Combined Use of Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging in the Differential Diagnosis of Sellar Tumors: A Single-Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Acquisition and Processing
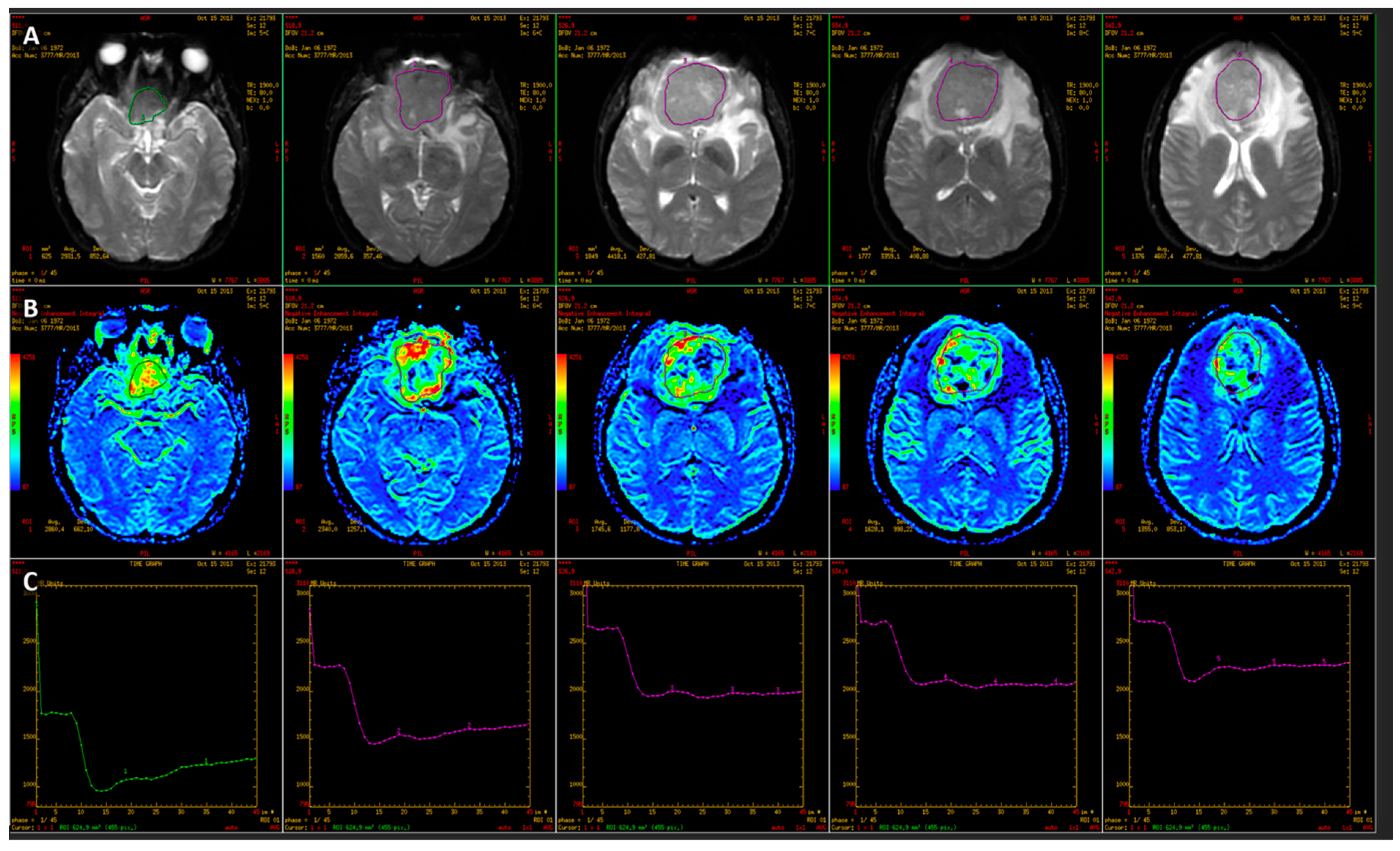
2.2.1. Diffusion-Weighted Imaging
2.2.2. Dynamic Susceptibility Contrast Perfusion-Weighted Imaging
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gittleman, H.; Ostrom, Q.T.; Farah, P.D.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Kruchko, C.; Singer, J.; Kshettry, V.R.; Laws, E.R.; et al. Descriptive Epidemiology of Pituitary Tumors in the United States, 2004–2009: Clinical Article. J. Neurosurg. 2014, 121, 527–535. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Miki, Y. Imaging of Pituitary Tumors: An Update with the 5th WHO Classifications—Part 2. Neoplasms Other than PitNET and Tumor-Mimicking Lesions. Jpn. J. Radiol. 2023, 41, 808–829. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Bano, S. Imaging of the Pituitary: Recent Advances. Indian J. Endocr. Metab. 2011, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, J.-F. Magnetic Resonance Imaging of Pituitary Tumors. In Frontiers of Hormone Research; Buchfelder, M., Guaraldi, F., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 45, pp. 97–120. ISBN 978-3-318-02737-2. [Google Scholar]
- Ilie, M.D.; Lasolle, H.; Raverot, G. Emerging and Novel Treatments for Pituitary Tumors. J. Clin. Med. 2019, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Buchfelder, M.; Schlaffer, S.M.; Zhao, Y. The Optimal Surgical Techniques for Pituitary Tumors. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101299. [Google Scholar] [CrossRef]
- Molitch, M.E. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA 2017, 317, 516. [Google Scholar] [CrossRef]
- Reeves, R.A.; Parekh, M. Pituitary Gland Imaging. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pierallini, A.; Caramia, F.; Falcone, C.; Tinelli, E.; Paonessa, A.; Ciddio, A.B.; Fiorelli, M.; Bianco, F.; Natalizi, S.; Ferrante, L.; et al. Pituitary Macroadenomas: Preoperative Evaluation of Consistency with Diffusion-Weighted MR Imaging—Initial Experience. Radiology 2006, 239, 223–231. [Google Scholar] [CrossRef]
