Outcomes, Sequelae, and Ventilatory Strategies in Long COVID Patients with Severe ARDS: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
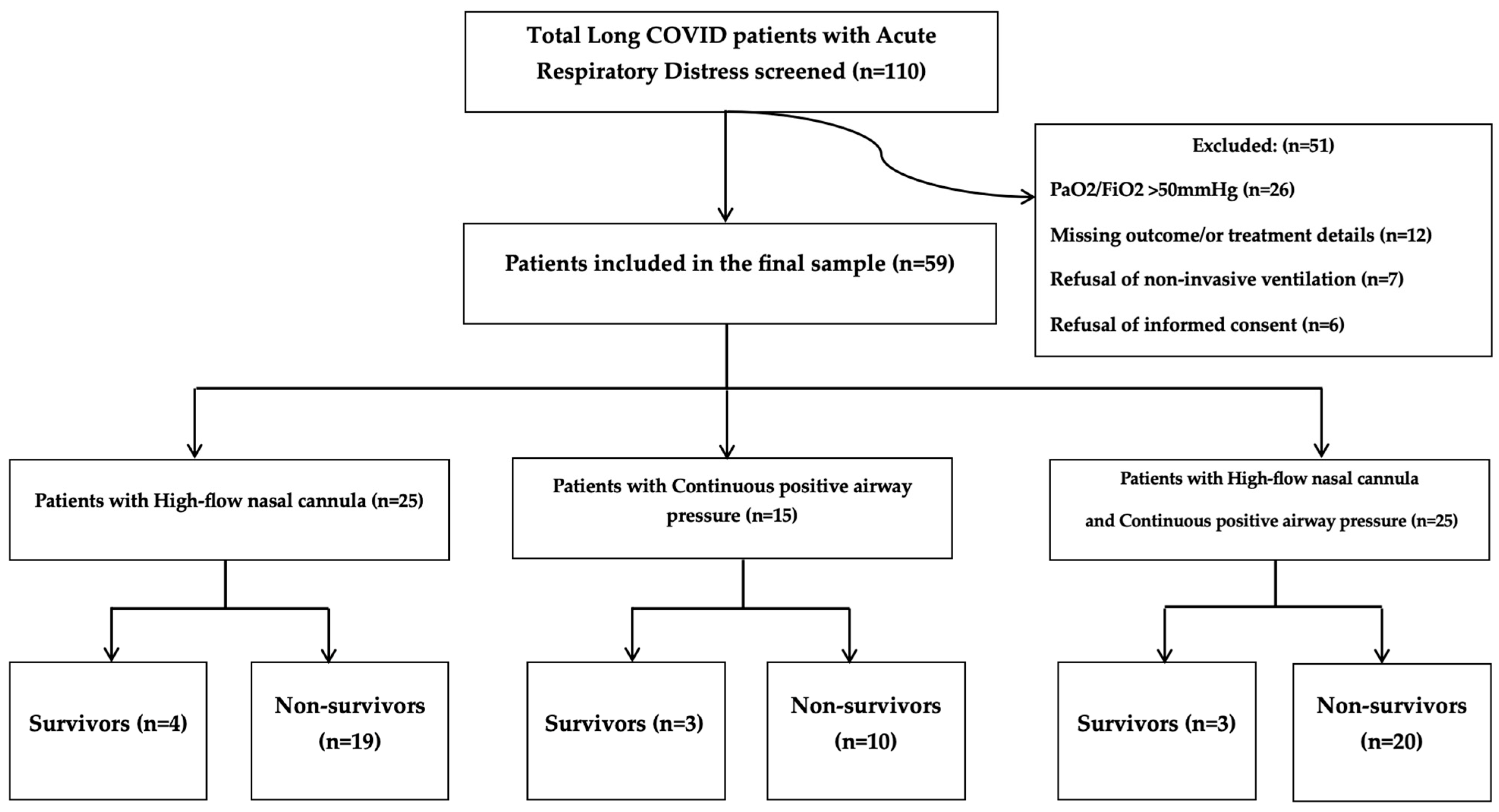
2.1. Study Design and Participants
2.2. Interventions
2.2.1. Continuous Positive Airway Pressure (CPAP)
2.2.2. High-Flow Nasal Cannula (HFNC)
2.3. Statistical Analysis
3. Results
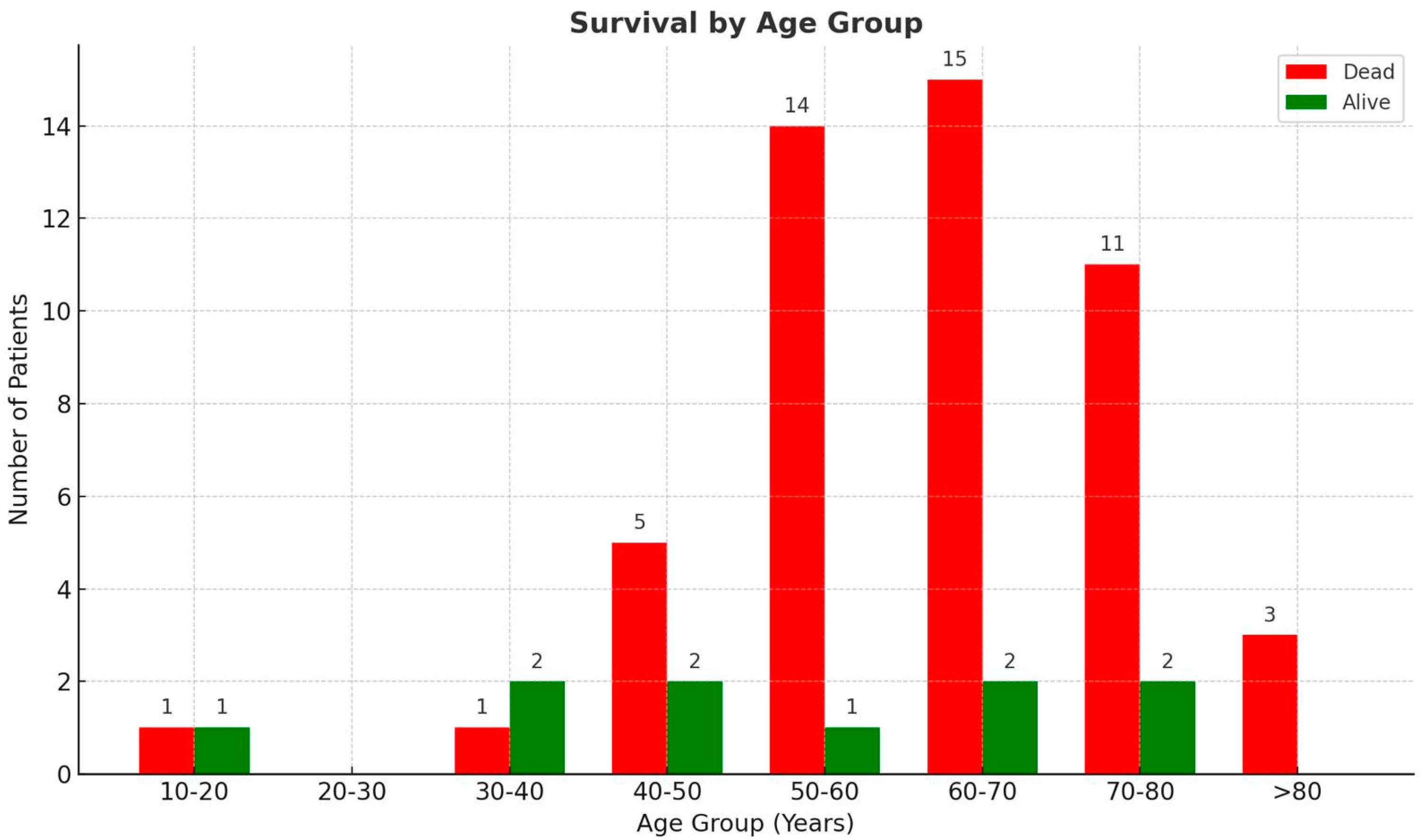
3.1. Patient Demographics
3.2. Comorbidity Analysis
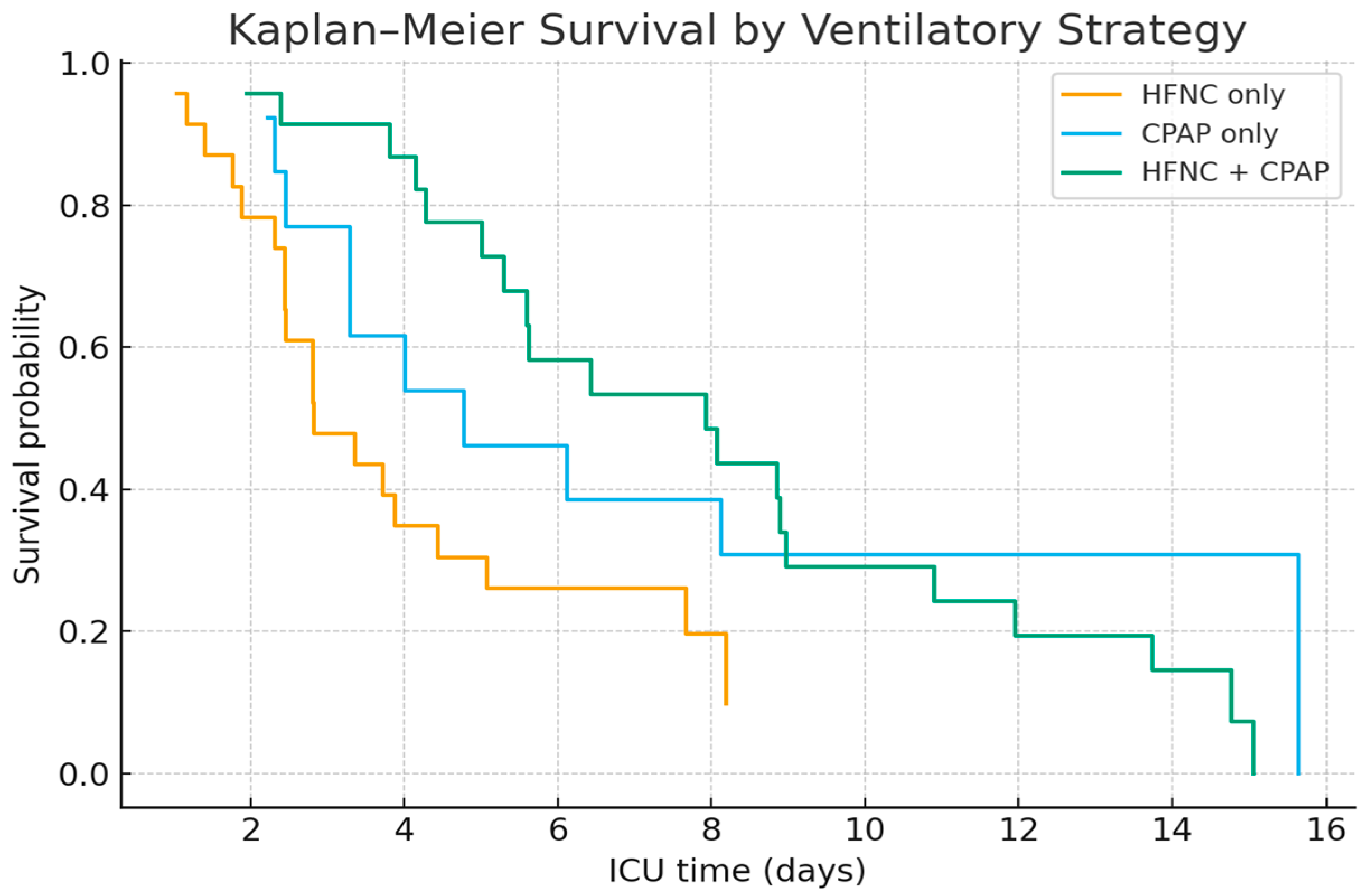
3.3. Ventilatory Support and Treatment Outcomes
4. Discussion
Study Strengths, Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azagew, A.W.; Beko, Z.W.; Ferede, Y.M.; Mekonnen, H.S.; Abate, H.K.; Mekonnen, C.K. Global prevalence of COVID-19-induced acute respiratory distress syndrome: Systematic review and meta-analysis. Syst. Rev. 2023, 12, 212. [Google Scholar] [CrossRef]
- Radovanovic, D.; Coppola, S.; Franceschi, E.; Gervasoni, F.; Duscio, E.; Chiumello, D.A.; Santus, P. Mortality and clinical outcomes in patients with COVID-19 pneumonia treated with non-invasive respiratory support: A rapid review. J. Crit. Care 2021, 65, 1–8. [Google Scholar] [CrossRef]
- World Health Organization. Post COVID-19 Condition (Long COVID). 2024. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-condition (accessed on 6 March 2025).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Patton, M.J.; Benson, D.; Robison, S.W.; Raval, D.; Locy, M.L.; Patel, K.; Grumley, S.; Levitan, E.B.; Morris, P.; Might, M.; et al. Characteristics and determinants of pulmonary long COVID. JCI Insight 2024, 9, e177518. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, S.; Vidal, M.A.; Hernandez, M.A.; Henriquez-Beltran, M.E.; Cabrera, C.; Quiroga, R.; Antilef, B.E.; Aguilar, K.P.; Castillo, D.A.; Llerena, F.J.; et al. Clinical and pulmonary function analysis in long-COVID revealed that long-term pulmonary dysfunction is associated with vascular inflammation pathways and metabolic syndrome. Front. Med. 2023, 10, 1271863. [Google Scholar] [CrossRef]
