Objective and Subjective Voice Outcomes in Post-COVID-19 Dysphonia: A High-Speed Videoendoscopy Pre–Post Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Patient-Reported Outcomes (PROs)
2.4. Laryngological Examination and Imaging
2.4.1. High-Speed Videoendoscopy (HSV) Acquisition
2.4.2. Rater Blinding and Reliability
2.4.3. Post-Processing and Kymographic Analysis
2.5. Treatment Protocol
2.6. Outcomes
2.7. Statistical Analysis
3. Results
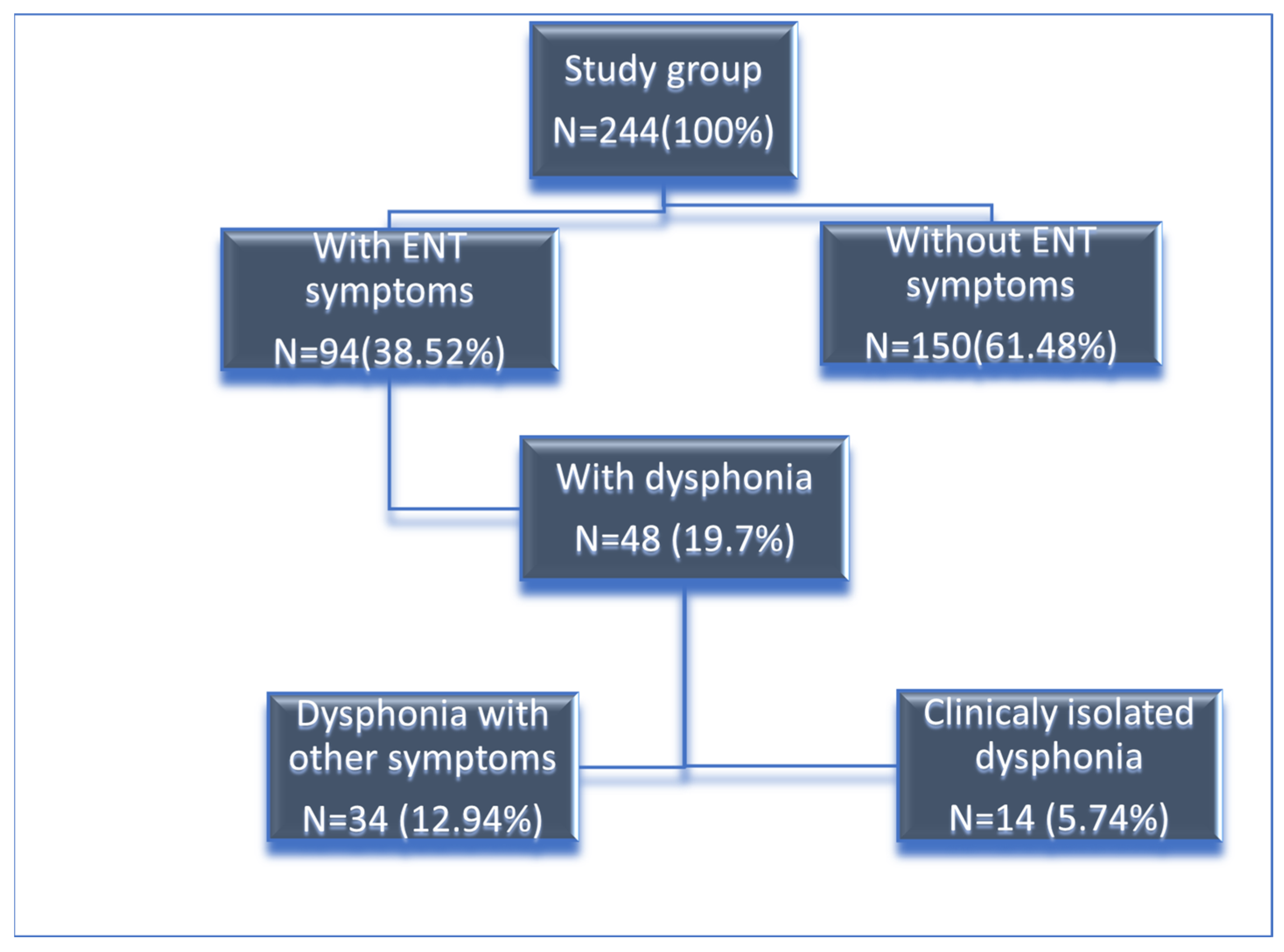
3.1. Patient Overview
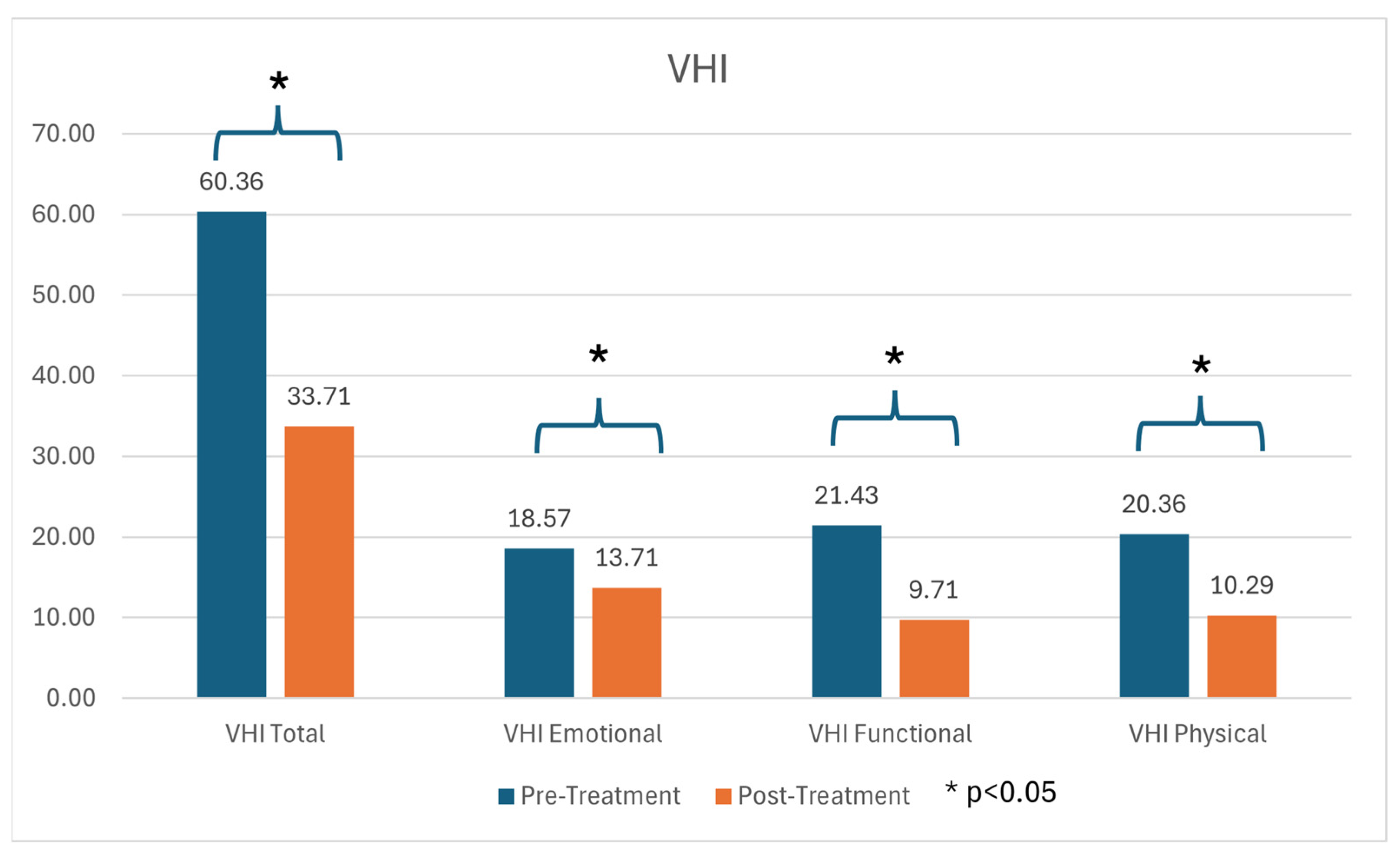
3.2. Subjective Voice Assessment
3.3. Objective Voice Assessment
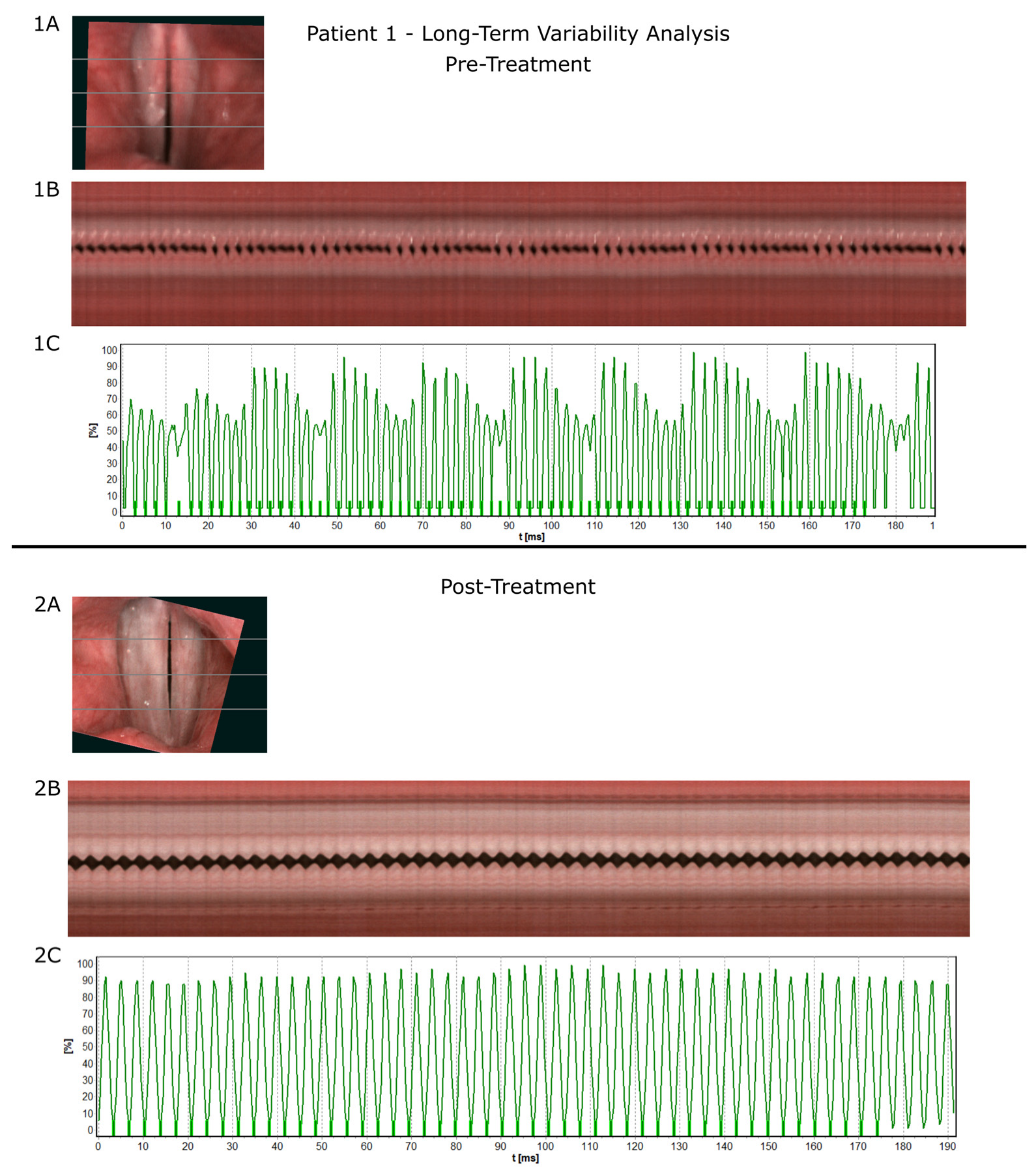
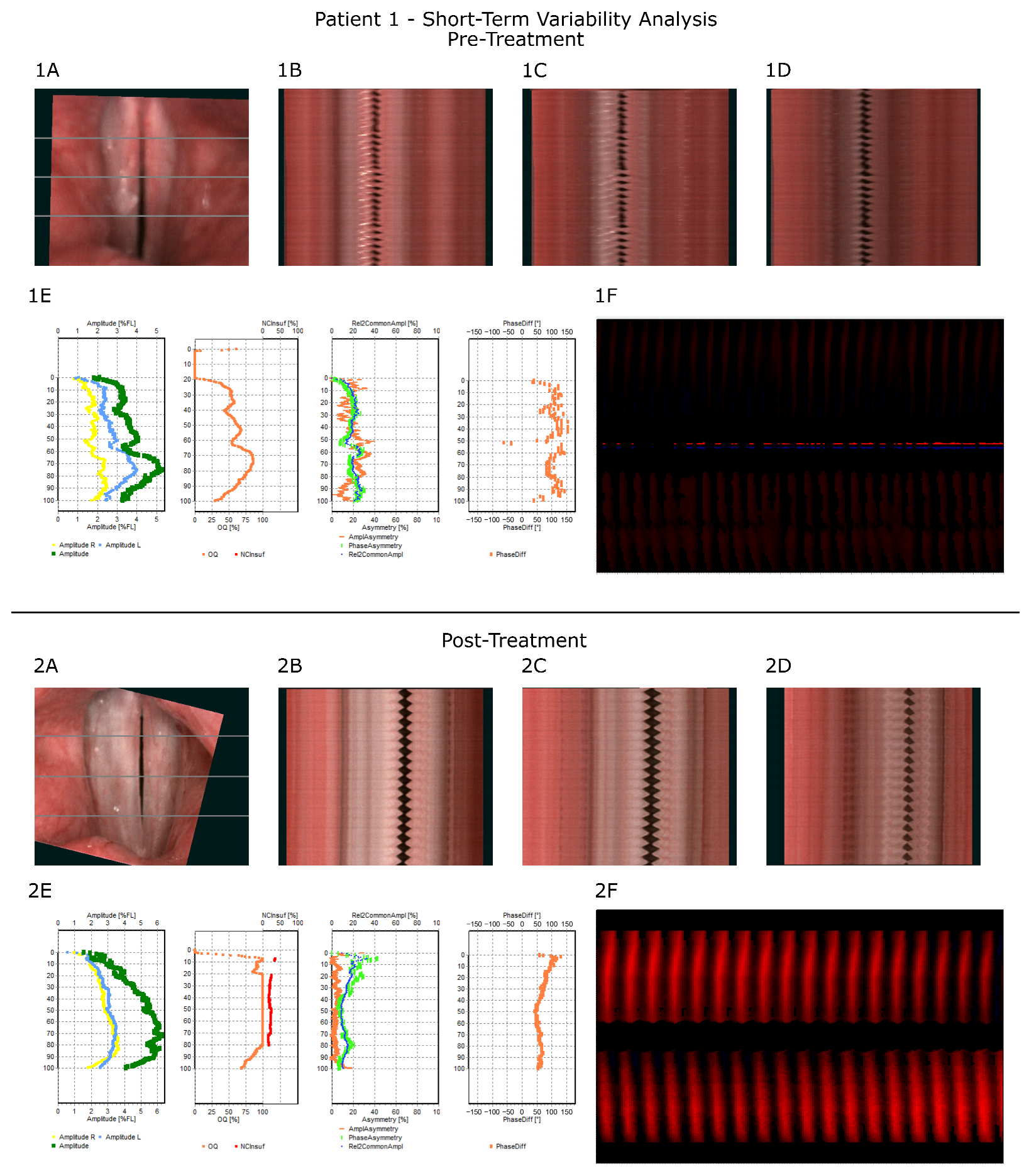
3.4. Illustration of Treatment Process—Representative Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. In National Institute for Health and Care Excellence: Clinical Guidelines; National Institute for Health and Care Excellence (NICE): London, UK, 2024; ISBN 978-1-4731-3943-5.
- Pfaff, E.R.; Madlock-Brown, C.; Baratta, J.M.; Bhatia, A.; Davis, H.; Girvin, A.; Hill, E.; Kelly, E.; Kostka, K.; Loomba, J.; et al. Coding Long COVID: Characterizing a New Disease through an ICD-10 Lens. BMC Med. 2023, 21, 58. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, J.J.; García-Castillo, V.; Cuellar, S.V.; Campillay-Véliz, C.P.; Salazar-Ardiles, C.; Avellaneda, A.M.; Muñoz, C.A.; Retamal-Díaz, A.; Bueno, S.M.; González, P.A.; et al. New Insights into the Pathogenesis of SARS-CoV-2 during and after the COVID-19 Pandemic. Front. Immunol. 2024, 15, 1363572. [Google Scholar] [CrossRef]
- Ozonoff, A.; Jayavelu, N.D.; Liu, S.; Melamed, E.; Milliren, C.E.; Qi, J.; Geng, L.N.; McComsey, G.A.; Cairns, C.B.; Baden, L.R.; et al. Features of Acute COVID-19 Associated with Post-Acute Sequelae of SARS-CoV-2 Phenotypes: Results from the IMPACC Study. Nat. Commun. 