- Korbecki, A.; Machaj, W.; Korbecka, J.; Sobański, M.; Kaczorowski, M.; Tabakow, P.; Hałoń, A.; Trybek, G.; Podgórski, P.; Bladowska, J. Evaluation of the Value of Perfusion-Weighted Magnetic Resonance Imaging in the Differential Diagnosis of Sellar and Parasellar Tumors. J. Clin. Med. 2023, 12, 2957. [Google Scholar] [CrossRef]
- Hansen, T.M.; Batra, S.; Lim, M.; Gallia, G.L.; Burger, P.C.; Salvatori, R.; Wand, G.; Quinones-Hinojosa, A.; Kleinberg, L.; Redmond, K.J. Invasive Adenoma and Pituitary Carcinoma: A SEER Database Analysis. Neurosurg. Rev. 2014, 37, 279–286. [Google Scholar] [CrossRef]
- Korbecki, A.; Wagel, J.; Zacharzewska-Gondek, A.; Gewald, M.; Korbecka, J.; Sobański, M.; Kacała, A.; Zdanowicz-Ratajczyk, A.; Kaczorowski, M.; Hałoń, A.; et al. Role of Diffusion-Weighted Imaging in the Diagnosis of Pituitary Region Tumors. Neuroradiology 2025, 67, 437–447. [Google Scholar] [CrossRef]
- Kunii, N.; Abe, T.; Kawamo, M.; Tanioka, D.; Izumiyama, H.; Moritani, T. Rathke’s Cleft Cysts: Differentiation from Other Cystic Lesions in the Pituitary Fossa by Use of Single-Shot Fast Spin-Echo Diffusion-Weighted MR Imaging. Acta Neurochir. 2007, 149, 759–769. [Google Scholar] [CrossRef]
- Mahmoud, O.M.; Tominaga, A.; Amatya, V.J.; Ohtaki, M.; Sugiyama, K.; Saito, T.; Sakoguchi, T.; Kinoshita, Y.; Shrestha, P.; Abe, N.; et al. Role of PROPELLER Diffusion Weighted Imaging and Apparent Diffusion Coefficient in the Diagnosis of Sellar and Parasellar Lesions. Eur. J. Radiol. 2010, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, O.M.; Tominaga, A.; Amatya, V.J.; Ohtaki, M.; Sugiyama, K.; Sakoguchi, T.; Kinoshita, Y.; Takeshima, Y.; Abe, N.; Akiyama, Y.; et al. Role of PROPELLER Diffusion-Weighted Imaging and Apparent Diffusion Coefficient in the Evaluation of Pituitary Adenomas. Eur. J. Radiol. 2011, 80, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Maeda, M.; Hori, K.; Kozuka, Y.; Sakuma, H.; Taki, W.; Takeda, K. Apparent Diffusion Coefficient of Pituitary Macroadenoma Evaluated with Line-Scan Diffusion-Weighted Imaging. J. Neuroradiol. 2007, 34, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Bladowska, J.; Zimny, A.; Guziński, M.; Hałoń, A.; Tabakow, P.; Czyż, M.; Czapiga, B.; Jarmundowicz, W.; Sąsiadek, M.J. Usefulness of Perfusion Weighted Magnetic Resonance Imaging with Signal-Intensity Curves Analysis in the Differential Diagnosis of Sellar and Parasellar Tumors: Preliminary Report. Eur. J. Radiol. 2013, 82, 1292–1298. [Google Scholar] [CrossRef]
