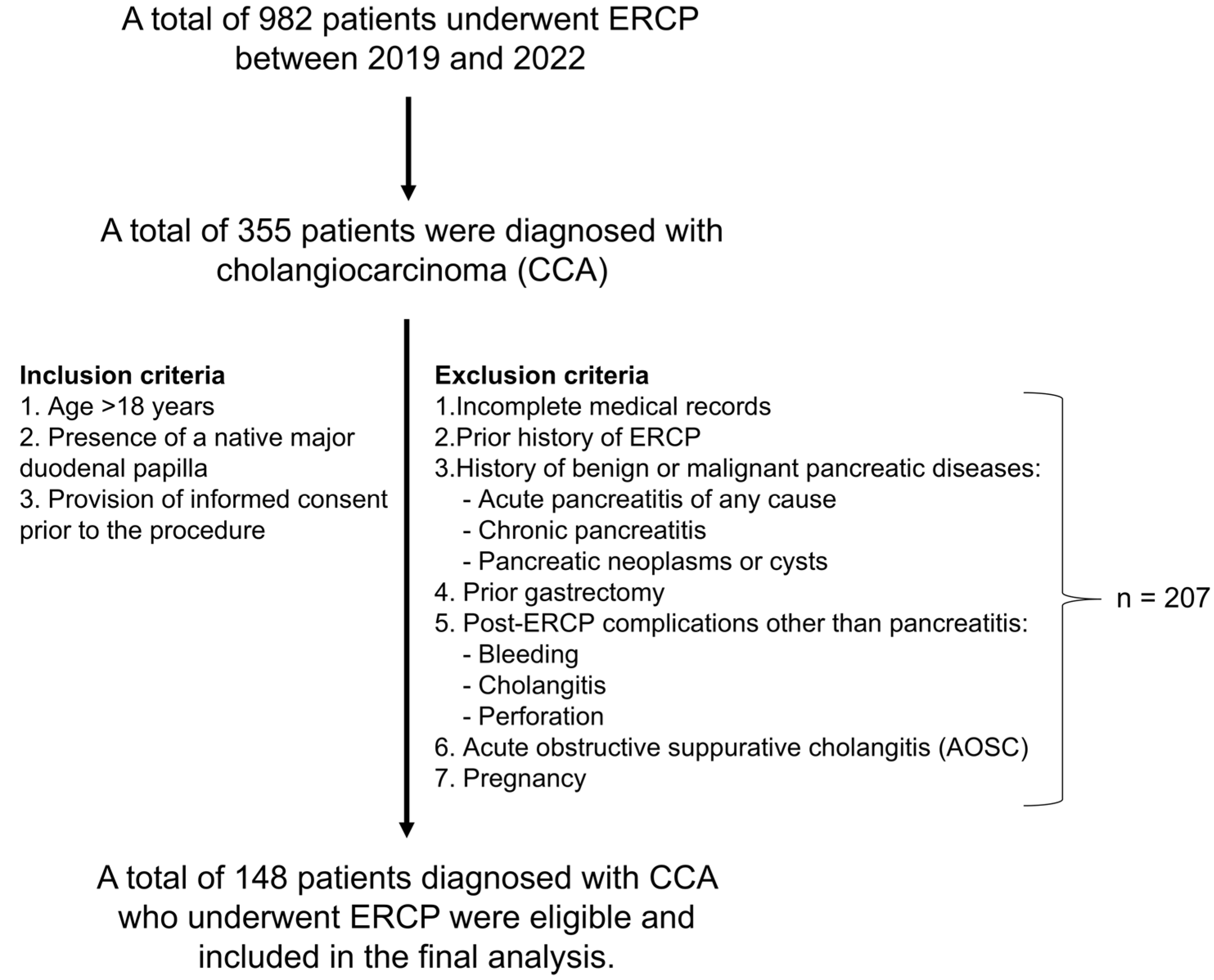
2.1. Study Design and Patient Population
2.2. Data Collection
2.3. Assessment of Post-ERCP Pancreatitis (PEP)
- Abdominal pain: New-onset or worsening abdominal pain suggestive of pancreatitis.
- Laboratory: Serum amylase level elevated to at least three times the upper limit of normal, measured more than 24 h after ERCP.
- Hospitalization: Hospitalization for more than one night attributable to the pancreatitis episode.