- Šitum, I.; Hrvoić, L.; Erceg, A.; Mandarić, A.; Karmelić, D.; Mamić, G.; Džaja, N.; Babić, A.; Mihaljević, S.; Mažar, M.; et al. CPAP vs. HFNC in treatment of patients with COVID-19 ARDS: A retrospective propensity-matched study. Can. J. Respir. Ther. 2024, 60, 164–172. [Google Scholar] [CrossRef]
- Al Hashim, A.H.; Al Reesi, A.; Al Lawati, N.M.; Burad, J.; Al Khabori, M.; Chandwani, J.; Al Lawati, R.; Al Masroori, Y.; Al Balushi, A.A.; Al Masroori, S.; et al. Comparison of Noninvasive Mechanical Ventilation with High-Flow Nasal Cannula, Face-Mask, and Helmet in Hypoxemic Respiratory Failure in Patients with COVID-19: A Randomized Controlled Trial. Crit. Care Med. 2023, 51, 1515–1526. [Google Scholar] [CrossRef]
- Mirunalini, G.; Anand, K.; Pushparani, A.; Kadirvelu, G. Comparison of High Flow Nasal Cannula and Continuous Positive Airway Pressure in COVID-19 Patients with Acute Respiratory Distress Syndrome in Critical Care Unit: A Randomized Control Study. Cureus 2023, 15, e45798. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, L.; Guo, L.; Wu, L.; Alwalid, O.; Liu, J.; Zheng, Y.; Chen, L.; Wu, W.; Li, H.; et al. Long-term radiological and pulmonary function abnormalities at 3 years after COVID-19 hospitalisation: A longitudinal cohort study. Eur. Respir. J. 2024, 64, 2301612. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Q.; Yuan, X.; Lu, C.; Wang, S.; Yuan, Y. Diagnostic value of lung function tests in long COVID: Analysis of positive bronchial provocation test outcomes. Front. Med. 2024, 11, 1512658. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Cianciulli, A.; Santoro, E.; Manente, R.; Pacifico, A.; Comunale, G.; Finizio, M.; Capunzo, M.; De Caro, F.; Franci, G.; Moccia, G.; et al. Validation of a Questionnaire on the Post-COVID-19 Condition (Long COVID): A Cross-Sectional Study in Italy. Infect. Dis. Rep. 2025, 17, 69. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Malin, J.J.; Spinner, C.D.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Gastmeier, P.; Langer, F.; Wepler, M.; Westhoff, M.; et al. Key summary of German national treatment guidance for hospitalized COVID-19 patients. Infection 2022, 50, 93–106. [Google Scholar]
- Gulick, R.M.; Pau, A.K.; Daar, E.; Evans, L.; Gandhi, R.T.; Tebas, P.; Ridzon, R.; Masur, H.; Lane, H.C.; NIH COVID-19 Treatment Guidelines Panel. National Institutes of Health COVID-19 Treatment Guidelines Panel: Perspectives and Lessons Learned. Ann. Intern. Med. 2024, 177, 1547–1557. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F. Mortality of Post-COVID-19 Condition: 2025 Update. COVID 2025, 5, 11. [Google Scholar] [CrossRef]
- Murad, M.; Atkin, S.L.; Wasif, P.; Behzad, A.A.; Husain, A.M.J.A.; Leahy, R.; d’Hellencourt, F.L.; Joury, J.; Aziz, M.A.; Valluri, S.R.; et al. Burden of acute and long-term COVID-19: A nationwide study in Bahrain. Front. Public Health 2025, 13, 1539453. [Google Scholar] [CrossRef]
- Cai, M.; Xie, Y.; Topol, E.J.; Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 2024, 30, 1564–1573. [Google Scholar] [CrossRef]
- Tsolaki, V.; Zakynthinos, G.E.; Mantzarlis, K.; Makris, D. Increased mortality among hypertensive COVID-19 patients: Pay a closer look on diuretics in mechanically ventilated patients. Heart Lung 2020, 49, 894–895. [Google Scholar] [CrossRef]