2024, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Sapna, F.; Deepa, F.; Sakshi, F.; Sonam, F.; Kiran, F.; Perkash, R.S.; Bendari, A.; Kumar, A.; Rizvi, Y.; Suraksha, F.; et al. Unveiling the Mysteries of Long COVID Syndrome: Exploring the Distinct Tissue and Organ Pathologies Linked to Prolonged COVID-19 Symptoms. Cureus 2023, 15, e44588. [Google Scholar] [CrossRef]
- Peluso, M.J.; Swank, Z.N.; Goldberg, S.A.; Lu, S.; Dalhuisen, T.; Borberg, E.; Senussi, Y.; Luna, M.A.; Chang Song, C.; Clark, A.; et al. Plasma-Based Antigen Persistence in the Post-Acute Phase of COVID-19. Lancet Infect. Dis. 2024, 24, e345–e347. [Google Scholar] [CrossRef]
- Proal, A.D.; Aleman, S.; Bomsel, M.; Brodin, P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; Deeks, S.G.; et al. Targeting the SARS-CoV-2 Reservoir in Long COVID. Lancet Infect. Dis. 2025, 25, e294–e306. [Google Scholar] [CrossRef]
- Swank, Z.; Borberg, E.; Chen, Y.; Senussi, Y.; Chalise, S.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Henrich, T.J.; et al. Measurement of Circulating Viral Antigens Post-SARS-CoV-2 Infection in a Multicohort Study. Clin. Microbiol. Infect. 2024, 30, 1599–1605. [Google Scholar] [CrossRef]
- Antar, A.A.R.; Yu, T.; Demko, Z.O.; Hu, C.; Tornheim, J.A.; Blair, P.W.; Thomas, D.L.; Manabe, Y.C. Long COVID Brain Fog and Muscle Pain Are Associated with Longer Time to Clearance of SARS-CoV-2 RNA from the Upper Respiratory Tract during Acute Infection. Front. Immunol. 2023, 14, 1147549. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Satapathy, A.; Naidu, M.M.; Mukhopadhyay, S.; Sharma, S.; Barton, L.M.; Stroberg, E.; Duval, E.J.; Pradhan, D.; Tzankov, A.; et al. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and Coronavirus Disease 19 (COVID-19)—Anatomic Pathology Perspective on Current Knowledge. Diagn. Pathol. 2020, 15, 103. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 Infection and Persistence in the Human Body and Brain at Autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- del Rio, C.; Collins, L.F.; Malani, P. Long-Term Health Consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef]