- Mild PEP: Hospitalization for 2–3 days without the need for additional interventions.
- Moderate PEP: Hospitalization lasting 4–10 days.
- Severe PEP: Hospitalization exceeding 10 days, or the presence of local complications (e.g., pancreatic necrosis, pseudocyst, or other severe complications requiring percutaneous drainage or surgery), consistent with the Cotton criteria [16].
2.4. Diagnosis of Other Complications
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Severity and Clinical Outcomes of PEP
3.3. Risk Factors Associated with PEP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERCP | Endoscopic Retrograde Cholangiopancreatography |
| PEP | Post-ERCP pancreatitis |
| iCCA | Intrahepatic cholangiocarcinoma |
| pCCA | Perihilar cholangiocarcinoma |
| dCCA | Distal cholangiocarcinoma |
| ECOG | Eastern Cooperative Oncology Group performance status |
References
- Vatanasapt, V.; Uttaravichien, T.; Mairiang, E.O.; Pairojkul, C.; Chartbanchachai, W.; Haswell-Elkins, M. Cholangiocarcinoma in north-east Thailand. Lancet 1990, 335, 116–117. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- de Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Cidon, E.U. Resectable Cholangiocarcinoma: Reviewing the Role of Adjuvant Strategies. Clin. Med. Insights Oncol. 2016, 10, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Khuntikeo, N.; Pugkhem, A.; Titapun, A.; Bhudhisawasdi, V. Surgical management of perihilar cholangiocarcinoma: A Khon Kaen experience. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 521–524. [Google Scholar] [CrossRef]
- Hu, Q.L.; Liu, J.B.; Ellis, R.J.; Liu, J.Y.; Yang, A.D.; D’Angelica, M.I.; Ko, C.Y.; Merkow, R.P. Association of preoperative biliary drainage technique with postoperative outcomes among patients with resectable hepatobiliary malignancy. HPB 2020, 22, 249–257. [Google Scholar] [CrossRef]
- Cohen, S.; Bacon, B.R.; Berlin, J.A.; Fleischer, D.; Hecht, G.A.; Loehrer, P.J., Sr.; McNair, A.E., Jr.; Mulholland, M.; Norton, N.J.; Rabeneck, L.; et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, 14–16 January 2002. Gastrointest. Endosc. 2002, 56, 803–809. [Google Scholar] [CrossRef]
- Patel, P.S.; Akshintala, V.S. Post-endoscopic retrograde cholangiopancreatography pancreatitis: A review. J. Pancreatol. 2024, 7, 28–34. [Google Scholar] [CrossRef]
- van der Gaag, N.A.; Rauws, E.A.J.; van Eijck, C.H.J.; Bruno, M.J.; van der Harst, E.; Kubben, F.J.G.M.; Gerritsen, J.J.G.M.; Greve, J.W.; Gerhards, M.F.; de Hingh, I.H.J.T.; et al. Preoperative Biliary Drainage for Cancer of the Head of the Pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef]
- Vanella, G.; Dell’Anna, G.; Bronswijk, M.; van Wanrooij, R.L.J.; Rizzatti, G.; Gkolfakis, P.; Larghi, A.; van der Merwe, S.; Arcidiacono, P.G. Endoscopic ultrasound-guided biliary drainage and gastrointestinal anastomoses: The journey from promising innovations to standard of care. Ann. Gastroenterol. 2022, 35, 441–451. [Google Scholar] [CrossRef]