- Dosbayeva, A.; Serikbayev, A.; Sharapiyeva, A.; Amrenova, K.; Krykpayeva, A.; Kairkhanova, Y.; Dyussupov, A.; Seitkabylov, A.; Zhumanbayeva, Z. Post-COVID-19 Syndrome: Incidence, Biomarkers, and Clinical Patterns in Kazakhstan. Georgian Med. News 2025, 363, 184–192. [Google Scholar]
- Vasiliu, L.; Dodi, G.; Onofriescu, M.; Diaconu, A.; Voroneanu, L.; Sascau, R.A.; Statescu, C.; Covic, A.C. The Impact of Prior COVID-19 on Long-Term Mortality and Echocardiographic Predictors in Chronic Kidney Disease Patients. Diagnostics 2025, 15, 678. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Mei, Q.; Walline, J.H.; Zhang, Z.; Liu, Y.; Zhu, H.; Du, B. Cardiovascular outcomes in long COVID-19: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2025, 12, 1450470. [Google Scholar] [CrossRef]
- Brîndușe, L.A.; Eclemea, I.; Neculau, A.E.; Păunescu, B.A.; Bratu, E.C.; Cucu, M.A. Rural versus urban healthcare through the lens of health behaviors and access to primary care: A post-hoc analysis of the Romanian health evaluation survey. BMC Health Serv. Res. 2024, 24, 1341. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; De Soyza, A.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients with Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA 2022, 327, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Maritescu, A.; Crisan, A.F.; Pescaru, C.C.; Stoicescu, E.R.; Oancea, C.; Iacob, D. Effectiveness of Combined Pulmonary Rehabilitation and Progressive Muscle Relaxation in Treating Long-Term COVID-19 Symptoms: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 6237. [Google Scholar] [CrossRef] [PubMed]
- Florian, C.A.; Corina, P.C.; Adelina, M.; Vlad, C.; Cristian, O.; Emanuela, V. Respiratory Muscle Training and Its Impact on Balance and Gait in Patients with Severe COPD. Medicina 2024, 60, 257. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar]

