Allergic Contact Dermatitis: Immunopathology and Potential Therapeutic Strategies
Abstract
1. Introduction
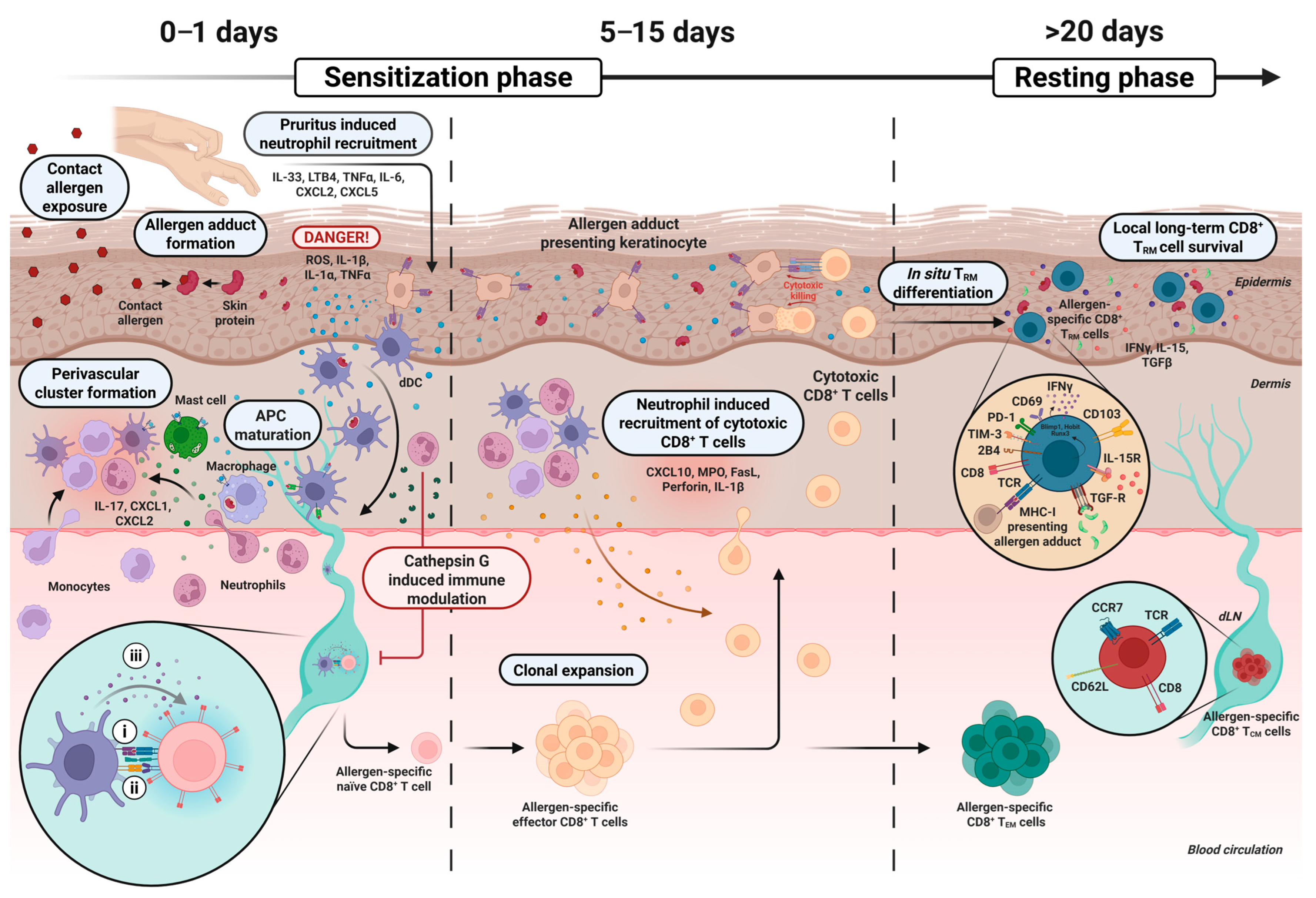
2. The Sensitization Phase and Development of CD8+ TRM Cells
3. The Role of Neutrophils in the Sensitization Phase
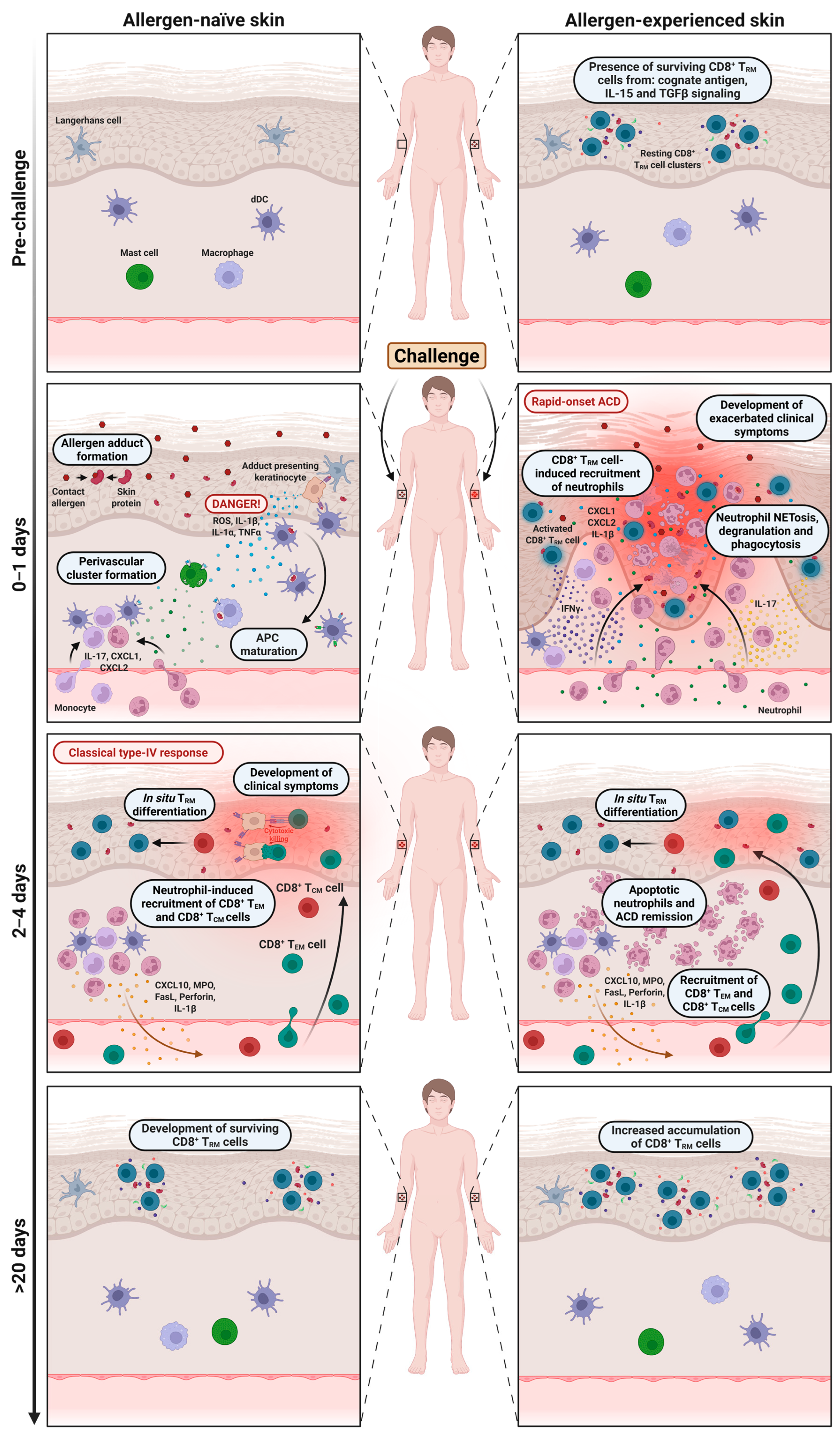
4. The Function of Memory CD8+ T Cells and Neutrophils During the Challenge Phase
| Feature | Challenge with Contact Allergen on: | ||
|---|---|---|---|
| Allergen-Naïve Skin | Allergen-Experienced Skin | Refs. | |
| Resulting skin reaction | Classical delayed-type (type IV) hypersensitivity reaction. Typically seen when patch testing patients. | Rapid-onset and exacerbated reaction. Typically seen in daily life situations where patients are re-exposed at the same skin site repeatedly. | [5,6,7,13,15] |
| Presence of allergen-specific TRM cells | Absent. | CD8+ TRM cells enriched in epidermis. | [7,13,15] |
| Kinetics | Delayed-type response (peaks ~48–72 h post challenge). | Rapid-onset response (<24 h post challenge). | [5,6,7,13,15] |
| Memory T cell subsets involved in inflammatory response | Circulating and effector memory T cell subsets (TCM and TEM subsets). | CD8+ TRM cells (predominantly Tc1 and Tc17 subsets). | [7,13,15,79] |
| Cytokines/molecules involved | IL-1β, IL-12, and CXCL10 (late induction); IFN-γ, IL-17, and TNF-α (produced after T cell recruitment). | IL-1β, IFN-γ, IL-17, TNF-α, and granzyme B, perforin (produced locally and early); CXCL1 and CXCL2 (recruitment of neutrophils). | [7,13,15,22,36,44,49,50,80] |
5. Mediators of Long-Term Persistence of Skin-Resident CD8+ TRM Cells
6. Potential Therapeutic Targets Against Rapid-Onset ACD
6.1. Targeting the IL-1R-CXCR2 Neutrophil Axis
6.2. Neutralization Inflammatory Cytokines (IL-17/IFN-γ)
6.3. JAK Inhibitors
6.4. Modulation of CD8+ TRM Cell Survival and Function
6.5. Stimulation of Inhibitory Checkpoint Receptor Signaling
6.6. Targeting Metabolic Pathways
6.7. Anti-Pruritic Strategies
6.8. Redox Modulation and Allergen Metabolism
| Therapeutic Strategy | Mechanism | Limitations | Reported/Potential Side Effects | Refs. |
|---|---|---|---|---|
| Anti-IL-1 and anti-IL-1R blockade (e.g., Anakinra, CXCR2 inhibitors) | Reduces neutrophil recruitment and downstream CD8+ TRM activation | Requires near-immediate administration after exposure; limited data in ACD | Neutropenia, increased infection risk; may transiently impair host defense | [13,22,33,96,97,98,99,100,101,102] |
| Cytokine neutralization (anti-IL-17/anti-IFN-γ) (e.g., Secukinumab, Emapalumab) | Blocks CD8+ TRM-induced inflammation and downstream release of neutrophil-recruiting chemokines | Secukinumab showed limited efficacy in a nickel allergy trial; unknown efficacy against rapid-onset ACD | IL-17 inhibitors: increased risk of mucocutaneous candidiasis; IFN-γ blockade: increased infection risk | [7,71,103,104,105,106,107,108,109,110] |
| JAK inhibitors (e.g., Tofacitinib, Ruxolitinib, Abrocitinib, Upadacitinib, Baricitinib) | Broad inhibition of cytokine signaling (IL-15, IL-17, IFN-γ, IL-4) and inflammation | Systemic use limited by safety concerns; efficacy in ACD unknown; topical efficacy in ACD is unexplored | Systemic: thromboembolism, cytopenia, and serious infections; topical: local irritation and unknown long-term safety | [111,112,113,114,115,116] |
| CD8+ TRM modulation (e.g., anti–IL-15, anti–TGF-β, IL-4 administration) | Reduces CD8+ TRM survival and persistence | No ACD trials; IL-15 and TGF-β are pleiotropic; IL-4 may worsen or cause development of type 2 autoimmunity. | IL-15 blockade may impair antiviral/antitumor immunity; TGF-β blockade increases risk of autoimmunity/inflammation; IL-4 may exacerbate AD/asthma | [83,89,117,118,147] |
| Checkpoint receptor agonists (e.g., PD-1, CTLA-4 agonists) | Inhibit T cell activation and dampen TRM-driven inflammation | No clinical trials in skin disease; systemic immunosuppression risk; topical formulations not available | Increased infection and malignancy risk with systemic administration | [70,119,120,121] |
| Metabolic pathway modulation (AhR modulators e.g., Tapinarof; mTOR inhibitors, e.g., rapamycin) | Alters TRM activation/inflammation and survival; Tapinarof is shown to reduce IL-17 expression | No studies in ACD; paradoxical of contact dermatitis with Tapinarof; systemic rapamycin toxicity | Tapinarof: folliculitis, nasopharyngitis, and contact dermatitis; rapamycin: infections, dyslipidemia, mouth ulcers, and renal/metabolic toxicity | [66,122,123,124,125,126,127,128,129,130,131,132,133,134,148] |
| Anti-pruritic strategies (e.g., H1-antihistamines, TRPA1 antagonists, LTRAs, IL-33 blockade) | Reduce scratching-induced barrier damage and neutrophil infiltration | Limited efficacy in non-histaminergic itch; mixed efficacy in AD (IL-33); no ACD trials | H1-antihistamines: sedation, fatigue, and cognitive impairment; IL-33 blockade: rare thrombosis; TRPA1 antagonists: possible off-target effects; LTRAs: safe in asthma, but untested in ACD | [57,58,59,60,135,136,137,138,139,140,141,142,143] |
| Redox modulation/allergen metabolism (e.g., Nrf2 activators: sulforaphane, curcumin) | Enhances detoxification of allergen adducts and reduces oxidative stress | Only preclinical data; systemic administration may cause off-target effects | Safety in ACD not established | [28,31,69,144,145,146] |