- Sawlani, V.; Patel, M.D.; Davies, N.; Flintham, R.; Wesolowski, R.; Ughratdar, I.; Pohl, U.; Nagaraju, S.; Petrik, V.; Kay, A.; et al. Multiparametric MRI: Practical Approach and Pictorial Review of a Useful Tool in the Evaluation of Brain Tumours and Tumour-like Lesions. Insights Imaging 2020, 11, 84. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Mukundan, S.; Barboriak, D.P. Diffusion-Weighted and Perfusion MR Imaging for Brain Tumor Characterization and Assessment of Treatment Response. Radiology 2006, 239, 632–649. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Feng, Y.; Yu, T.; Guo, Y.; Bai, X.; Ye, Z. Diffusion and Perfusion Weighted Magnetic Resonance Imaging for Tumor Volume Definition in Radiotherapy of Brain Tumors. Radiat. Oncol. 2016, 11, 123. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Jo, Y.; Yoo, R.-E.; Choi, S.H.; Nam, S.J.; Kim, J.H. Radiomics Prognostication Model in Glioblastoma Using Diffusion- and Perfusion-Weighted MRI. Sci. Rep. 2020, 10, 4250. [Google Scholar] [CrossRef]
- Valles, F.E.; Perez-Valles, C.L.; Regalado, S.; Barajas, R.F.; Rubenstein, J.L.; Cha, S. Combined Diffusion and Perfusion MR Imaging as Biomarkers of Prognosis in Immunocompetent Patients with Primary Central Nervous System Lymphoma. AJNR Am. J. Neuroradiol. 2013, 34, 35–40. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Rogg, J.M.; Donahue, J.E.; Machan, J.T.; Goldman, M.A.; Doberstein, C.E. Preoperative MRI Evaluation of Pituitary Macroadenoma: Imaging Features Predictive of Successful Transsphenoidal Surgery. Am. J. Roentgenol. 2010, 195, 720–728. [Google Scholar] [CrossRef]
- Tamrazi, B.; Pekmezci, M.; Aboian, M.; Tihan, T.; Glastonbury, C.M. Apparent Diffusion Coefficient and Pituitary Macroadenomas: Pre-Operative Assessment of Tumor Atypia. Pituitary 2017, 20, 195–200. [Google Scholar] [CrossRef]
- Yamasaki, F.; Kurisu, K.; Satoh, K.; Arita, K.; Sugiyama, K.; Ohtaki, M.; Takaba, J.; Tominaga, A.; Hanaya, R.; Yoshioka, H.; et al. Apparent Diffusion Coefficient of Human Brain Tumors at MR Imaging. Radiology 2005, 235, 985–991. [Google Scholar] [CrossRef]
- Bladowska, J.; Ssiadek, M. Diagnostic Imaging of the Pituitary and Parasellar Region. In Pituitary Adenomas; Rahimi-Movaghar, V., Ed.; InTech: Berlin, Germany, 2012; ISBN 978-953-51-0041-6. [Google Scholar]