| Parameter | Survivor (n = 10, 15%) | Non-Survivor (n = 49, 85%) |
|---|---|---|
| Female (n, %) | 2 (20%) | 12 (25.45%) |
| Male (n, %) | 8 (80%) | 37 (74.55%) |
| Days in ICU (mean ± SD, max) | 11 ± 10.02 (max 39) | 6.36 ± 5.85 (max 33) |
| Smoking (n, %) | 0 | 2 (4%) |
| Comorbidities | Survivor (n = 10) | Non-Survivor (n = 49) |
|---|---|---|
| Number of comorbidities (mean ± SD) | 3.2 ± 0.6 | 3.33 ± 1.49 |
| Range of comorbidities (min–max) | 2–4 | 0–6 |
| Diabetes Mellitus (n, %) | 6 (60%) | 31 (56%) |
| BMI > 30 (n, %) | 4 (40%) | 23 (42%) |
| Arterial hypertension (n, %) | 8 (80%) | 32 (71%) |
| Pulmonary diseases (n, %) | 1 (10%) | 6 (11%) |
| Other cardiovascular diseases (n, %) | 4 (40%) | 32 (58%) |
| Hepatic diseases (n, %) | 3 (30%) | 16 (29%) |
| Onco- and hematological disease (n, %) | 3 (30%) | 17 (31%) |
| Neurological disorders (n, %) | 2 (20%) | 10 (18%) |
| Chronic kidney disease (n, %) | 1 (10%) | 9 (16%) |
| Patients’ Therapy | ||
|---|---|---|
| Therapy (HFNC) | Survivor | Non-Survivor |
| Number of patients (n, %) | 4 (17.4%) | 19 (82.6%) |
| On ICU (in days) (mean, SD) | 6.5 ± 3.28 | 3.84 ± 2.48 |
| Endotracheal intubation (n, %) | 0 | 3 (15.79%) |
| PaCO2 at admission (mean, SD) | 35.48 ± 2.25 mmHg | 41.87 ± 21.93 mmHg |
| PaO2 at admission (mean SD) | 53.95 ± 24.9 mmHg | 55.35 ± 26.49 mmHg |
| Therapy (CPAP) | ||
| Number of patients (n, %) | 3 (23.08%) | 10 (76.92%) |
| On ICU (in days) (mean, SD) | 11 ± 2.16 | 5.9 ± 4.16 |
| Endotracheal intubation (n, %) | 2 (66.67%) | 3 (30%) |
| PaCO2 at admission (mean, SD) | 45.55 ± 0.45 mmHg | 31.79 ± 4.43 mmHg |
| PaO2 at admission (mean SD) | 29.85 ± 3.65 mmHg | 54.83 ± 25.25 mmHg |
