Accelerated CXL Versus Accelerated Contact-Lens Assisted CXL Treatment for Progressive Keratoconus—A 3-Year Retrospective Comparative Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
Eligibility Criteria
2.2. Data Collection
2.3. Statistical Analysis
2.4. Surgical Technique
2.5. Postoperative Follow-Up
2.6. Main Outcome Measures
3. Results
3.1. Efficacy
3.2. Regression and Progression
3.3. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CXL | Corneal Cross-Linking |
| A-CACXL | Contact lens-assisted accelerated corneal cross-linking |
| Kmax | Maximum keratometry |
| D | Diopters |
| Kmean | Mean keratometry |
| K | Keratometry |
| MCT | Minimum corneal thickness |
| BAD-D | Belin–Ambrósio enhanced ectasia display total deviation |
| UDVA | Uncorrected distance visual acuity |
| BCVA | Best-corrected visual acuity |
| ISV | Index of surface variance |
| IHD | Index of height decentration |
References
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Alió Del Barrio, J.L.; Alió, J.L. Corneal surgery in keratoconus: Which type, which technique, which outcomes? Eye Vis. 2016, 3, 2. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- O’Brart, D.P.S. Corneal collagen cross-linking: A review. J. Optom. 2014, 7, 113–124. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J.; Loukas, Y.L.; Asimellis, G. Cross-Linking Biomechanical Effect in Human Corneas by Same Energy, Different UV-A Fluence: An Enzymatic Digestion Comparative Evaluation. Cornea 2016, 35, 557–561. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Giacomin, N.T.; Bueno, R.L.; Ghanem, R.C.; Moraes, H.V.; Santhiago, M.R. Accelerated corneal collagen crosslinking: Technique, efficacy, safety, and applications. J. Cataract. Refract. Surg. 2016, 42, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, M.M.; Einollahi, B.; Baradaran-Rafii, A.; Roshandel, D.; Hasani, H.; Nazeri, M. Accelerated versus conventional corneal collagen cross-linking in patients with keratoconus: An intrapatient comparative study. Int. Ophthalmol. 2016, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spörl, E.; Reber, F.; Pillunat, L.; Funk, R. Corneal Endothelial Cytotoxicity of Riboflavin/UVA Treatment in vitro. Ophthalmic Res. 2003, 35, 324–328. [Google Scholar] [CrossRef]