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACD | Allergic contact dermatitis |
| AhR | Aryl hydrocarbon receptor |
| APC | Antigen-presenting cell |
| CCR7 | C-C chemokine receptor type 7 |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | (C-X-C motif) receptor |
| dDC | Dermal dendritic cell |
| dLN | Draining lymph node |
| DNFB | Dinitrofluorobenzene |
| FasL | Fas ligand |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| IL-1R | Interleukin-1 receptor |
| JAK | Janus kinase |
| LTB4 | Leukotriene B4 |
| LTRA MHC | Leukotriene receptor antagonist Major histocompatibility complex |
| MPO | Myeloperoxidase |
| mTOR | Mechanistic target of rapamycin |
| NETosis | Neutrophil extracellular trap formation |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PD-1 | Programmed cell death protein 1 |
| ROS | Reactive oxygen species |
| TCR | T cell receptor |
| TCM | Central memory T cell |
| TEM | Effector memory T cell |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| TRM | Tissue-resident memory T cell |
| TRP | Transient receptor potential |
References
- Scheinman, P.L.; Vocanson, M.; Thyssen, J.P.; Johansen, J.D.; Nixon, R.L.; Dear, K.; Botto, N.C.; Morot, J.; Goldminz, A.M. Contact Dermatitis. Nat. Rev. Dis. Prim. 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Ofenloch, R.F.; Bruze, M.; Naldi, L.; Cazzaniga, S.; Elsner, P.; Goncalo, M.; Schuttelaar, M.-L.A.; Diepgen, T.L. Prevalence of Skin Disease in a Population-Based Sample of Adults from Five European Countries. Br. J. Dermatol. 2018, 178, 1111–1118. [Google Scholar] [CrossRef]
- Tramontana, M.; Hansel, K.; Bianchi, L.; Sensini, C.; Malatesta, N.; Stingeni, L. Advancing the Understanding of Allergic Contact Dermatitis: From Pathophysiology to Novel Therapeutic Approaches. Front. Med. 2023, 10, 1184289. [Google Scholar] [CrossRef]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavõ, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis Guideline for Diagnostic Patch Testing—Recommendations on Best Practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Menné, T.; Thyssen, J.P.; Johansen, J.D. Nickel Allergy in a Danish Population 25 Years after the First Nickel Regulation. Contact Dermat. 2017, 76, 325–332. [Google Scholar] [CrossRef]
- Hindsén, M.; Bruze, M.; Christensen, O.B. The Significance of Previous Allergic Contact Dermatitis for Elicitation of Delayed Hypersensitivity to Nickel. Contact Dermat. 1997, 37, 101–106. [Google Scholar] [CrossRef]
- Schmidt, J.D.; Ahlström, M.G.; Johansen, J.D.; Dyring-Andersen, B.; Agerbeck, C.; Nielsen, M.M.; Poulsen, S.S.; Woetmann, A.; Ødum, N.; Thomsen, A.R.; et al. Rapid Allergen-induced Interleukin-17 and Interferon-γ Secretion by Skin-resident Memory CD8 + T Cells. Contact Dermat. 2017, 76, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Bonefeld, C.M.; Schwensen, J.F.B.; Thyssen, J.P.; Uter, W. Novel Insights into Contact Dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Frosch, P.J.; Kügler, K. Occupational Contact Dermatitis. In Contact Dermatitis; Springer: Berlin, Heidelberg, 2011; pp. 831–839. ISBN 9783642038266. [Google Scholar]
- Pesonen, M.; Jolanki, R.; Larese Filon, F.; Wilkinson, M.; Kręcisz, B.; Kieć-Świerczyńska, M.; Bauer, A.; Mahler, V.; John, S.M.; Schnuch, A.; et al. Patch Test Results of the European Baseline Series among Patients with Occupational Contact Dermatitis across Europe—Analyses of the European Surveillance System on Contact Allergy Network, 2002–2010. Contact Dermat. 2015, 72, 154–163. [Google Scholar] [CrossRef]
- Zuberbier, T.; Lötvall, J.; Simoens, S.; Subramanian, S.V.; Church, M.K. Economic Burden of Inadequate Management of Allergic Diseases in the European Union: A GA(2)LEN Review. Allergy 2014, 69, 1275–1279. [Google Scholar] [CrossRef]
- Saetterstrøm, B.; Olsen, J.; Johansen, J.D. Cost-of-Illness of Patients with Contact Dermatitis in Denmark. Contact Dermat. 2014, 71, 154–161. [Google Scholar] [CrossRef]
- Funch, A.B.; Mraz, V.; Gadsbøll, A.Ø.; Jee, M.H.; Weber, J.F.; Ødum, N.; Woetmann, A.; Johansen, J.D.; Geisler, C.; Bonefeld, C.M. CD8+ Tissue-Resident Memory T Cells Recruit Neutrophils That Are Essential for Flare-Ups in Contact Dermatitis. Allergy 2022, 77, 513–524. [Google Scholar] [CrossRef]
- Mraz, V.; Funch, A.B.; Jee, M.H.; Gadsbøll, A.-S.Ø.; Weber, J.F.; Yeung, K.; Lohmann, R.K.D.; Hawkes, A.; Ødum, N.; Woetmann, A.; et al. CD100 Boosts the Inflammatory Response in the Challenge Phase of Allergic Contact Dermatitis in Mice. Contact Dermat. 2023, 89, 446–452. [Google Scholar] [CrossRef]
- Funch, A.B.; Weber, J.F.; Lohmann, R.K.D.; Mraz, V.; Yeung, K.; Jee, M.H.; Ødum, N.; Woetmann, A.; Johansen, J.D.; Geisler, C.; et al. CD4+ T Cells Inhibit the Generation of CD8+ Epidermal-Resident Memory T Cells Directed against Clinically Relevant Contact Allergens. Contact Dermat. 2023, 88, 425–437. [Google Scholar] [CrossRef]
- Funch, A.B.; Ahlström, M.G.; Johansen, J.D.; Geisler, C.; Bonefeld, C.M. Neutrophil Infiltration in Allergic Contact Dermatitis to Nickel. Br. J. Dermatol. 2024, 190, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.; Trautmann, A.; Voss, A.; Bröcker, E.B.; Toksoy, A.; Gillitzer, R. Differential and Sequential Expression of Multiple Chemokines during Elicitation of Allergic Contact Hypersensitivity. Am. J. Pathol. 2001, 158, 431–440. [Google Scholar] [CrossRef]