- Doai, M.; Tonami, H.; Matoba, M.; Tachibana, O.; Iizuka, H.; Nakada, S.; Yamada, S. Pituitary Macroadenoma: Accuracy of Apparent Diffusion Coefficient Magnetic Resonance Imaging in Grading Tumor Aggressiveness. Neuroradiol. J. 2019, 32, 86–91. [Google Scholar] [CrossRef]
- Ma, Z.; He, W.; Zhao, Y.; Yuan, J.; Zhang, Q.; Wu, Y.; Chen, H.; Yao, Z.; Li, S.; Wang, Y. Predictive Value of PWI for Blood Supply and T1-Spin Echo MRI for Consistency of Pituitary Adenoma. Neuroradiology 2016, 58, 51–57. [Google Scholar] [CrossRef]
- Filippi, C.G.; Edgar, M.A.; Ulug, A.M.; Heier, L.A.; Zimmerman, R.D. Appearance of Meningiomas on Diffusion-Weighted Images: Correlating Diffusion Constants with Histopathologic Findings. Am. J. Neuroradiol. 2001, 22, 65–72. [Google Scholar] [PubMed]
- Santelli, L.; Ramondo, G.; Della Puppa, A.; Ermani, M.; Scienza, R.; d’Avella, D.; Manara, R. Diffusion-Weighted Imaging Does Not Predict Histological Grading in Meningiomas. Acta Neurochir. 2010, 152, 1315–1319. [Google Scholar] [CrossRef]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R.J. Diffusion and Perfusion MRI to Differentiate Treatment-Related Changes Including Pseudoprogression from Recurrent Tumors in High-Grade Gliomas with Histopathologic Evidence. AJNR Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef]
- Du, X.; He, Y.; Lin, W. Diagnostic Accuracy of the Diffusion-Weighted Imaging Method Used in Association With the Apparent Diffusion Coefficient for Differentiating Between Primary Central Nervous System Lymphoma and High-Grade Glioma: Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 882334. [Google Scholar] [CrossRef] [PubMed]

| Patient Age | n | M ± SD | Me (Q1; Q3) | Range |
|---|---|---|---|---|
| Age | 88 | 59.28 ± 15.79 | 63.50 (51.00; 68.25) | 21.00–86.00 |
| Tumor size | n | M ± SD | Me (Q1; Q3) | Range |
| TR | 88 | 2.79 ± 1.11 | 2.70 (2.00; 3.50) | 0.70–6.60 |
| AP | 88 | 2.58 ± 1.02 | 2.40 (1.80; 3.30) | 1.20–5.90 |
| CC | 88 | 2.82 ± 1.33 | 2.65 (1.78; 3.52) | 0.60–7.30 |
| volume | 88 | 14.64 ± 16.13 | 9.61 (3.44; 20.70) | 0.35–77.12 |
| Tumor type | n | % | ||
| Non-functional solid adenomas | 25 | 28.4 | ||
| Invasive pituitary adenomas | 22 | 25 | ||
| Meningiomas | 16 | 18.2 | ||
| Prolactinomas | 13 | 14.8 | ||
| Adamantinomatous craniopharyngiomas | 12 | 13.6 | ||
| DWI and PWI parameters: | n | M ± SD | Me (Q1; Q3) | Range |
| ADCmin | 88 | 0.76 ± 0.35 | 0.66 (0.54; 0.83) | 0.37–1.96 |