- Yaffe, J.A.; Matlov Kormas, R.; Malyugin, B.E.; Boyko, M.; Tuuminen, R.; Knyazer, B. Ethnicity, Progressive Keratoconus, and Outcomes after Corneal Cross-Linking in Southern Israel. Life 2023, 13, 2294. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Mrochen, M.; Iseli, H.P.; Seiler, T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J. Cataract. Refract. Surg. 2009, 35, 621–624. [Google Scholar] [CrossRef]
- Cantemir, A.; Alexa, A.I.; Galan, B.G.; Anton, N.; Ciuntu, R.E.; Danielescu, C.; Chiselita, D.; Costin, D. Outcomes of iontophoretic corneal collagen crosslinking in keratoconic eyes with very thin corneas. Medicine 2017, 96, e8758. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, M.S.; Gupta, D.; Sachdev, G.; Sachdev, R. Tailored stromal expansion with a refractive lenticule for crosslinking the ultrathin cornea. J. Cataract. Refract. Surg. 2015, 41, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Mencucci, R. Transepithelial corneal collagen cross-linking in ultrathin keratoconic corneas. Clin. Ophthalmol. 2012, 6, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Kling, S.; Gilardoni, F.; Hafezi, N.; Hillen, M.; Abrishamchi, R.; Gomes, J.A.P.; Mazzotta, C.; Randleman, J.B.; Torres-Netto, E.A. Individualized Corneal Cross-linking With Riboflavin and UV-A in Ultrathin Corneas: The Sub400 Protocol. Am. J. Ophthalmol. 2021, 224, 133–142. [Google Scholar] [CrossRef]
- Jacob, S.; Kumar, D.A.; Agarwal, A.; Basu, S.; Sinha, P.; Agarwal, A. Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J. Refract. Surg. 2014, 30, 366–372. [Google Scholar] [CrossRef]
- Mazzotta, C.; Jacob, S.; Agarwal, A.; Kumar, D.A. In Vivo Confocal Microscopy After Contact Lens-Assisted Corneal Collagen Cross-linking for Thin Keratoconic Corneas. J. Refract. Surg. 2016, 32, 326–331. [Google Scholar] [CrossRef]
- Matlov Kormas, R.; Abu Tailakh, M.; Chorny, A.; Jacob, S.; Knyazer, B. Accelerated CXL Versus Accelerated Contact Lens-Assisted CXL for Progressive Keratoconus in Adults. J. Refract. Surg. 2021, 37, 623–630. [Google Scholar] [CrossRef]
- Randleman, J.B.; Santhiago, M.R.; Kymionis, G.D.; Hafezi, F. Corneal Cross-Linking (CXL): Standardizing Terminology and Protocol Nomenclature. J. Refract. Surg. 2017, 33, 727–729. [Google Scholar] [CrossRef]
- Spoerl, E.; Mrochen, M.; Sliney, D.; Trokel, S.; Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007, 26, 385–389. [Google Scholar] [CrossRef]
- Borges-Rodríguez, Y.; Morales-Cueto, R.; Rivillas-Acevedo, L. Effect of the Ultraviolet Radiation on the Lens. Curr. Protein Pept. Sci. 2023, 24, 215–228. [Google Scholar] [CrossRef]