- Cotton, P.B.; Garrow, D.A.; Gallagher, J.; Romagnuolo, J. Risk factors for complications after ERCP: A multivariate analysis of 11,497 procedures over 12 years. Gastrointest. Endosc. 2009, 70, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Barthet, M.; Lesavre, N.; Desjeux, A.; Gasmi, M.; Berthezene, P.; Berdah, S.; Viviand, X.; Grimaud, J.C. Complications of endoscopic sphincterotomy: Results from a single tertiary referral center. Endoscopy 2002, 34, 991–997. [Google Scholar] [CrossRef] [PubMed]
- El Nakeeb, A.; El Hanafy, E.; Salah, T.; Atef, E.; Hamed, H.; Sultan, A.M.; Hamdy, E.; Said, M.; El Geidie, A.A.; Kandil, T.; et al. Post-endoscopic retrograde cholangiopancreatography pancreatitis: Risk factors and predictors of severity. World J. Gastrointest. Endosc. 2016, 8, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Lazaraki, G.; Chatzimavroudis, G.; Gkagkalis, S.; Vasiliadis, I.; Papaeuthimiou, A.; Terzoudis, S.; Pilpilidis, I.; Zavos, C.; Kountouras, J. Risk factors for therapeutic ERCP-related complications: An analysis of 2715 cases performed by a single endoscopist. Ann. Gastroenterol. 2014, 27, 65–72. [Google Scholar]
- Chandrasekhara, V.; Khashab, M.A.; Muthusamy, V.R.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Gurudu, S.R.; et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef]
- Cotton, P.B.; Lehman, G.; Vennes, J.; Geenen, J.E.; Russell, R.C.G.; Meyers, W.C.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef]
- Miyatani, H.; Mashima, H.; Sekine, M.; Matsumoto, S. Post-ERCP biliary complications in patients with biliary type sphincter of Oddi dysfunction. Sci. Rep. 2018, 8, 9951. [Google Scholar] [CrossRef]
- Wu, H.M.; Dixon, E.; May, G.R.; Sutherland, F.R. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): A population-based review. HPB 2006, 8, 393–399. [Google Scholar] [CrossRef]
- Szary, N.M.; Al-Kawas, F.H. Complications of endoscopic retrograde cholangiopancreatography: How to avoid and manage them. Gastroenterol. Hepatol. 2013, 9, 496–504. [Google Scholar]
- Chi, J.Y.; Ma, L.Y.; Zou, J.C.; Ma, Y.F. Risk factors of pancreatitis after endoscopic retrograde cholangiopancreatography in patients with biliary tract diseases. BMC Surg. 2023, 23, 62. [Google Scholar] [CrossRef]
- Omar, M.A.; Ahmed, A.E.; Said, O.A.; El-Amin, H. Risk factors for post-ERCP pancreatitis: A prospective multicenter study in upper Egypt. Egypt. J. Surg. 2015, 34, 1–10. [Google Scholar] [CrossRef]
- Noda, J.; Takano, Y.; Tamai, N.; Yamawaki, M.; Azami, T.; Niiya, F.; Maruoka, N.; Nagahama, M. Difficult biliary cannulation during endoscopic retrograde cholangiopancreatography for distal malignant biliary obstruction caused by pancreatic cancer: An observational study. DEN Open 2025, 5, e70092. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Horhat, A.; Mois, E.; Graur, F.; Tefas, C.; Craciun, R.; Nenu, I.; Spârchez, M.; Sparchez, Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World J. Gastrointest. Oncol. 2021, 13, 2050–2063. [Google Scholar] [CrossRef] [PubMed]
- Syrén, E.; Eriksson, S.; Enochsson, L.; Eklund, A.; Sandblom, G. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography. BJS Open 2019, 3, 485–489. [Google Scholar] [CrossRef]
- Ergin, E.; Oruç, N.; Ersöz, G.; Tekeşin, O.; Özütemiz, Ö. Prognosis and risk factors of ERCP pancreatitis in elderly. Sci. Rep. 2021, 11, 15930. [Google Scholar] [CrossRef]