| rADCmin | 88 | 0.96 ± 0.42 | 0.84 (0.69; 1.08) | 0.49–2.38 |
| rCBV | 88 | 3.08 ± 1.93 | 2.69 (1.48; 4.32) | 0.29–11.33 |
| rPH | 88 | 1.75 ± 1.24 | 1.48 (0.76; 2.26) | 0.20–5.92 |
| rCBVmax | 88 | 4.15 ± 2.63 | 3.78 (2.17; 5.40) | 0.42–17.77 |
| rPHmax | 88 | 2.55 ± 1.65 | 2.08 (1.37; 3.35) | 0.29–8.16 |
| Variable | Group | n | M ± SD | Me (Q1; Q3) | Range |
|---|---|---|---|---|---|
| ADCmin | Non-functional solid adenomas | 25 | 0.67 ± 0.18 | 0.70 (0.53; 0.76) | 0.37–1.06 |
| ADCmin | Invasive pituitary adenomas | 22 | 0.58 ± 0.10 | 0.56 (0.52; 0.64) | 0.43–0.92 |
| ADCmin | Meningiomas | 16 | 0.78 ± 0.19 | 0.73 (0.65; 0.84) | 0.55–1.20 |
| ADCmin | Prolactinomas | 13 | 0.60 ± 0.20 | 0.57 (0.44; 0.67) | 0.39–1.00 |
| ADCmin | Adamantinomatous craniopharyngiomas | 12 | 1.45 ± 0.36 | 1.42 (1.29; 1.70) | 0.87–1.96 |
| rADCmin | Non-functional solid adenomas | 25 | 0.85 ± 0.22 | 0.87 (0.69; 0.97) | 0.49–1.29 |
| rADCmin | Invasive pituitary adenomas | 22 | 0.74 ± 0.12 | 0.73 (0.67; 0.81) | 0.54–1.08 |
| rADCmin | Meningiomas | 16 | 0.95 ± 0.21 | 0.90 (0.82; 1.03) | 0.62–1.41 |
| rADCmin | Prolactinomas | 13 | 0.77 ± 0.23 | 0.69 (0.59; 0.85) | 0.51–1.23 |
| rADCmin | Adamantinomatous craniopharyngiomas | 12 | 1.83 ± 0.44 | 1.92 (1.56; 2.23) | 1.13–2.38 |
| rCBVmax | Non-functional solid adenomas | 25 | 3.37 ± 1.99 | 3.27 (1.85; 4.36) | 0.42–7.50 |
| rCBVmax | Invasive pituitary adenomas | 22 | 4.03 ± 2.23 | 3.98 (1.96; 5.47) | 1.29–8.84 |
| rCBVmax | Meningiomas | 16 | 7.05 ± 3.34 | 5.82 (5.17; 7.41) | 3.78–17.77 |
| rCBVmax | Prolactinomas | 13 | 4.04 ± 1.53 | 3.75 (2.81; 5.04) | 2.01–7.08 |
| rCBVmax | Adamantinomatous craniopharyngiomas | 12 | 2.22 ± 0.95 | 2.15 (1.84; 2.54) | 0.58–3.97 |
| rCBV | Non-functional solid adenomas | 25 | 2.62 ± 1.52 | 2.55 (1.28; 3.65) | 0.29–5.97 |
| rCBV | Invasive pituitary adenomas | 22 | 3.03 ± 1.76 | 2.60 (1.33; 4.57) | 0.97–5.88 |
| rCBV | Meningiomas | 16 | 5.23 ± 2.16 | 4.53 (3.90; 6.32) | 2.53–11.33 |
| rCBV | Prolactinomas | 13 | 2.84 ± 1.21 | 2.39 (1.78; 3.83) | 1.46–4.91 |
| rCBV | Adamantinomatous craniopharyngiomas | 12 | 1.52 ± 0.77 | 1.39 (1.22; 1.84) | 0.39–2.96 |
| rPHmax | Non-functional solid adenomas | 25 | 2.09 ± 1.83 | 1.40 (0.85; 2.57) | 0.29–6.96 |
| rPHmax | Invasive pituitary adenomas | 22 | 2.57 ± 1.27 | 2.12 (1.70; 3.54) | 0.86–5.18 |
| rPHmax | Meningiomas | 16 | 3.43 ± 1.99 | 3.00 (2.28; 4.50) | 0.62–8.16 |