- Srivatsa, S.; Jacob, S.; Agarwal, A. Contact lens assisted corneal cross linking in thin ectatic corneas—A review. Indian J. Ophthalmol. 2020, 68, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spörl, E.; Herbst, H. Biomechanical efficacy of contact lens-assisted collagen cross-linking in porcine eyes. Acta Ophthalmol. 2019, 97, e84–e90. [Google Scholar] [CrossRef]
- Zhang, H.; Roozbahani, M.; Piccinini, A.L.; Golan, O.; Hafezi, F.; Scarcelli, G.; Randleman, J.B. Depth-Dependent Reduction of Biomechanical Efficacy of Contact Lens–Assisted Corneal Cross-linking Analyzed by Brillouin Microscopy. J. Refract. Surg. 2019, 35, 721–728. [Google Scholar] [CrossRef]
- Kling, S.; Richoz, O.; Hammer, A.; Tabibian, D.; Jacob, S.; Agarwal, A.; Hafezi, F. Increased Biomechanical Efficacy of Corneal Cross-linking in Thin Corneas Due to Higher Oxygen Availability. J. Refract. Surg. 2015, 31, 840–846. [Google Scholar] [CrossRef]
- Malhotra, C.; Gupta, B.; Jain, A.K.; Dhar, S.; Gupta, A.; Balyan, M. Comparison of contact lens-assisted and transepithelial corneal crosslinking with standard epithelium-off crosslinking for progressive keratoconus: 24-month clinical results. J. Cataract. Refract. Surg. 2022, 48, 199–207. [Google Scholar] [CrossRef]
- Knyazer, B.; Kormas, R.M.; Chorny, A.; Lifshitz, T.; Achiron, A.; Mimouni, M. Corneal Cross-linking in Thin Corneas: 1-Year Results of Accelerated Contact Lens-Assisted Treatment of Keratoconus. J. Refract. Surg. 2019, 35, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Hersh, P.S.; Greenstein, S.A.; Fry, K.L. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J. Cataract. Refract. Surg. 2011, 37, 149–160. [Google Scholar] [CrossRef]
- Godefrooij, D.A.; Soeters, N.; Imhof, S.M.; Wisse, R.P.L. Corneal Cross-Linking for Pediatric Keratoconus: Long-Term Results. Cornea 2016, 35, 954–958. [Google Scholar] [CrossRef]
- Wittig-Silva, C.; Chan, E.; Islam, F.M.A.; Wu, T.; Whiting, M.; Snibson, G.R. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: Three-year results. Ophthalmology 2014, 121, 812–821. [Google Scholar] [CrossRef]
- Kuechler, S.J.; Tappeiner, C.; Epstein, D.; Frueh, B.E. Keratoconus Progression After Corneal Cross-Linking in Eyes With Preoperative Maximum Keratometry Values of 58 Diopters and Steeper. Cornea 2018, 37, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
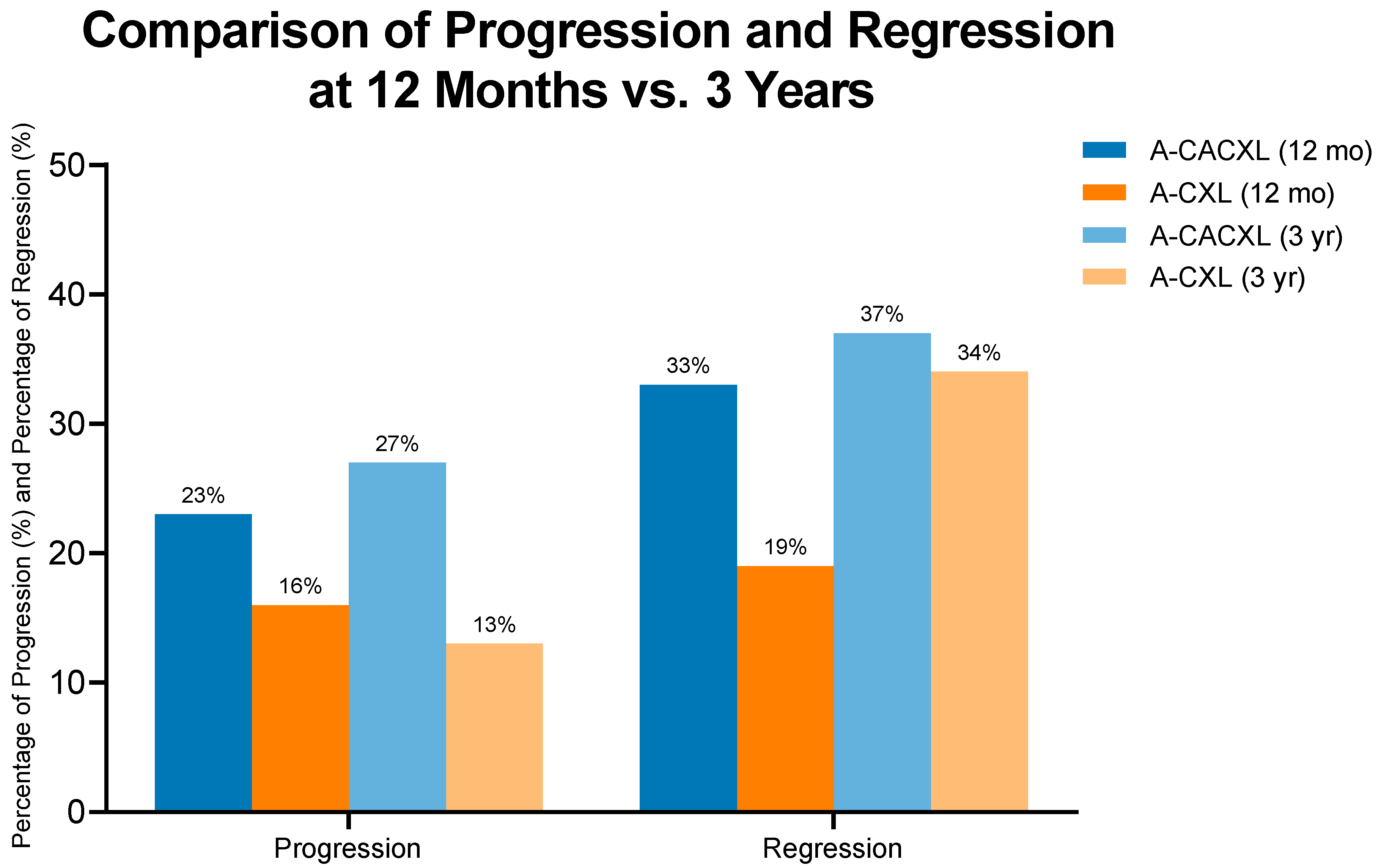
| Parameter | Variable |
|---|---|
| Treatment target | Keratoconus |