- Kabil, S.E.; Behairy, R.; Sayed, M.; El Sharkawy, M.; Hassanin, H.E.; Elsaeed, M.; Yousef, I.H.; Ewis, A.M.; Wahba, A.H.; Omar, F.; et al. Sensorineural Hearing Loss in Post-COVID-19 Patients. Electron. J. Gen. Med. 2024, 21, em609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alcalde-Herraiz, M.; Güell, K.L.; Chen, L.; Mateu, L.; Li, C.; Ali, R.; Wareham, N.; Paredes, R.; Prieto-Alhambra, D.; et al. Refinement of Post-COVID Condition Core Symptoms, Subtypes, Determinants, and Health Impacts: A Cohort Study Integrating Real-World Data and Patient-Reported Outcomes. eBioMedicine 2024, 111, 105493. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, G.; Aldè, M.; Consonni, D.; Zuccotti, G.; Berardino, F.D.; Barozzi, S.; Bertoli, S.; Battezzati, A.; Zanetti, D.; Pignataro, L. Prevalence of Dysphonia in Non Hospitalized Patients with COVID-19 in Lombardy, the Italian Epicenter of the Pandemic. J. Voice 2023, 37, 605–609. [Google Scholar] [CrossRef]
- Suwannutsiri, T.; Arreenich, P.; Sombuntham, P. Prevalence and Associated Factors of Dysphonia in Non-Hospitalized Thai COVID-19 Patients: A Descriptive Study with Thai-VHI10 Assessment. Asian Biomed. (Res. Rev. News) 2024, 18, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.H.G.; de Azevedo, E.S.; Müller, J.V.C.; Loli, A. Dysphonia and COVID-19: A Review. J. Voice 2025, S0892-1997(24)00419-3. [Google Scholar] [CrossRef]
- Pietruszewska, W.; Just, M.; Morawska, J.; Malinowski, J.; Hoffman, J.; Racino, A.; Barańska, M.; Kowalczyk, M.; Niebudek-Bogusz, E. Comparative Analysis of High-Speed Videolaryngoscopy Images and Sound Data Simultaneously Acquired from Rigid and Flexible Laryngoscope: A Pilot Study. Sci. Rep. 2021, 11, 20480. [Google Scholar] [CrossRef]