| Therapy (HFNC + CPAP) | ||
| Number of patients (n, %) | 3 (13.04%) | 20 (86.96%) |
| On ICU (in days) (mean, SD) | 17 ± 15.9 | 9.1 ± 7.56 |
| Endotracheal intubation (n, %) | 2 (66.67%) | 18 (90%) |
| PaCO2 at admission (mean, SD) | 34.63 ± 6.47 mmHg | 33.78 ± 10.05 mmHg |
| PaO2 at admission (mean SD) | 60.23 ± 32.54 mmHg | 48.12 ± 30.63 mmHg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mîțu, D.-A.; Buleu, F.; Popa, D.-I.; Trebuian, C.; Sutoi, D.; Coman, A.; Lighezan, D.F.; Buleu, T.; Sliman, N.; Radbea, O.R.; et al. Outcomes, Sequelae, and Ventilatory Strategies in Long COVID Patients with Severe ARDS: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 7223. https://doi.org/10.3390/jcm14207223
Mîțu D-A, Buleu F, Popa D-I, Trebuian C, Sutoi D, Coman A, Lighezan DF, Buleu T, Sliman N, Radbea OR, et al. Outcomes, Sequelae, and Ventilatory Strategies in Long COVID Patients with Severe ARDS: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(20):7223. https://doi.org/10.3390/jcm14207223
Chicago/Turabian StyleMîțu, Diana-Alexandra, Florina Buleu, Daian-Ionel Popa, Cosmin Trebuian, Dumitru Sutoi, Adina Coman, Daniel Florin Lighezan, Tiberiu Buleu, Natheer Sliman, Oana Raluca Radbea, and et al. 2025. "Outcomes, Sequelae, and Ventilatory Strategies in Long COVID Patients with Severe ARDS: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 20: 7223. https://doi.org/10.3390/jcm14207223
APA StyleMîțu, D.-A., Buleu, F., Popa, D.-I., Trebuian, C., Sutoi, D., Coman, A., Lighezan, D. F., Buleu, T., Sliman, N., Radbea, O. R., & Mederle, O. A. (2025). Outcomes, Sequelae, and Ventilatory Strategies in Long COVID Patients with Severe ARDS: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(20), 7223. https://doi.org/10.3390/jcm14207223