- Larsen, J.M.; Bonefeld, C.M.; Poulsen, S.S.; Geisler, C.; Skov, L. IL-23 and T(H)17-Mediated Inflammation in Human Allergic Contact Dermatitis. J. Allergy Clin. Immunol. 2009, 123, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.C.; Németh, T.; Csepregi, J.Z.; Dudeck, A.; Roers, A.; Ozsvári, B.; Oswald, E.; Puskás, L.G.; Jakob, T.; Mócsai, A.; et al. Neutrophils Are Required for Both the Sensitization and Elicitation Phase of Contact Hypersensitivity. J. Exp. Med. 2015, 212, 15–22. [Google Scholar] [CrossRef]
- Cattani, F.; Gallese, A.; Mosca, M.; Buanne, P.; Biordi, L.; Francavilla, S.; Coletti, G.; Pellegrini, L.; Melillo, G.; Bertini, R. The Role of CXCR2 Activity in the Contact Hypersensitivity Response in Mice. Eur. Cytokine Netw. 2006, 17, 42–48. [Google Scholar]
- Kish, D.D.; Gorbachev, A.V.; Parameswaran, N.; Gupta, N.; Fairchild, R.L. Neutrophil Expression of Fas Ligand and Perforin Directs Effector CD8 T Cell Infiltration into Antigen-Challenged Skin. J. Immunol. 2012, 189, 2191–2202. [Google Scholar] [CrossRef]
- Kish, D.D.; Min, S.; Dvorina, N.; Baldwin, W.M.; Stohlman, S.A.; Fairchild, R.L. Neutrophil Cathepsin G Regulates Dendritic Cell Production of IL-12 during Development of CD4 T Cell Responses to Antigens in the Skin. J. Immunol. 2019, 202, 1045–1056. [Google Scholar] [CrossRef]
- Dilulio, N.A.; Engeman, T.; Armstrong, D.; Tannenbaum, C.; Hamilton, T.A.; Fairchild, R.L. Groα-Mediated Recruitment of Neutrophils Is Required for Elicitation of Contact Hypersensitivity. Eur. J. Immunol. 1999, 29, 3485–3495. [Google Scholar] [CrossRef]
- Engeman, T.; Gorbachev, A.V.; Kish, D.D.; Fairchild, R.L. The Intensity of Neutrophil Infiltration Controls the Number of Antigen-Primed CD8 T Cells Recruited into Cutaneous Antigen Challenge Sites. J. Leukoc. Biol. 2004, 76, 941–949. [Google Scholar] [CrossRef]
- Jee, M.H.; Funch, A.B.; Weber, J.F.; Yeung, K.; Mraz, V.; Gadsbøll, A.-S.Ø.; Song, T.; Woetmann, A.; Ødum, N.; Johansen, J.D.; et al. Pre-Existing Inflammation Reduces the Response to Contact Allergens in Tmem79-Deficient Mice. Allergy 2024, 79, 1978–1981. [Google Scholar] [CrossRef]
- Helou, D.G.; Noël, B.; Gaudin, F.; Groux, H.; El Ali, Z.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Cutting Edge: Nrf2 Regulates Neutrophil Recruitment and Accumulation in Skin during Contact Hypersensitivity. J. Immunol. 2019, 202, 2189–2194. [Google Scholar] [CrossRef]
- Pesqué, D.; Andrades, E.; Berenguer-Molins, P.; Perera-Bel, J.; Clarós, M.; Bódalo-Torruella, M.; González-Farré, M.; Gallardo, F.; Pujol, R.M.; Giménez-Arnau, A.M. Transcriptomic Analysis of Allergic Patch Test Reactions in Non-Atopic Patients: A Comparative Study Across Multiple Allergens. Allergy 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.; Dooms-Goossens, A.; Karlberg, A.T.; Lepoittevin, J.P. The Chemistry of Contact Allergy: Why Is a Molecule Allergenic? Contact Dermat. 1995, 32, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Funch, A.B.; Weber, J.F.; Mraz, V.; Kongsbak-Wismann, M.; Lohmann, R.K.D.; Jee, M.H.; Vaher, H.; Yeung, K.; Gadsbøll, A.Ø.; Ødum, N.; et al. CD8+ Skin-Resident Memory T Cells Require TCR Signaling for Their Persistence in a Mouse Model of Allergic Contact Dermatitis. J. Investig. Dermatol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, J.P.; Leblond, I. Hapten-Peptide-T Cell Receptor Interactions: Molecular Basis for the Recognition of Haptens by T Lymphocytes. Eur. J. Dermatol. 1997, 7, 151–154. [Google Scholar]
- Yeung, K.; Mraz, V.; Geisler, C.; Skov, L.; Bonefeld, C.M. The Role of Interleukin-1β in the Immune Response to Contact Allergens. Contact Dermat. 2021, 85, 387–397. [Google Scholar] [CrossRef]
- Watanabe, H.; Gaide, O.; Pétrilli, V.; Martinon, F.; Contassot, E.; Roques, S.; Kummer, J.A.; Tschopp, J.; French, L.E. Activation of the IL-1beta-Processing Inflammasome Is Involved in Contact Hypersensitivity. J. Investig. Dermatol. 2007, 127, 1956–1963. [Google Scholar] [CrossRef]
- Enk, A.H.; Angeloni, V.L.; Udey, M.C.; Katz, S.I. An Essential Role for Langerhans Cell-Derived IL-1 Beta in the Initiation of Primary Immune Responses in Skin. J. Immunol. 1993, 150, 3698–3704. [Google Scholar] [CrossRef] [PubMed]
- Enk, A.H.; Katz, S.I. Early Molecular Events in the Induction Phase of Contact Sensitivity. Proc. Natl. Acad. Sci. USA 1992, 89, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, C.; Cumberbatch, M.; Mee, J.B.; Dearman, R.J.; Wei, X.-Q.; Liew, F.Y.; Kimber, I.; Groves, R.W. IL-18 Is a Key Proximal Mediator of Contact Hypersensitivity and Allergen-Induced Langerhans Cell Migration in Murine Epidermis. J. Leukoc. Biol. 2008, 83, 361–367. [Google Scholar] [CrossRef]
- Cumberbatch, M.; Dearman, R.J.; Kimber, I. Langerhans Cells Require Signals from Both Tumour Necrosis Factor Alpha and Interleukin 1 Beta for Migration. Adv. Exp. Med. Biol. 1997, 417, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Nakajima, S.; Egawa, G.; Ogasawara, K.; Malissen, B.; Miyachi, Y.; Kabashima, K. Compensatory Role of Langerhans Cells and Langerin-Positive Dermal Dendritic Cells in the Sensitization Phase of Murine Contact Hypersensitivity. J. Allergy Clin. Immunol. 2010, 125, 1154–1156. [Google Scholar] [CrossRef]