- Radetic, M.; Ladna, M.; Shen, S.; Daneshmand, A.; Seoud, T.; Dixit, D.; Qumseya, A.; Novikov, A.; Qumseya, B. Risk of post-ERCP pancreatitis due to placement of biliary self-expandable metal stents (SEMS): A single-center retrospective study. Gastrointest Endosc. 2025, in press. [Google Scholar] [CrossRef]
- Mandai, K.; Tsuchiya, T.; Kawakami, H.; Ryozawa, S.; Saitou, M.; Iwai, T.; Ogawa, T.; Tamura, T.; Doi, S.; Okabe, Y.; et al. Fully covered metal stents vs. plastic stents for preoperative biliary drainage in patients with resectable pancreatic cancer without neoadjuvant chemotherapy: A multicenter, prospective, randomized controlled trial. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 1185–1194. [Google Scholar] [CrossRef]
- Tol, J.A.; van Hooft, J.E.; Timmer, R.; Kubben, F.J.; van der Harst, E.; de Hingh, I.H.; Vleggaar, F.P.; Molenaar, I.Q.; Keulemans, Y.C.; Boerma, D.; et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut 2016, 65, 1981–1987. [Google Scholar] [CrossRef]
- Kato, S.; Kuwatani, M.; Hayashi, T.; Eto, K.; Ono, M.; Ehira, N.; Yamato, H.; Sano, I.; Taya, Y.; Onodera, M.; et al. Inutility of endoscopic sphincterotomy to prevent pancreatitis after biliary metal stent placement in the patients without pancreatic duct obstruction. Scand. J. Gastroenterol. 2020, 55, 503–508. [Google Scholar] [CrossRef]
- Kawakubo, K.; Isayama, H.; Nakai, Y.; Togawa, O.; Sasahira, N.; Kogure, H.; Sasaki, T.; Matsubara, S.; Yamamoto, N.; Hirano, K.; et al. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg. Endosc. 2012, 26, 771–776. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Yu, B.; Li, J.-S. Self-expandable metal stents and the risk of post-ERCP pancreatitis. Gastrointest. Endosc. 2025, 102, 620. [Google Scholar] [CrossRef]
- Kim, G.H.; Ryoo, S.K.; Park, J.K.; Park, J.K.; Lee, K.H.; Lee, K.T.; Lee, J.K. Risk Factors for Pancreatitis and Cholecystitis after Endoscopic Biliary Stenting in Patients with Malignant Extrahepatic Bile Duct Obstruction. Clin. Endosc. 2019, 52, 598–605. [Google Scholar] [CrossRef]
- Saito, H.; Kakuma, T.; Matsushita, I. Risk factors for the development of post-endoscopic retrograde cholangiopancreatography pancreatitis in patients with asymptomatic common bile duct stones. World J. Gastrointest. Endosc. 2019, 11, 515–522. [Google Scholar] [CrossRef]
- Saito, H.; Kadono, Y.; Shono, T.; Kamikawa, K.; Urata, A.; Nasu, J.; Uehara, M.; Matsushita, I.; Kakuma, T.; Hashigo, S.; et al. Synergistic effect of independent risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis: A multicenter retrospective study in Japan. Clin. Endosc. 2024, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Todor, S.B.; Hasegan, A.; Bacila, C.; Solomon, A.; Cristian, A.; Dura, H. Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. J. Pers. Med. 2023, 13, 1356. [Google Scholar] [CrossRef]
- Freeman, M.L.; DiSario, J.A.; Nelson, D.B.; Fennerty, M.B.; Lee, J.G.; Bjorkman, D.J.; Overby, C.S.; Aas, J.; Ryan, M.E.; Bochna, G.S.; et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest Endosc 2001, 54, 425–434. [Google Scholar] [CrossRef]
| Variable | Total (n = 148) | Non-PEP n = 109 (%) | PEP n = 39 (%) | p-Value |
|---|---|---|---|---|