- Malinowski, J.; Pietruszewska, W.; Stawiski, K.; Kowalczyk, M.; Barańska, M.; Rycerz, A.; Niebudek-Bogusz, E. High-Speed Videoendoscopy Enhances the Objective Assessment of Glottic Organic Lesions: A Case-Control Study with Multivariable Data-Mining Model Development. Cancers 2023, 15, 3716. [Google Scholar] [CrossRef]
- Al-Ani, R.M.; Rashid, R.A. Prevalence of Dysphonia Due to COVID-19 at Salahaddin General Hospital, Tikrit City, Iraq. Am. J. Otolaryngol. 2021, 42, 103157. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Bitter, T.; Takes, R.; Lee, V.H.F.; Saba, N.F.; Mäkitie, A.A.; Kowalski, L.P.; Nixon, I.J.; Ferlito, A. Post COVID-19 and Long COVID Symptoms in Otorhinolaryngology—A Narrative Review. J. Clin. Med. 2025, 14, 506. [Google Scholar] [CrossRef]
- Iannella, G.; Magliulo, G.; Lechien, J.R.; Maniaci, A.; Perrone, T.; Frasconi, P.C.; De Vito, A.; Martone, C.; Ferlito, S.; Cocuzza, S.; et al. Impact of COVID-19 Pandemic on the Incidence of Otitis Media with Effusion in Adults and Children: A Multicenter Study. Eur. Arch. Otorhinolaryngol. 2022, 279, 2383–2389. [Google Scholar] [CrossRef]
- Shah, H.P.; Bourdillon, A.T.; Panth, N.; Ihnat, J.; Kohli, N. Long-Term Laryngological Sequelae and Patient-Reported Outcomes after COVID-19 Infection. Am. J. Otolaryngol. 2023, 44, 103780. [Google Scholar] [CrossRef]
- Allisan-Arrighi, A.E.; Rapoport, S.K.; Laitman, B.M.; Bahethi, R.; Mori, M.; Woo, P.; Genden, E.; Courey, M.; Kirke, D.N. Long-Term Upper Aerodigestive Sequelae as a Result of Infection with COVID-19. Laryngoscope Investig. Otolaryngol. 2022, 7, 476–485. [Google Scholar] [CrossRef]
- Hoffman, J.; Barańska, M.; Niebudek-Bogusz, E.; Pietruszewska, W. Comparative Evaluation of High-Speed Videoendoscopy and Laryngovideostroboscopy for Functional Laryngeal Assessment in Clinical Practice. J. Clin. Med. 2025, 14, 1723. [Google Scholar] [CrossRef]