- Noordegraaf, M.; Flacher, V.; Stoitzner, P.; Clausen, B.E. Functional Redundancy of Langerhans Cells and Langerin+ Dermal Dendritic Cells in Contact Hypersensitivity. J. Investig. Dermatol. 2010, 130, 2752–2759. [Google Scholar] [CrossRef]
- Martin, S.; Ortmann, B.; Pflugfelder, U.; Birsner, U.; Weltzien, H.U. Role of Hapten-Anchoring Peptides in Defining Hapten-Epitopes for MHC-Restricted Cytotoxic T Cells. Cross-Reactive TNP-Determinants on Different Peptides. J. Immunol. 1992, 149, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; von Bonin, A.; Fessler, C.; Pflugfelder, U.; Weltzien, H.U. Structural Complexity of Antigenic Determinants for Class I MHC-Restricted, Hapten-Specific T Cells. Two Qualitatively Differing Types of H-2Kb-Restricted TNP Epitopes. J. Immunol. 1993, 151, 678–687. [Google Scholar]
- Lanier, L.L.; O’Fallon, S.; Somoza, C.; Phillips, J.H.; Linsley, P.S.; Okumura, K.; Ito, D.; Azuma, M. CD80 (B7) and CD86 (B70) Provide Similar Costimulatory Signals for T Cell Proliferation, Cytokine Production, and Generation of CTL. J. Immunol. 1995, 154, 97–105. [Google Scholar] [CrossRef]
- Riemann, H.; Loser, K.; Beissert, S.; Fujita, M.; Schwarz, A.; Schwarz, T.; Grabbe, S. IL-12 Breaks Dinitrothiocyanobenzene (DNTB)-Mediated Tolerance and Converts the Tolerogen DNTB into an Immunogen. J. Immunol. 2005, 175, 5866–5874. [Google Scholar] [CrossRef]
- Xu, H.; DiIulio, N.A.; Fairchild, R.L. T Cell Populations Primed by Hapten Sensitization in Contact Sensitivity Are Distinguished by Polarized Patterns of Cytokine Production: Interferon Gamma-Producing (Tc1) Effector CD8+ T Cells and Interleukin (Il) 4/Il-10-Producing (Th2) Negative Regulator. J. Exp. Med. 1996, 183, 1001–1012. [Google Scholar] [CrossRef]
- Larsen, J.M.; Geisler, C.; Nielsen, M.W.; Boding, L.; Von Essen, M.; Hansen, A.K.; Skov, L.; Bonefeld, C.M. Cellular Dynamics in the Draining Lymph Nodes during Sensitization and Elicitation Phases of Contact Hypersensitivity. Contact Dermat. 2007, 57, 300–308. [Google Scholar] [CrossRef]
- Vocanson, M.; Hennino, A.; Rozières, A.; Poyet, G.; Nicolas, J.-F. Effector and Regulatory Mechanisms in Allergic Contact Dermatitis. Allergy 2009, 64, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Rajagopal, S.; Atwater, A.R. Chemokine Signaling in Allergic Contact Dermatitis: Toward Targeted Therapies. Dermat. Contact Atopic Occup. Drug 2018, 29, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.; Boorsma, D.M.; Van Beek, P.J.; Nieboer, C.; Stoof, T.J.; Willemze, R.; Tensen, C.P. Differential Expression of CXCR3 Targeting Chemokines CXCL10, CXCL9, and CXCL11 in Different Types of Skin Inflammation. J. Pathol. 2001, 194, 398–405. [Google Scholar] [CrossRef]
- Flier, J.; Boorsma, D.M.; Bruynzeel, D.P.; Van Beck, P.J.; Stoof, T.J.; Scheper, R.J.; Willemze, R.; Tensen, C.P. The CXCR3 Activating Chemokines IP-10, Mig, and IP-9 Are Expressed in Allergic but Not in Irritant Patch Test Reactions. J. Investig. Dermatol. 1999, 113, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Dudda, J.C.; Lembo, A.; Bachtanian, E.; Huehn, J.; Siewert, C.; Hamann, A.; Kremmer, E.; Förster, R.; Martin, S.F. Dendritic Cells Govern Induction and Reprogramming of Polarized Tissue-Selective Homing Receptor Patterns of T Cells: Important Roles for Soluble Factors and Tissue Microenvironments. Eur. J. Immunol. 2005, 35, 1056–1065. [Google Scholar] [CrossRef]
- Zaid, A.; Hor, J.L.; Christo, S.N.; Groom, J.R.; Heath, W.R.; Mackay, L.K.; Mueller, S.N. Chemokine Receptor-Dependent Control of Skin Tissue-Resident Memory T Cell Formation. J. Immunol. 2017, 199, 2451–2459. [Google Scholar] [CrossRef]
- Strzepa, A.; Gurski, C.J.; Dittel, L.J.; Szczepanik, M.; Pritchard, K.A.; Dittel, B.N. Neutrophil-Derived Myeloperoxidase Facilitates Both the Induction and Elicitation Phases of Contact Hypersensitivity. Front. Immunol. 2020, 11, 608871. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Gasperini, S.; Calzetti, F.; Bertagnin, A.; Luster, A.D.; McDonald, P.P. Regulated Production of the Interferon-γ-Inducible Protein-L0 (IP-10) Chemokine by Human Neutrophils. Eur. J. Immunol. 1997, 27, 111–115. [Google Scholar] [CrossRef]
- Jiang, X.; Park, C.O.; Geddes Sweeney, J.; Yoo, M.J.; Gaide, O.; Kupper, T.S. Dermal Γδ T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity. PLoS ONE 2017, 12, e0169397. [Google Scholar] [CrossRef]
- Kish, D.D.; Gorbachev, A.V.; Fairchild, R.L. IL-1 Receptor Signaling Is Required at Multiple Stages of Sensitization and Elicitation of the Contact Hypersensitivity Response. J. Immunol. 2012, 188, 1761–1771. [Google Scholar] [CrossRef]
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A.; et al. TRPA1 Controls Inflammation and Pruritogen Responses in Allergic Contact Dermatitis. FASEB J. 2013, 27, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Ishida, T.; Sato, K.; Mandokoro, K.; Yabe, S.; Sato, F.; Chiba, Y.; Kon, R.; Ikarashi, N.; Kamei, J. Interference of Skin Scratching Attenuates Accumulation of Neutrophils in Murine Allergic Contact Dermatitis Model. Inflammation 2019, 42, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; He, R.; Li, Y.; Mondal, S.; Yoon, J.; Afshar, R.; Chen, M.; Lee, D.M.; Luo, H.R.; Luster, A.D.; et al. Leukotriene B4-Driven Neutrophil Recruitment to the Skin Is Essential for Allergic Skin Inflammation. Immunity 2012, 37, 747–758. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Y.; Liu, B.; Zhang, Y.; Yin, C.; Wang, J.; Nie, H.; Xu, R.; Tai, Y.; He, X.; et al. Neutrophil-Derived Oxidative Stress Contributes to Skin Inflammation and Scratching in a Mouse Model of Allergic Contact Dermatitis via Triggering pro-Inflammatory Cytokine and Pruritogen Production in Skin. Biochem. Pharmacol. 2024, 223, 116163. [Google Scholar] [CrossRef]