| Age, yr (median (range)) | 66 (34–89) | 64 (34–89) | 68 (39–86) | 0.021 * |
| <66 | 69 (46.6) | 57 (52.3) | 12 (30.6) | |
| ≥66 | 79 (53.4) | 52 (47.7) | 27 (69.4) | |
| Gender | 0.493 | |||
| Male | 94 (63.5) | 71 (65.1) | 23 (59) | |
| Female | 54 (36.5) | 38 (34.9) | 16 (41) | |
| BMI (kg/m2), mean ± SD | 0.508 | |||
| <18.5 | 20 (13.5) | 13 (11.9) | 7 (17.9) | |
| 18.5–24.9 | 94 (63.5) | 72 (66.1) | 22 (56.4) | |
| >25 | 34 (23.0) | 24 (22) | 10 (25.6) | |
| Underlying disease a | 0.547 b | |||
| NO | 89 (60) | 63 (57.8) | 26 (66.7) | |
| T2DM | 41 (28) | 30 (27.5) | 11 (28.1) | |
| Hypertension | 15 (10) | 13 (11.9) | 2 (5.1) | |
| Combine | 3 (2) | 3 (2.8) | 0 (0) | |
| ECOG | 0.118 | |||
| 1 | 69 (46.6) | 47 (43.1) | 22 (56.4) | |
| 2 | 60 (40.5) | 49 (45.0) | 11 (28.2) | |
| 3/4 | 19 (12.9) | 13 (11.9) | 6 (15.4) | |
| Total bilirubin (mg/dL), median (range) | 15 (0.2–40.7) | 19 (0.2–40.4) | 14.9 (0.3–40.7) | 0.031 * |
| <15 | 73 (49.3) | 48 (44) | 25 (64.1) | |
| ≥15 | 75 (50.7) | 61 (56) | 14 (35.9) | |
| Location of CCA | 0.985 | |||
| iCCA | 24 (16.2) | 18 (16.5) | 6 (15.4) | |
| pCCA | 101 (68.2) | 74 (67.9) | 27 (69.6) | |
| dCCA | 23 (15.6) | 17 (15.6) | 6 (15.4) | |
| Sphincterotomy | 0.733 | |||
| No | 113 (76.4) | 84 (77.1) | 29 (74.4) | |
| Yes | 35 (23.6) | 25 (22.9) | 10 (25.6) | |
| Pancreatic duct stent | 1.000 b | |||
| No | 143 (96.6) | 105 (96.3) | 38 (97.4) | |
| Yes | 5 (3.4) | 4 (3.7) | 1 (2.6) | |
| Stent type | 0.020 b,* | |||
| No stent | 29 (19.6) | 25 (22.9) | 4 (10.3) | |
| ENBD | 23 (15.5) | 19 (17.4) | 4 (10.3) | |
| Plastic stent | 18 (12.2) | 16 (14.7) | 2 (5.1) | |
| Metallic stent | 78 (52.7) | 49 (45) | 29 (74.3) | |
| Technically success | 0.013 * | |||
| No | 27 (18.2) | 25 (22.9) | 2 (5.1) | |
| Yes | 121 (81.8) | 84 (77.1) | 37 (94.9) | |
| Total procedure time (min.), median (range) | 60 (18–140) | 60 (18–120) | 60 (20–140) | 0.849 |
| <60 | 55 (37.2) | 41 (37.6) | 14 (35.9) | |
| ≥60 | 93 (62.8) | 68 (62.4) | 25 (64.1) | |
| Length of stay (days), median (range) | 4 (2–36) | 3 (2–36) | 7 (3–21) | <0.001 * |
| <4 | 63 (42.6) | 59 (54.1) | 4 (10.3) | |
| ≥4 | 85 (57.4) | 50 (45.9) | 35 (89.7) | |
| Post-ERCP complication (cholangitis) | 0.042 * | |||
| No | 122 (82.4) | 94 (86.2) | 28 (71.8) | |
| Yes | 26 (17.6) | 15 (13.8) | 11 (28.2) | |
| Mortality (in hospital) | 148 | 0 | 1 (0.7%) | - |
| Variable | Total PEP n = 39 (100%) | Mild/Moderate n = 28 (71.8%) | Severe n = 11 (28.2%) |
|---|---|---|---|
| Age ≥ 66 years, n (%) | 30 (76.9) | 21 (75) | 9 (81.8) |
| Male sex, n (%) | 23 (59.0) | 18 (64.3) | 5 (45.5) |
| Overweight/Obese, n (%) | 10 (25.6) | 5 (17.9) | 5 (45.5) |
| pCCA, n (%) | 27 (69) | 20 (71) | 7 (63) |
| Total bilirubin ≥ 15 mg/dL, n (%) | 14 (35.9) | 8 (28.6) | 6 (54.5) |
| Metallic stent, n (%) | 29 (74.3) | 19 (67.9) | 10 (90.9) |
| Sphincterotomy, n (%) | 10 (25.6) | 7 (25.0) | 3 (27.3) |
| ECOG 3–4, n (%) | 6 (15.4) | 3 (10.7) | 3 (27.7) |
| Length of Stay ≥ 4 days, n (%) | 35 (89.7) | 24 (85.7) | 11 (100) |
| Post-ERCP cholangitis, n (%) | 11 (28.2) | 8 (28.6) | 3 (27.3) |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age, yr (Median) | ||||||
| <66 | 1 | 1 | ||||