- Patel, R.; Dailey, S.; Bless, D. Comparison of High-Speed Digital Imaging with Stroboscopy for Laryngeal Imaging of Glottal Disorders. Ann. Otol. Rhinol. Laryngol. 2008, 117, 413–424. [Google Scholar] [CrossRef]
- Sielska-Badurek, E.M.; Jędra, K.; Sobol, M.; Niemczyk, K.; Osuch-Wójcikiewicz, E. Laryngeal Stroboscopy-Normative Values for Amplitude, Open Quotient, Asymmetry and Phase Difference in Young Adults. Clin. Otolaryngol. 2019, 44, 158–165. [Google Scholar] [CrossRef]
- Mehta, D.D.; Hillman, R.E. Current Role of Stroboscopy in Laryngeal Imaging. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 429. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Bhatta, S.; Ganesuni, D.; Ghanpur, A.D.; Saindani, S.J. High-Speed Videolaryngoscopy in Early Glottic Carcinoma Patients Following Transoral CO2 LASER Cordectomy. Eur. Arch. Otorhinolaryngol. 2021, 278, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Woo, P.; Murry, T. Spectral Analysis of Digital Kymography in Normal Adult Vocal Fold Vibration. J. Voice 2014, 28, 356–361. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Isotani, S.; Pimenta, R.A.; Dajer, M.E.; Hachiya, A.; Tsuji, D.H.; Tayama, N.; Yokonishi, H.; Imagawa, H.; Yamauchi, A.; et al. High-Speed Videolaryngoscopy: Quantitative Parameters of Glottal Area Waveforms and High-Speed Kymography in Healthy Individuals. J. Voice 2017, 31, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Imagawa, H.; Yokonishi, H.; Sakakibara, K.-I.; Tayama, N. Multivariate Analysis of Vocal Fold Vibrations on Various Voice Disorders Using High-Speed Digital Imaging. Appl. Sci. 2021, 11, 6284. [Google Scholar] [CrossRef]
- Schlegel, P.; Kniesburges, S.; Dürr, S.; Schützenberger, A.; Döllinger, M. Machine Learning Based Identification of Relevant Parameters for Functional Voice Disorders Derived from Endoscopic High-Speed Recordings. Sci. Rep. 2020, 10, 10517. [Google Scholar] [CrossRef]