- Arnason, B.G.; Waksman, B.H. The Retest Reaction in Delayed Sensitivity. Lab. Investig. 1963, 12, 737–747. [Google Scholar]
- Nakagawa, S.; Fukushiro, S.; Gotoh, M.; Kohda, M.; Namba, M.; Tanioku, K. Studies on the Retest Reaction in Contact Sensitivity to DNCB. Dermatologica 1978, 157, 13–20. [Google Scholar] [CrossRef]
- Scheper, R.J.; von Blomberg, M.; Boerrigter, G.H.; Bruynzeel, D.; van Dinther, A.; Vos, A. Induction of Immunological Memory in the Skin. Role of Local T Cell Retention. Clin. Exp. Immunol. 1983, 51, 141–148. [Google Scholar]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T Cells in Nonlymphoid Tissue That Provide Enhanced Local Immunity during Infection with Herpes Simplex Virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common Clonal Origin of Central and Resident Memory T Cells Following Skin Immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Gadsbøll, A.-S.Ø.; Jee, M.H.; Funch, A.B.; Alhede, M.; Mraz, V.; Weber, J.F.; Callender, L.A.; Carroll, E.C.; Bjarnsholt, T.; Woetmann, A.; et al. Pathogenic CD8+ Epidermis-Resident Memory T Cells Displace Dendritic Epidermal T Cells in Allergic Dermatitis. J. Investig. Dermatol. 2020, 140, 806–815.e5. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two Subsets of Memory T Lymphocytes with Distinct Homing Potentials and Effector Functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T.; Bergström, M.A.; Börje, A.; Luthman, K.; Nilsson, J.L.G. Allergic Contact Dermatitis—Formation, Structural Requirements, and Reactivity of Skin Sensitizers. Chem. Res. Toxicol. 2008, 21, 53–69. [Google Scholar] [CrossRef]
- Gamradt, P.; Laoubi, L.; Nosbaum, A.; Mutez, V.; Lenief, V.; Grande, S.; Redoulès, D.; Schmitt, A.-M.; Nicolas, J.-F.; Vocanson, M. Inhibitory Checkpoint Receptors Control CD8+ Resident Memory T Cells to Prevent Skin Allergy. J. Allergy Clin. Immunol. 2019, 143, 2147–2157.e9. [Google Scholar] [CrossRef]
- He, D.; Wu, L.; Kim, H.K.; Li, H.; Elmets, C.A.; Xu, H. IL-17 and IFN-Gamma Mediate the Elicitation of Contact Hypersensitivity Responses by Different Mechanisms and Both Are Required for Optimal Responses. J. Immunol. 2009, 183, 1463–1470. [Google Scholar] [CrossRef]
- Albanesi, C.; Cavani, A.; Girolomoni, G. Interferon-Gamma-Stimulated Human Keratinocytes Express the Genes Necessary for the Production of Peptide-Loaded MHC Class II Molecules. J. Investig. Dermatol. 1998, 110, 138–142. [Google Scholar] [CrossRef]
- Trautmann, A.; Akdis, M.; Kleemann, D.; Altznauer, F.; Simon, H.U.; Graeve, T.; Noll, M.; Bröcker, E.B.; Blaser, K.; Akdis, C.A. T Cell-Mediated Fas-Induced Keratinocyte Apoptosis Plays a Key Pathogenetic Role in Eczematous Dermatitis. J. Clin. Investig. 2000, 106, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Z.; Tan, K.W.; Wong, F.H.S.; Chua, Y.L.; Tang, Y.; Ng, L.G.; Angeli, V.; Kemeny, D.M. CD8 T Cells Regulate Allergic Contact Dermatitis by Modulating CCR2-Dependent TNF/INOS-Expressing Ly6C+ CD11b+ Monocytic Cells. J. Investig. Dermatol. 2014, 134, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Kish, D.D.; Li, X.; Fairchild, R.L. CD8 T Cells Producing IL-17 and IFN-γ Initiate the Innate Immune Response Required for Responses to Antigen Skin Challenge. J. Immunol. 2009, 182, 5949–5959. [Google Scholar] [CrossRef]
- Kish, D.D.; Volokh, N.; Baldwin, W.M.; Fairchild, R.L. Hapten Application to the Skin Induces an Inflammatory Program Directing Hapten-Primed Effector CD8 T Cell Interaction with Hapten-Presenting Endothelial Cells. J. Immunol. 2011, 186, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Kursawe Larsen, C.; Funch, A.B.; Vaher, H.; Lohmann, R.K.D.; Jee, M.H.; Schwensen, J.F.B.; Zachariae, C.; Svedman, C.; Bergendorff, O.; Bonefeld, C.M.; et al. Cross-Reactivity between Thiuram Disulfides and Dithiocarbamates. A Study of TETD and ZDEC Using Mouse Models. Contact Dermat. 2024, 92, 137–144. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Iwata, Y.; Fukushima, H.; Tanaka, Y.; Watanabe, S.; Saito, K.; Ito, H.; Sugiura, M.; Akiyama, M.; Sugiura, K. Neutrophil Extracellular Traps Are Involved in Enhanced Contact Hypersensitivity Response in IL-36 Receptor Antagonist-Deficient Mice. Sci. Rep. 2022, 12, 13384. [Google Scholar] [CrossRef]
- Azeem, M.; Helal, M.; Klein-Hessling, S.; Serfling, E.; Goebeler, M.; Muhammad, K.; Kerstan, A. NFATc1 Fosters Allergic Contact Dermatitis Responses by Enhancing the Induction of IL-17-Producing CD8 Cells. J. Investig. Dermatol. 2025, 145, 1995–2006.e5. [Google Scholar] [CrossRef]
- Watanabe, H.; Gehrke, S.; Contassot, E.; Roques, S.; Tschopp, J.; Friedmann, P.S.; French, L.E.; Gaide, O. Danger Signaling through the Inflammasome Acts as a Master Switch between Tolerance and Sensitization. J. Immunol. 2008, 180, 5826–5832. [Google Scholar] [CrossRef]