| rPHmax | Prolactinomas | 13 | 3.01 ± 1.51 | 2.83 (1.84; 4.08) | 0.72–6.59 |
| rPHmax | Adamantinomatous craniopharyngiomas | 12 | 1.80 ± 0.89 | 1.80 (1.45; 2.05) | 0.54–4.02 |
| rPH | Non-functional solid adenomas | 25 | 1.63 ± 1.48 | 1.21 (0.57; 2.18) | 0.20–5.92 |
| rPH | Invasive pituitary adenomas | 22 | 1.84 ± 1.03 | 1.65 (1.14; 2.45) | 0.46–4.16 |
| rPH | Meningiomas | 16 | 2.26 ± 1.28 | 2.10 (1.49; 2.45) | 0.49–5.60 |
| rPH | Prolactinomas | 13 | 2.02 ± 1.16 | 1.75 (1.40; 2.65) | 0.29–4.21 |
| rPH | Adamantinomatous craniopharyngiomas | 12 | 0.89 ± 0.50 | 0.77 (0.47; 1.24) | 0.26–1.86 |
| Meningiomas vs. Solid Non-Functional Adenomas | ||||||
|---|---|---|---|---|---|---|
| ADCmin and rPHmax | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| ADCmin | 0.54 | 0.675 (0.515; 0.818) | 1 | 0.32 | 0.59 | 0.050 |
| rPHmax | 1.62 | 0.740 (0.578; 0.880) | 0.88 | 0.6 | 0.71 | 0.031 |
| ADCmin and rPHmax | 0.818 (0.690; 0.920) | 0.88 | 0.76 | 0.8 | 0.000 | |
| Meningioms vs. invasive adenomas | ||||||
| ADCmin and rCBV | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| ADCmin | 0.64 | 0.872 (0.743; 0.972) | 0.81 | 0.82 | 0.82 | 0.000 |
| rCBV | 2.83 | 0.790 (0.625; 0.918) | 0.94 | 0.59 | 0.74 | 0.001 |
| ADCmin and rCBV | 0.830 (0.699; 0.946) | 0.75 | 0.91 | 0.84 | 0.000 | |
| ADCmin and rCBVmax | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| ADCmin | 0.64 | 0.872 (0.743; 0.972) | 0.81 | 0.82 | 0.82 | 0.000 |
| rCBVmax | 4.54 | 0.804 (0.659; 0.923) | 0.94 | 0.59 | 0.74 | 0.001 |
| ADCmin and rCBVmax | 0.815 (0.676; 0.923) | 0.81 | 0.82 | 0.82 | 0.000 | |
| rADCmin and rCBV | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| rADCmin | 0.83 | 0.824 (0.670; 0.952) | 0.75 | 0.82 | 0.79 | 0.000 |
| rCBV | 2.83 | 0.790 (0.625; 0.918) | 0.94 | 0.59 | 0.74 | 0.001 |
| rADCmin and rCBV | 0.767 (0.633; 0.892) | 0.62 | 0.91 | 0.79 | 0.000 | |
| rADCmin and rCBVmax | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| rADCmin | 0.83 | 0.824 (0.670; 0.952) | 0.75 | 0.82 | 0.79 | 0.000 |
| rCBVmax | 4.54 | 0.804 (0.659; 0.923) | 0.94 | 0.59 | 0.74 | 0.001 |
| rADCmin and rCBVmax | 0.753 (0.608; 0.892) | 0.69 | 0.82 | 0.76 | 0.001 | |
| Meningiomas vs. adamantinomatous craniopharyngiomas | ||||||
| rADCmin and rCBV | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| rADCmin | 1.13 | 0.964 (0.891; 1.000) | 0.81 | 1 | 0.89 | 0.000 |