- Berti, L.C.; Gauy, M.; da Silva, L.C.S.; Rios, J.V.V.; Morais, V.B.; de Almeida, T.C.; Sossolete, L.S.; Quirino, J.H.d.M.; Martins, C.F.P.; Fernandes-Svartman, F.R.; et al. Acoustic Characteristics of Voice and Speech in Post-COVID-19. Healthcare 2025, 13, 63. [Google Scholar] [CrossRef]
- Bonilha, H.S.; Deliyski, D.D.; Whiteside, J.P.; Gerlach, T.T. Vocal Fold Phase Asymmetries in Patients With Voice Disorders: A Study Across Visualization Techniques. Am. J. Speech Lang. Pathol. 2012, 21, 3–15. [Google Scholar] [CrossRef]
- Eysholdt, U.; Rosanowski, F.; Hoppe, U. Vocal Fold Vibration Irregularities Caused by Different Types of Laryngeal Asymmetry. Eur. Arch. Otorhinolaryngol. 2003, 260, 412–417. [Google Scholar] [CrossRef]
- Tierney, W.S.; Xiao, R.; Milstein, C.F. Characterization of Functional Dysphonia: Pre- and Post-Treatment Findings. Laryngoscope 2021, 131, E1957–E1964. [Google Scholar] [CrossRef]
- Franz, L.; Da Canal, A.; Spinato, G.; Baracca, G.; Lucchini, E.; de Filippis, C.; Marioni, G. Tele-Voice Therapy. Application of the Tele-Medicine Paradigm to Speech Therapy for Dysphonia: A Systematic Review. Am. J. Otolaryngol. 2025, 46, 104626. [Google Scholar] [CrossRef]
- Espina González, C.; Núñez Batalla, F.; Mackers Iglesias, P.; Sumarroca Trouboul, A.; Cantón Bascuas, M.; García Lorenzo, J. Dysphonia and Other Voice Alterations Associated with COVID-19: Systematic Review. Acta Otorrinolaringol. (Engl. Ed.) 2024, 75, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, M.M.; Kałuża-Olszewska, J.; Niebudek-Bogusz, E.; Klepaczko, A.; Pietruszewska, W. High-Speed Videoendoscopy and Stiffness Mapping for AI-Assisted Glottic Lesion Differentiation. Cancers 2025, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
| Pre-Treatment | Post-Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Mean | Median | SD | Mean | Median | SD | p value |
| AmpAvg [%FL] | 6.721 | 5.450 | 4.243 | 9.279 | 7.300 | 5.861 | 0.00850 |