- Hirai, T.; Yang, Y.; Zenke, Y.; Li, H.; Chaudhri, V.K.; De La Cruz Diaz, J.S.; Zhou, P.Y.; Nguyen, B.A.-T.; Bartholin, L.; Workman, C.J.; et al. Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche. Immunity 2021, 54, 84–98.e5. [Google Scholar] [CrossRef]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair Follicle-Derived IL-7 and IL-15 Mediate Skin-Resident Memory T Cell Homeostasis and Lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Wynne-Jones, E.; Freestone, D.; Pellicci, D.G.; Mielke, L.A.; Newman, D.M.; Braun, A.; Masson, F.; Kallies, A.; Belz, G.T.; et al. T-Box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 2015, 43, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Christo, S.N.; Evrard, M.; Park, S.L.; Gandolfo, L.C.; Burn, T.N.; Fonseca, R.; Newman, D.M.; Alexandre, Y.O.; Collins, N.; Zamudio, N.M.; et al. Discrete Tissue Microenvironments Instruct Diversity in Resident Memory T Cell Function and Plasticity. Nat. Immunol. 2021, 22, 1140–1151. [Google Scholar] [CrossRef]
- Cheuk, S.; Schlums, H.; Gallais Sérézal, I.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Zapata, L.; Garg, M.; Riding, R.L.; Refat, M.A.; Fan, X.; Azzolino, V.; Tovar-Garza, A.; Tsurushita, N.; et al. Antibody Blockade of IL-15 Signaling Has the Potential to Durably Reverse Vitiligo. Sci. Transl. Med. 2018, 10, eaam7710. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; De Jong, A.; Harel, S.; Destefano, G.M.; Rothman, L.; et al. Alopecia Areata Is Driven by Cytotoxic T Lymphocytes and Is Reversed by JAK Inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Evrard, M.; Wynne-Jones, E.; Peng, C.; Kato, Y.; Christo, S.N.; Fonseca, R.; Park, S.L.; Burn, T.N.; Osman, M.; Devi, S.; et al. Sphingosine 1-Phosphate Receptor 5 (S1PR5) Regulates the Peripheral Retention of Tissue-Resident Lymphocytes. J. Exp. Med. 2022, 219, e20210116. [Google Scholar] [CrossRef]
- Mora-Buch, R.; Lake, M.E.; Sama, A.; Chasse, A.Y.; Akbaba, H.; Mani, V.; Bromley, S.K. IL-4 Impairs the Formation of Skin-Resident Memory CD8+ T Cells. Nat. Immunol. 2025, 26, 1329–1338. [Google Scholar] [CrossRef]
- Mackay, L.K.; Stock, A.T.; Ma, J.Z.; Jones, C.M.; Kent, S.J.; Mueller, S.N.; Heath, W.R.; Carbone, F.R.; Gebhardt, T. Long-Lived Epithelial Immunity by Tissue-Resident Memory T (TRM) Cells in the Absence of Persisting Local Antigen Presentation. Proc. Natl. Acad. Sci. USA 2012, 109, 7037–7042. [Google Scholar] [CrossRef]
- Abdelbary, M.; Hobbs, S.J.; Gibbs, J.S.; Yewdell, J.W.; Nolz, J.C. T Cell Receptor Signaling Strength Establishes the Chemotactic Properties of Effector CD8+ T Cells That Control Tissue-Residency. Nat. Commun. 2023, 14, 3928. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.N.; Mooster, J.L.; Kilgore, A.M.; Osborn, J.F.; Nolz, J.C. Local Antigen in Nonlymphoid Tissue Promotes Resident Memory CD8+ T Cell Formation during Viral Infection. J. Exp. Med. 2016, 213, 951–966. [Google Scholar] [CrossRef]
- Muschaweckh, A.; Buchholz, V.R.; Fellenzer, A.; Hessel, C.; König, P.-A.; Tao, S.; Tao, R.; Heikenwälder, M.; Busch, D.H.; Korn, T.; et al. Antigen-Dependent Competition Shapes the Local Repertoire of Tissue-Resident Memory CD8+ T Cells. J. Exp. Med. 2016, 213, 3075–3086. [Google Scholar] [CrossRef]
- Jacob, S.E.; Castanedo-Tardan, M.P. Pharmacotherapy for Allergic Contact Dermatitis. Expert Opin. Pharmacother. 2007, 8, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Lenief, V.; Lefevre, M.-A.; Cuzin, R.; Guironnet-Paquet, A.; Mosnier, A.; Nosbaum, A.; Nicolas, J.-F.; Vocanson, M. Topical Corticosteroids Inhibit Allergic Skin Inflammation but Are Ineffective in Impeding the Formation and Expansion of Resident Memory T Cells. Allergy 2024, 79, 52–64. [Google Scholar] [CrossRef]
- Natsuaki, Y.; Egawa, G.; Nakamizo, S.; Ono, S.; Hanakawa, S.; Okada, T.; Kusuba, N.; Otsuka, A.; Kitoh, A.; Honda, T.; et al. Perivascular Leukocyte Clusters Are Essential for Efficient Activation of Effector T Cells in the Skin. Nat. Immunol. 2014, 15, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Goksøyr, L.; Funch, A.B.; Okholm, A.K.; Theander, T.G.; de Jongh, W.A.; Bonefeld, C.M.; Sander, A.F. Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis. Vaccines 2022, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Sitaru, S.; Budke, A.; Bertini, R.; Sperandio, M. Therapeutic Inhibition of CXCR1/2: Where Do We Stand? Intern. Emerg. Med. 2023, 18, 1647–1664. [Google Scholar] [CrossRef]
- Jurcevic, S.; Humfrey, C.; Uddin, M.; Warrington, S.; Larsson, B.; Keen, C. The Effect of a Selective CXCR2 Antagonist (AZD5069) on Human Blood Neutrophil Count and Innate Immune Functions. Br. J. Clin. Pharmacol. 2015, 80, 1324–1336. [Google Scholar] [CrossRef]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef]
- Kirsten, A.M.; Förster, K.; Radeczky, E.; Linnhoff, A.; Balint, B.; Watz, H.; Wray, H.; Salkeld, L.; Cullberg, M.; Larsson, B. The Safety and Tolerability of Oral AZD5069, a Selective CXCR2 Antagonist, in Patients with Moderate-to-Severe COPD. Pulm. Pharmacol. Ther. 2015, 31, 36–41. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and Safety of a CXCR2 Antagonist, AZD5069, in Patients with Uncontrolled Persistent Asthma: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Secukinumab: A Review in Psoriatic Arthritis. Drugs 2021, 81, 483–494. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Emapalumab: First Global Approval. Drugs 2019, 79, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.; Matheson, R.; Zachariae, C.; Cameron, G.; Li, L.; Edson-Heredia, E.; Braun, D.; Banerjee, S. Anti–Interleukin-17 Monoclonal Antibody Ixekizumab in Chronic Plaque Psoriasis. N. Engl. J. Med. 2012, 366, 1190–1199. [Google Scholar] [CrossRef]