| Fluence (total) J/cm2 | 5.4 |
| Soak time and interval (minutes) | 30 (q2) |
| Intensity (mW) | 9 |
| Treatment time (minutes) | 10 |
| Epithelium status | Off |
| Chromophore | Riboflavin 0.1% (Medio-Cross; Peschke Meditrade, Switzerland) |
| Chromophore carrier | Dextran 5% (Medio-Cross; Peshke Meditrade, Switzerland) |
| Chromophore osmolarity | Iso-osmolar |
| Chromophore concentration | 0.1% |
| Light source | LightLink-CXL, LightMed, San Clemente, CA, USA |
| Irradiation mode (interval) | Continuous |
| Protocol modifications | Contact lens-assisted |
| Characteristic |
A-CACXL,
N = 30 |
A-CXL,
N = 32 | p -Value |
|---|---|---|---|
| Age Mean ± SD (N) | 25.2 ± 7.1 (30) | 21.8 ± 4.5 (32) | 0.022 |
| Sex, n (%) Female Male | 13 (43%) 17 (57%) | 14 (44%) 18 (56%) | 0.9 |
| Origin, n (%) Jewish Bedouin | 9 (30%) 21 (70%) | 14 (44%) 18 (56%) | 0.2 |
| Eye, n (%) Right Left | 15 (50%) 15 (50%) | 11 (34%) 21 (66%) | 0.2 |
| Kmax (D) Mean ± SD (N) | 61.0 ± 6.0 | 55.1 ± 4.2 | <0.001 |
| Anterior Kmean (D) Mean ± SD (N) | 51.7 ± 4.3 | 47.7 ± 3.0 | <0.001 |
| Anterior Astigmatism (D) Mean ± SD (N) | 5.06 ± 2.53 | 3.96 ± 1.94 | 0.044 |
| MCT (μm) Mean ± SD (N) | 398 ± 32 | 463 ± 31 | <0.001 |
| BAD-D (D) Mean ± SD (N) | 12.4 ± 3.7 | 8.28 ± 2.96 | <0.001 |
| Observational period (years) Mean ± SD (N) | 4.10 ± 1.42 (29) | 3.72 ± 1.20 (30) | 0.3 |
| A-CXL Group | A-CACXL Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD n = 32 | 12 Months Mean ± SD n = 32 | 3 years Mean ± SD n = 32 | 12 Months p-Value | 3 Years p-Value | Baseline Mean ± SD n=30 | 12 Months Mean ± SD n = 30 | 3 Years Mean ± SD n = 30 | 12 Months p-Value | 3 Years p-Value | |
| UDVA (LogMAR) | 0.63 ± 0.41 | 0.43 ± 0.31 | 0.44 ± 0.28 | 0.029 | 0.031 | 0.93 ± 0.58 | 0.74 ± 0.49 | 0.66 ± 0.51 | 0.2 | 0.056 |
| BCVA (LogMAR) | 0.32 ± 0.18 | 0.24 ± 0.15 | 0.18 ± 0.15 | 0.09 | 0.001 | 0.51 ± 0.30 | 0.40 ± 0.49 | 0.33 ± 0.34 | 0.3 | 0.037 |
| Kmax (D) | 55.1 ± 4.2 | 55.0 ± 4.2 | 54.5 ± 3.9 | 0.9 | 0.5 | 61.0 ± 6.0 | 60.2 ± 6.6 | 60.2 ± 6.6 | 0.7 | 0.8 |
| K_mean_front (D) | 47.65 ± 2.97 | 47.88 ± 2.95 | 47.46 ± 2.86 | 0.7 | 0.8 | 51.7 ± 4.3 | 51.9 ± 4.4 | 51.8 ± 4.5 | 0.8 | 0.9 |
| K steep front (D) | 49.74 ± 3.37 | 49.72 ± 3.38 | 49.26 ± 3.24 | 0.9 | 0.6 | 54.5 ± 4.6 | 54.4 ± 5.0 | 54.1 ± 4.9 | 0.9 | 0.7 |
| K_flat_front (D) | 45.78 ± 2.89 | 46.22 ± 2.83 | 45.81 ± 2.82 | 0.5 | 0.9 | 49.4 ± 4.1 | 49.8 ± 4.1 | 49.7 ± 4.3 | 0.7 | 0.8 |