| AmpAvg_2/3 [%FL] | 7.257 | 6.400 | 4.789 | 10.200 | 7.950 | 5.920 | 0.00996 |
| Non-opening [%FL] | 2.743 | 0.000 | 5.414 | 1.600 | 0.600 | 3.461 | 0.40081 * |
| RGGA [%] | 11.943 | 2.400 | 21.186 | 8.814 | 1.550 | 14.300 | 0.22707 |
| OQAvg [%] | 63.700 | 61.150 | 14.674 | 64.893 | 57.950 | 19.361 | 0.66035 * |
| OQAvg_2/3 [%] | 61.807 | 58.200 | 18.688 | 69.550 | 64.850 | 21.301 | 0.06115 |
| AmplAsymAvg [%] | 15.521 | 11.650 | 9.545 | 14.443 | 12.900 | 7.883 | 0.02176 |
| AmplAsymAvg_2/3 [%] | 15.457 | 14.150 | 10.444 | 11.279 | 10.300 | 6.395 | 0.75315 * |
| PhaseAsymAvg [%] | 16.529 | 12.850 | 12.344 | 16.814 | 18.200 | 10.799 | 0.45919 |
| PhaseAsymAvg_2/3 [%] | 16.950 | 10.900 | 15.461 | 18.293 | 16.050 | 14.058 | 0.35913 |
| AbsPhaseDiffAvg [°] | 63.979 | 62.200 | 25.212 | 48.993 | 52.100 | 26.724 | 0.01142 |
| Pre-Treatment | Post-Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | p value | |
| F0Avg [Hz] | 258.857 | 215.050 | 135.641 | 233.936 | 212.500 | 119.514 | 0.11585 * |
| PPF [%] | 7.456 | 3.615 | 9.525 | 2.585 | 1.025 | 4.112 | 0.09609 * |
| Jitt [%] | 7.651 | 3.715 | 10.256 | 2.469 | 1.020 | 3.817 | 0.03924 * |
| Jita [ms] | 0.309 | 0.135 | 0.364 | 0.136 | 0.045 | 0.248 | 0.02771 * |
| APF [%] | 14.336 | 6.415 | 14.146 | 3.946 | 2.360 | 5.959 | 0.01075 * |
| Shimmer [%] | 12.669 | 6.425 | 11.243 | 3.930 | 2.345 | 5.971 | 0.00604 * |
| PRAP [%] | 4.192 | 2.135 | 5.412 | 1.461 | 0.710 | 2.182 | 0.05462 * |
| ARAP [%] | 7.015 | 3.425 | 6.556 | 2.287 | 1.255 | 3.404 | 0.01310 * |
| PPQ3 [%] | 4.069 | 2.075 | 5.021 | 1.506 | 0.725 | 2.283 | 0.05462 * |
| PPQ5 [%] | 5.186 | 2.380 | 6.701 | 1.767 | 0.820 | 2.667 | 0.07474 * |
| APQ3 [%] | 7.786 | 3.425 | 7.900 | 2.321 | 1.265 | 3.472 | 0.01206 * |
| APQ5 [%] | 8.844 | 4.110 | 8.657 | 2.420 | 1.360 | 3.559 | 0.00713 * |
| Patient 1 | Long-Term Variability Parameters | |
|---|---|---|
| Pre-Treatment | Post-Treatment | |
| F0Avg [Hz] | 427.8 | 286.8 |