- Wu, D.; Hou, S.-Y.; Zhao, S.; Hou, L.-X.; Jiao, T.; Xu, N.-N.; Zhang, N. Efficacy and Safety of Interleukin-17 Antagonists in Patients with Plaque Psoriasis: A Meta-Analysis from Phase 3 Randomized Controlled Trials. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.; Smith, M.P.; Thibodeaux, Q.; Reddy, V.; Liao, W.; Bhutani, T. Anti IL-17 in Psoriasis. Expert Rev. Clin. Immunol. 2019, 15, 1185–1194. [Google Scholar] [CrossRef]
- Todberg, T.; Zachariae, C.; Krustrup, D.; Skov, L. The Effect of Anti-IL-17 Treatment on the Reaction to a Nickel Patch Test in Patients with Allergic Contact Dermatitis. Int. J. Dermatol. 2019, 58, e58–e61. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, B.; Wang, Z.; Xu, G.; Wang, L.; Zhang, T.; Zhang, Y. Risk of Candida Infection and Serious Infections in Patients with Moderate-to-Severe Psoriasis Receiving Biologics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Clin. Pract. 2022, 2022, 2442603. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, L.; Song, Z.; Zhang, R.; Kang, Y. Biologic Therapy and Superficial Fungal Infection Risk in Moderate-to-Severe Psoriasis: A Meta-Analysis. Mycoses 2025, 68, e70081. [Google Scholar] [CrossRef]
- Qi, F.; Liu, F.; Gao, L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 2021, 12, 790125. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia Areata. Nat. Rev. Dis. Prim. 2017, 3, 17011. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shen, C.; Wang, J.; Tian, J. Janus Kinase Inhibitors for Alopecia Areata: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2320351. [Google Scholar] [CrossRef]
- Traidl, S.; Freimooser, S.; Werfel, T. Janus Kinase Inhibitors for the Therapy of Atopic Dermatitis. Allergol. Sel. 2021, 5, 293–304. [Google Scholar] [CrossRef]
- Ingrassia, J.P.; Maqsood, M.H.; Gelfand, J.M.; Weber, B.N.; Bangalore, S.; Lo Sicco, K.I.; Garshick, M.S. Cardiovascular and Venous Thromboembolic Risk With JAK Inhibitors in Immune-Mediated Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2024, 160, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Isufi, D.; Jensen, M.B.; Loft, N.; Skov, L.; Elberling, J.; Alinaghi, F. Risk of Infections during Treatment with Oral Janus Kinase Inhibitors in Randomized Placebo-Controlled Trials: A Systematic Review and Meta-Analysis. JAAD Int. 2025, 18, 106–116. [Google Scholar] [CrossRef]
- Tieu, R.; Zeng, Q.; Zhao, D.; Zhang, G.; Feizi, N.; Manandhar, P.; Williams, A.L.; Popp, B.; Wood-Trageser, M.A.; Demetris, A.J.; et al. Tissue-Resident Memory T Cell Maintenance during Antigen Persistence Requires Both Cognate Antigen and Interleukin-15. Sci. Immunol. 2023, 8, eadd8454. [Google Scholar] [CrossRef]
- Teicher, B.A. TGFβ-Directed Therapeutics: 2020. Pharmacol. Ther. 2021, 217, 107666. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Biemond, M.; Vremec, D.; Gray, D.H.D.; Hodgkin, P.D.; Heinzel, S. Programmed Death Receptor 1 (PD-1) Ligand Fc Fusion Proteins Reduce T-Cell Proliferation in Vitro Independently of PD-1. Immunol. Cell Biol. 2024, 102, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Tetsu, H.; Nakayama, K.; Nishijo, T.; Yuki, T.; Miyazawa, M. CTLA-4 Suppresses Hapten-Induced Contact Hypersensitivity in Atopic Dermatitis Model Mice. Sci. Rep. 2023, 13, 7936. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.W.; Helm, E.Y.; Fu, Z.; Xiong, L.; Sun, N.; Oliff, K.N.; Muehlbauer, M.; Avram, D.; Zhou, L. The Aryl Hydrocarbon Receptor Cell Intrinsically Promotes Resident Memory CD8+ T Cell Differentiation and Function. Cell Rep. 2023, 42, 111963. [Google Scholar] [CrossRef]
- Correia, M.P.; Jeong, M.; Ast, V.; Platten, M.; Sexl, V.; Mogler, C. AhR-Mediated Activation of Innate Lymphocytes Restrains Tissue-Resident Memory-like CD8 + T Cell Responses during Contact Hypersensitivity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Chi, H. Regulation and Function of MTOR Signalling in T Cell Fate Decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof Is a Natural AhR Agonist That Resolves Skin Inflammation in Mice and Humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Bobonich, M.; Gorelick, J.; Aldredge, L.; Bruno, M.J.; DiRuggiero, D.; Martin, G.; Tallman, A.M.; Gold, L.S. Tapinarof, a Novel, First-in-Class, Topical Therapeutic Aryl Hydrocarbon Receptor Agonist for the Management of Psoriasis. J. Drugs Dermatol. 2023, 22, 779–784. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Stein Gold, L.; Strober, B.; Papp, K.A.; Armstrong, A.W.; Bagel, J.; Kircik, L.; Ehst, B.; Hong, H.C.-H.; Soung, J.; et al. Phase 3 Trials of Tapinarof Cream for Plaque Psoriasis. N. Engl. J. Med. 2021, 385, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the Treatment of Psoriasis: A Review of the Unique Mechanism of Action of a Novel Therapeutic Aryl Hydrocarbon Receptor-Modulating Agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef]