| ≥66 | 3.65 | 1.59–8.42 | 0.002 * | 2.89 | 1.17–7.17 | 0.022 * |
| Gender | ||||||
| Male | 1 | - | ||||
| Female | 1.30 | 0.61–2.75 | 0.493 | - | - | - |
| BMI (kg/m2) | ||||||
| <18.5) | 1 | - | ||||
| 18.5–24.9 | 0.57 | 0.20–1.59 | 0.284 | - | - | - |
| >25 | 0.77 | 0.24–2.51 | 0.670 | - | - | - |
| Underlying disease a | ||||||
| No | 1 | - | ||||
| T2DM | 0.888 | 0.39–2.03 | 0.780 | - | - | - |
| Hypertension | 0.373 | 0.08–1.77 | 0.214 | - | - | - |
| Combine b | - | - | - | |||
| ECOG score | ||||||
| 1 | 1 | - | ||||
| 2 | 0.48 | 0.21–1.10 | 0.082 | - | - | - |
| 3–4 | 0.99 | 0.33–2.94 | 0.980 | - | - | - |
| Total bilirubin (mg/dL), median (range) | ||||||
| <15 | 1 | 1 | ||||
| ≥15 | 0.44 | 0.21–0.94 | 0.034 * | 0.41 | 0.17–0.92 | 0.037 * |
| Location of CCA | ||||||
| iCCA | 1 | - | ||||
| pCCA | 1.09 | 0.39–3.05 | 0.863 | - | - | - |
| dCCA | 1.06 | 0.29–3.93 | 0.932 | - | - | - |
| Sphincterotomy | ||||||
| No | 1 | - | ||||
| Yes | 1.16 | 0.49–2.70 | 0.733 | - | - | - |
| Stent type | ||||||
| Non-metallic stent c | 1 | 1 | ||||
| Metallic stent | 3.55 | 1.58–7.99 | 0.002 * | 3.46 | 1.30–7.39 | 0.013 * |
| Technically success | ||||||
| No | 1 | 1 | ||||
| Yes | 5.51 | 1.24–24.46 | 0.025 * | 12.51 | 0.59–18.07 | 0.177 |
| Total procedure time (min), median (range) | ||||||
| <60 | 1 | - | ||||
| ≥60 | 1.08 | 0.50–2.30 | 0.849 | - | - | - |
| Post-ERCP cholangitis | ||||||
| No | 1 | 1 | ||||
| Yes | 2.46 | 1.01–5.97 | 0.046 * | 3.41 | 1.21–9.66 | 0.021 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karntaumporn, T.; Thanasukarn, V.; Srisuk, T.; Luvira, V.; Tipwaratorn, T.; Jareanrat, A.; Rattanarak, K.; Kraphunpongsakul, K.; Khuntikeo, N.; Chindaprasirt, J.; et al. Prevalence and Related Factors of Post-ERCP Pancreatitis in Cholangiocarcinoma Patients: A Retrospective Study in Northeast Thailand. J. Clin. Med. 2025, 14, 7286. https://doi.org/10.3390/jcm14207286
Karntaumporn T, Thanasukarn V, Srisuk T, Luvira V, Tipwaratorn T, Jareanrat A, Rattanarak K, Kraphunpongsakul K, Khuntikeo N, Chindaprasirt J, et al. Prevalence and Related Factors of Post-ERCP Pancreatitis in Cholangiocarcinoma Patients: A Retrospective Study in Northeast Thailand. Journal of Clinical Medicine. 2025; 14(20):7286. https://doi.org/10.3390/jcm14207286
Chicago/Turabian StyleKarntaumporn, Tanapoom, Vasin Thanasukarn, Tharatip Srisuk, Vor Luvira, Theerawee Tipwaratorn, Apiwat Jareanrat, Krit Rattanarak, Khanisara Kraphunpongsakul, Natcha Khuntikeo, Jarin Chindaprasirt, and et al. 2025. "Prevalence and Related Factors of Post-ERCP Pancreatitis in Cholangiocarcinoma Patients: A Retrospective Study in Northeast Thailand" Journal of Clinical Medicine 14, no. 20: 7286. https://doi.org/10.3390/jcm14207286
APA StyleKarntaumporn, T., Thanasukarn, V., Srisuk, T., Luvira, V., Tipwaratorn, T., Jareanrat, A., Rattanarak, K., Kraphunpongsakul, K., Khuntikeo, N., Chindaprasirt, J., Eurboonyanun, K., Sa-Ngiamwibool, P., Loilome, W., Prajumwongs, P., & Titapun, A. (2025). Prevalence and Related Factors of Post-ERCP Pancreatitis in Cholangiocarcinoma Patients: A Retrospective Study in Northeast Thailand. Journal of Clinical Medicine, 14(20), 7286. https://doi.org/10.3390/jcm14207286