| rCBV | 3.26 | 0.984 (0.938; 1.000) | 0.88 | 1 | 0.93 | 0.000 |
| rADCmin and rCBV | 0.906 (0.812; 1.000) | 0.81 | 1 | 0.89 | 0.000 | |
| rADCmin and rPH | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| rADCmin | 1.13 | 0.964 (0.891; 1.000) | 0.81 | 1 | 0.89 | 0.000 |
| rPH | 1.53 | 0.891 (0.750; 0.990) | 0.75 | 0.92 | 0.82 | 0.000 |
| rADCmin and rPH | 0.844 (0.719; 0.938) | 0.69 | 1 | 0.82 | 0.000 | |
| rADCmin and rCBVmax | threshold | auc.CI | sensitivity | specificity | accuracy | p |
| rADCmin | 1.13 | 0.964 (0.891; 1.000) | 0.81 | 1 | 0.89 | 0.000 |
| rCBVmax | 4.33 | 0.995 (0.969; 1.000) | 0.94 | 1 | 0.96 | 0.000 |
| rADCmin and rCBVmax | 0.906 (0.812; 1.000) | 0.81 | 1 | 0.89 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, A.; Łukasiewicz, M.; Kacała, A.; Sobański, M.; Zdanowicz-Ratajczyk, A.; Szałata, K.; Dorochowicz, M.; Korbecka, J.; Trybek, G.; Zimny, A.; et al. Combined Use of Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging in the Differential Diagnosis of Sellar Tumors: A Single-Centre Experience. J. Clin. Med. 2025, 14, 7168. https://doi.org/10.3390/jcm14207168
Korbecki A, Łukasiewicz M, Kacała A, Sobański M, Zdanowicz-Ratajczyk A, Szałata K, Dorochowicz M, Korbecka J, Trybek G, Zimny A, et al. Combined Use of Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging in the Differential Diagnosis of Sellar Tumors: A Single-Centre Experience. Journal of Clinical Medicine. 2025; 14(20):7168. https://doi.org/10.3390/jcm14207168
Chicago/Turabian StyleKorbecki, Adrian, Marek Łukasiewicz, Arkadiusz Kacała, Michał Sobański, Agata Zdanowicz-Ratajczyk, Karolina Szałata, Mateusz Dorochowicz, Justyna Korbecka, Grzegorz Trybek, Anna Zimny, and et al. 2025. "Combined Use of Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging in the Differential Diagnosis of Sellar Tumors: A Single-Centre Experience" Journal of Clinical Medicine 14, no. 20: 7168. https://doi.org/10.3390/jcm14207168
APA StyleKorbecki, A., Łukasiewicz, M., Kacała, A., Sobański, M., Zdanowicz-Ratajczyk, A., Szałata, K., Dorochowicz, M., Korbecka, J., Trybek, G., Zimny, A., & Bladowska, J. (2025). Combined Use of Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging in the Differential Diagnosis of Sellar Tumors: A Single-Centre Experience. Journal of Clinical Medicine, 14(20), 7168. https://doi.org/10.3390/jcm14207168