- Alexis, A.F.; Kircik, L.; Chovatiya, R.; Rice, Z.P.; Soong, W.; Bhutani, T.; Brown, P.M.; Piscitelli, S.C.; Rubenstein, D.S.; Tallman, A.M.; et al. Tapinarof Cream for Adults and Children with Atopic Dermatitis-Efficacy by Race and Fitzpatrick Skin Type in Two Phase 3 Randomized Clinical Trials. Dermatol. Ther. 2025, 15, 2667–2682. [Google Scholar] [CrossRef]
- Simpson, E.L.; Hebert, A.A.; Browning, J.; Serrao, R.T.; Sofen, H.; Brown, P.M.; Piscitelli, S.C.; Rubenstein, D.S.; Tallman, A.M. Tapinarof Improved Outcomes and Sleep for Patients and Families in Two Phase 3 Atopic Dermatitis Trials in Adults and Children. Dermatol. Ther. 2025, 15, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Miao, J.; Cao, R.; Han, M.; Sun, Y.; Liu, X.; Guo, L. Rapamycin Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing the MTOR-STAT3 Pathway. Neurochem. Res. 2017, 42, 2831–2840. [Google Scholar] [CrossRef]
- Esposito, M.; Ruffini, F.; Bellone, M.; Gagliani, N.; Battaglia, M.; Martino, G.; Furlan, R. Rapamycin Inhibits Relapsing Experimental Autoimmune Encephalomyelitis by Both Effector and Regulatory T Cells Modulation. J. Neuroimmunol. 2010, 220, 52–63. [Google Scholar] [CrossRef]
- Fernandez, D.; Bonilla, E.; Mirza, N.; Niland, B.; Perl, A. Rapamycin Reduces Disease Activity and Normalizes T Cell Activation-Induced Calcium Fluxing in Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 2006, 54, 2983–2988. [Google Scholar] [CrossRef]
- Geier, C.; Perl, A. Therapeutic MTOR Blockade in Systemic Autoimmunity: Implications for Antiviral Immunity and Extension of Lifespan. Autoimmun. Rev. 2021, 20, 102984. [Google Scholar] [CrossRef]
- Capella, G.L.; Grigerio, E.; Altomare, G. A Randomized Trial of Leukotriene Receptor Antagonist Montelukast in Moderate-to-Severe Atopic Dermatitis of Adults. Eur. J. Dermatol. 2001, 11, 209–213. [Google Scholar] [PubMed]
- Veien, N.K.; Busch-Sørensen, M.; Stausbøl-Grøn, B. Montelukast Treatment of Moderate to Severe Atopic Dermatitis in Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Acad. Dermatol. 2005, 53, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Heber, S.; Gold-Binder, M.; Ciotu, C.I.; Witek, M.; Ninidze, N.; Kress, H.-G.; Fischer, M.J.M. A Human TRPA1-Specific Pain Model. J. Neurosci. 2019, 39, 3845–3855. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-Concept Clinical Trial of Etokimab Shows a Key Role for IL-33 in Atopic Dermatitis Pathogenesis. Sci. Transl. Med. 2019, 11, eaax2945. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Mustapa, M.N.; Reid, F.; Lei, A.; Smith, R.; Moate, R.; Kelly, A.; Chen, R.; Gavala, M.; Jimenez, E.; et al. Efficacy and Safety of Tozorakimab in Moderate-to-Severe Atopic Dermatitis: A Phase 2a Randomized Controlled Trial (FRONTIER-2). J. Eur. Acad. Dermatol. Venereol. 2025, 39, 1126–1133. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Church, D.S. Pharmacology of Antihistamines. Indian J. Dermatol. 2013, 58, 219–224. [Google Scholar] [CrossRef]
- Naicker, P.; Anoopkumar-Dukie, S.; Grant, G.D.; Kavanagh, J.J. The Effects of Antihistamines with Varying Anticholinergic Properties on Voluntary and Involuntary Movement. Clin. Neurophysiol. 2013, 124, 1840–1845. [Google Scholar] [CrossRef]
- van Ruissen, M.C.E.; van Kraaij, S.J.W.; Wolfova, J.; Herrmann, F.E.; Botilde, Y.; Wollin, L.; Klarenbeek, N.B. Proof of Pharmacology, Safety, and Pharmacokinetics of the Novel TRPA1 Antagonist BI 1839100: A Randomized, Placebo-Controlled, Parallel Group, First-In-Human Study in Healthy Male Participants. Clin. Transl. Sci. 2025, 18, e70290. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, J.-P. Metabolism versus Chemical Transformation or Pro- versus Prehaptens? Contact Dermat. 2006, 54, 73–74. [Google Scholar] [CrossRef] [PubMed]
- El Ali, Z.; Gerbeix, C.; Hemon, P.; Esser, P.R.; Martin, S.F.; Pallardy, M.; Kerdine-Römer, S. Allergic Skin Inflammation Induced by Chemical Sensitizers Is Controlled by the Transcription Factor Nrf2. Toxicol. Sci. 2013, 134, 39–48. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab Treatment Decreases Biomarkers and Improves Clinical Symptoms in Systemic Sclerosis Patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Balato, A.; Lembo, S.; Ayala, F.; Balato, N.; Caiazzo, G.; Raimondo, A.; Di Caprio, R.; Monfrecola, G. Mechanistic Target of Rapamycin Complex 1 Is Involved in Psoriasis and Regulated by Anti-TNF-α Treatment. Exp. Dermatol. 2017, 26, 325–327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funch, A.B.; Geisler, C.; Bonefeld, C.M. Allergic Contact Dermatitis: Immunopathology and Potential Therapeutic Strategies. J. Clin. Med. 2025, 14, 7175. https://doi.org/10.3390/jcm14207175
Funch AB, Geisler C, Bonefeld CM. Allergic Contact Dermatitis: Immunopathology and Potential Therapeutic Strategies. Journal of Clinical Medicine. 2025; 14(20):7175. https://doi.org/10.3390/jcm14207175
Chicago/Turabian StyleFunch, Anders Boutrup, Carsten Geisler, and Charlotte Menné Bonefeld. 2025. "Allergic Contact Dermatitis: Immunopathology and Potential Therapeutic Strategies" Journal of Clinical Medicine 14, no. 20: 7175. https://doi.org/10.3390/jcm14207175
APA StyleFunch, A. B., Geisler, C., & Bonefeld, C. M. (2025). Allergic Contact Dermatitis: Immunopathology and Potential Therapeutic Strategies. Journal of Clinical Medicine, 14(20), 7175. https://doi.org/10.3390/jcm14207175