| Anterior Astigmatism (D) | 3.96 ± 1.94 | 3.52 ± 1.96 | 3.47 ± 1.86 | 0.4 | 0.3 | 5.06 ± 2.53 | 4.66 ± 2.46 | 4.20 ± 2.41 | 0.5 | 0.2 |
| K steep back (D) | −7.40 ± 0.65 | −7.48 ± 0.68 | −7.49 ± 0.73 | 0.6 | 0.6 | −8.19 ± 0.94 | −8.16 ± 0.96 | −8.23 ± 1.01 | 0.9 | 0.9 |
| K_flat_back (D) | −6.75 ± 0.59 | −6.84 ± 0.54 | −6.71 ± 0.61 | 0.5 | 0.8 | −7.26 ± 0.79 | −7.35 ± 0.83 | −7.38 ± 0.92 | 0.7 | 0.6 |
| MCT (μm) | 463 ± 31 | 450 ± 35 | 453 ± 31 | 0.1 | 0.2 | 398 ± 32 | 388 ± 41 | 381 ± 44 | 0.3 | 0.07 |
| ISV (μm) | 90 ± 29 | 87 ± 29 | 84 ± 28 | 0.7 | 0.4 | 108 ± 31 | 104 ± 32 | 102 ± 34 | 0.6 | 0.5 |
| IHD (μm) | 0.11 ± 0.05 | 0.13 ± 0.05 | 0.15 ± 0.13 | 0.1 | 0.1 | 0.12 ± 0.06 | 0.14 ± 0.07 | 0.13 ± 0.08 | 0.4 | 0.6 |
| BAD-D | 8.28 ± 2.96 | 8.52 ± 2.91 | 8.40 ± 2.43 | 0.7 | 0.9 | 12.4 ± 3.7 | 12.4 ± 3.8 | 13.0 ± 4.0 | 0.9 | 0.5 |
| A-CACXL, N = 30 | A-CXL, N = 32 | p-Value | |
|---|---|---|---|
| Progression, n (%) | 8 (27%) | 4 (13%) | 0.2 |
| Regression, n (%) | 11 (37%) | 11 (34%) | >0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunin, A.; Kagasov, S.; Amitai, N.; Kerman, T.; Matlov Kormas, R.; Jacob, S.; Tuuminen, R.; Kravitz, L.; Knyazer, B. Accelerated CXL Versus Accelerated Contact-Lens Assisted CXL Treatment for Progressive Keratoconus—A 3-Year Retrospective Comparative Follow-Up. J. Clin. Med. 2025, 14, 7141. https://doi.org/10.3390/jcm14207141
Bunin A, Kagasov S, Amitai N, Kerman T, Matlov Kormas R, Jacob S, Tuuminen R, Kravitz L, Knyazer B. Accelerated CXL Versus Accelerated Contact-Lens Assisted CXL Treatment for Progressive Keratoconus—A 3-Year Retrospective Comparative Follow-Up. Journal of Clinical Medicine. 2025; 14(20):7141. https://doi.org/10.3390/jcm14207141
Chicago/Turabian StyleBunin, Anna, Shmuel Kagasov, Nir Amitai, Tomer Kerman, Ran Matlov Kormas, Soosan Jacob, Raimo Tuuminen, Liron Kravitz, and Boris Knyazer. 2025. "Accelerated CXL Versus Accelerated Contact-Lens Assisted CXL Treatment for Progressive Keratoconus—A 3-Year Retrospective Comparative Follow-Up" Journal of Clinical Medicine 14, no. 20: 7141. https://doi.org/10.3390/jcm14207141
APA StyleBunin, A., Kagasov, S., Amitai, N., Kerman, T., Matlov Kormas, R., Jacob, S., Tuuminen, R., Kravitz, L., & Knyazer, B. (2025). Accelerated CXL Versus Accelerated Contact-Lens Assisted CXL Treatment for Progressive Keratoconus—A 3-Year Retrospective Comparative Follow-Up. Journal of Clinical Medicine, 14(20), 7141. https://doi.org/10.3390/jcm14207141