| PPF [%] | 7.56 | 0.27 |
| Jitt [%] | 7.37 | 0.27 |
| Jita [ms] | 0.17 | 0.01 |
| APF [%] | 26.93 | 1.51 |
| Shimmer [%] | 19.29 | 1.51 |
| PRAP [%] | 3.73 | 0.17 |
| ARAP [%] | 8.07 | 0.9 |
| PPQ3 [%] | 3.79 | 0.17 |
| PPQ5 [%] | 5.46 | 0.16 |
| APQ3 [%] | 10.41 | 0.9 |
| APQ5 [%] | 13.87 | 0.86 |
| Patient 1 | Short-Term Variability Parameters | |
|---|---|---|
| Pre-Treatment | Post-Treatment | |
| AmpAvg [%FL] | 3.8 | 4.9 |
| AmpAvg_2/3 [%FL] | 3.5 | 5.5 |
| Non-opening [%FL] | 17.1 | 1.7 |
| RGGA [%] | 0 | 18.1 |
| OQAvg [%] | 49 | 90.9 |
| OQAvg_2/3 [%] | 58.6 | 100 |
| AmplAsymAvg [%] | 19.2 | 4.3 |
| AmplAsymAvg_2/3 [%] | 18.5 | 4.4 |
| PhaseAsymAvg [%] | 18.8 | 14.7 |
| PhaseAsymAvg_2/3 [%] | 18.3 | 10.2 |
| AbsPhaseDiffAvg [°] | 107 | 67.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeleniewska, J.; Malinowski, J.; Niebudek-Bogusz, E.; Pietruszewska, W. Objective and Subjective Voice Outcomes in Post-COVID-19 Dysphonia: A High-Speed Videoendoscopy Pre–Post Study. J. Clin. Med. 2025, 14, 6861. https://doi.org/10.3390/jcm14196861
Jeleniewska J, Malinowski J, Niebudek-Bogusz E, Pietruszewska W. Objective and Subjective Voice Outcomes in Post-COVID-19 Dysphonia: A High-Speed Videoendoscopy Pre–Post Study. Journal of Clinical Medicine. 2025; 14(19):6861. https://doi.org/10.3390/jcm14196861
Chicago/Turabian StyleJeleniewska, Joanna, Jakub Malinowski, Ewa Niebudek-Bogusz, and Wioletta Pietruszewska. 2025. "Objective and Subjective Voice Outcomes in Post-COVID-19 Dysphonia: A High-Speed Videoendoscopy Pre–Post Study" Journal of Clinical Medicine 14, no. 19: 6861. https://doi.org/10.3390/jcm14196861
APA StyleJeleniewska, J., Malinowski, J., Niebudek-Bogusz, E., & Pietruszewska, W. (2025). Objective and Subjective Voice Outcomes in Post-COVID-19 Dysphonia: A High-Speed Videoendoscopy Pre–Post Study. Journal of Clinical Medicine, 14(19), 6861. https://doi.org/10.3390/jcm14196861






