Altered Pattern of Serum N-Glycome in Subarachnoid Hemorrhage and Cerebral Vasospasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Patient Samples
2.3. N-Glycan Sample Preparation
2.4. UPLC-FLR-MS Analysis
2.5. Data Analysis
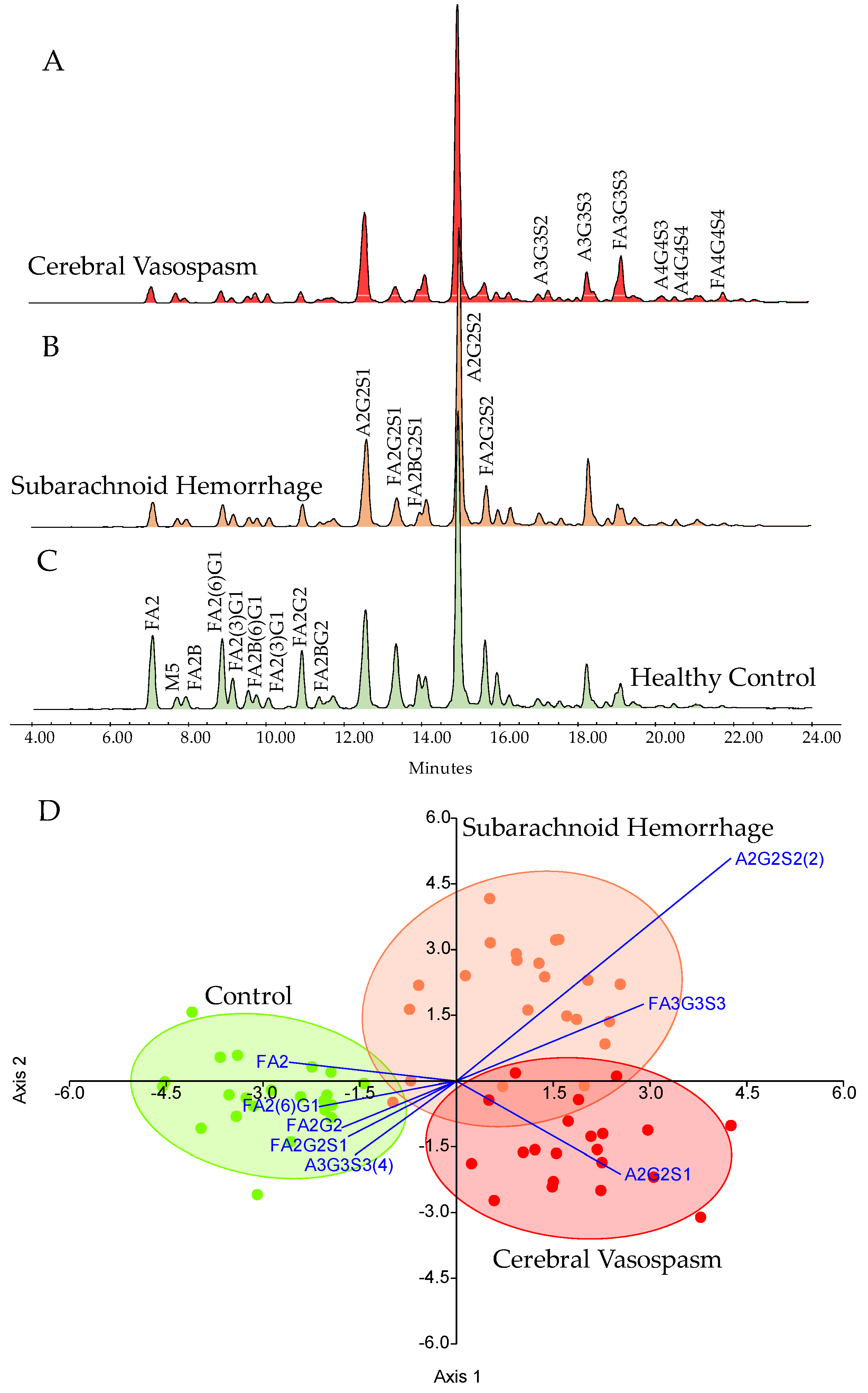
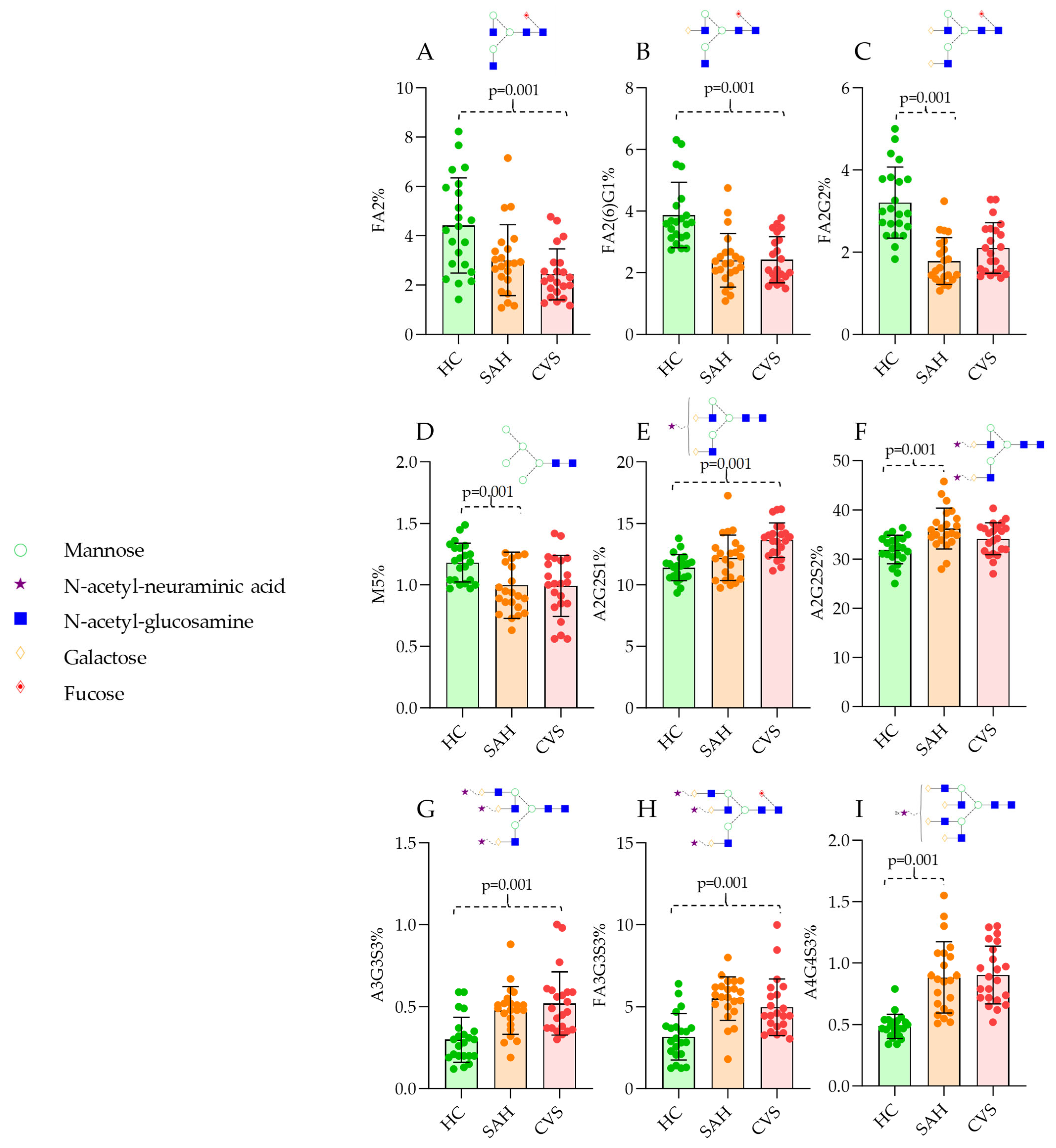
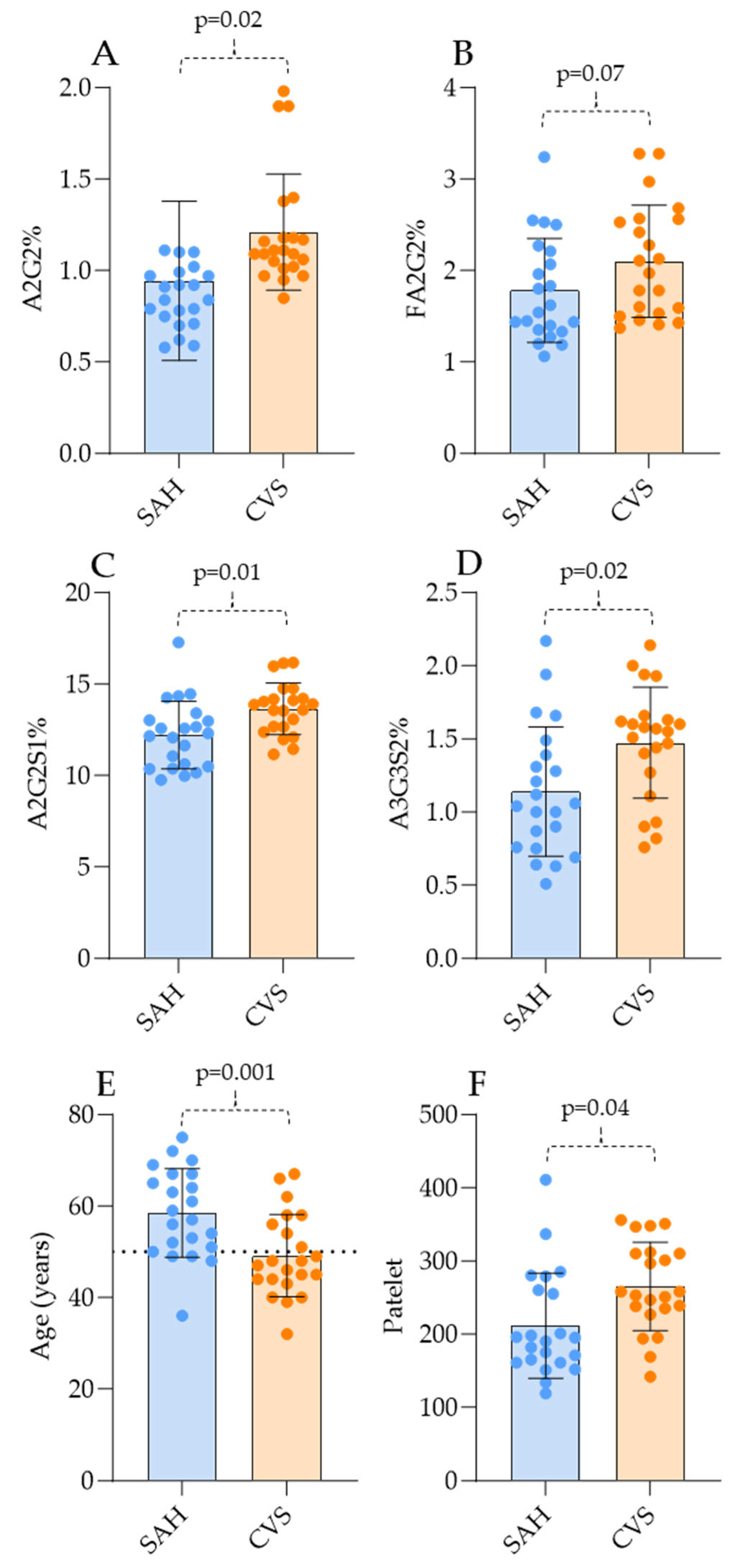
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Rooij, N.K.; Linn, F.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Śliwczyński, A.; Jewczak, M. An Analysis of the Incidence and Cost of Intracranial Aneurysm and Subarachnoid Haemorrhage Treatment between 2013 and 2021. J. Environ. Res. Public Health 2023, 20, 3828. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Rinkel, G.J.; Lawes, C.M.; Algra, A.; Bennett, D.A.; van Gijn, J.; Anderson, C.S. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke 2005, 36, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—Risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [Google Scholar] [CrossRef]
- Claassen, J.; Park, S. Spontaneous subarachnoid haemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef]
- Stauning, A.T.; Eriksson, F.; Benndorf, G.; Holst, A.V.; Hauerberg, J.; Stavngaard, T.; Poulsgaard, L.; Rochat, P.; Eskesen, V.; Birkeland, P.; et al. Mortality among patients treated for aneurysmal subarachnoid hemorrhage in Eastern Denmark 2017–2019. Acta Neurochir. 2022, 164, 2419–2430. [Google Scholar] [CrossRef]
- Bracard, S.; Schmitt, E. Vasospasm and delayed consequences. Interv. Neuroradiol. 2008, 14 (Suppl. S1), 17–22. [Google Scholar] [CrossRef]
- Lee, R.H.C.; Lee, M.H.H.; Wu, C.Y.C.; Couto, E.S.A.; Possoit, H.E.; Hsieh, T.H.; Minagar, A.; Lin, H.W. Cerebral ischemia and neuroregeneration. Neural Regen. Res. 2018, 13, 373–385. [Google Scholar] [CrossRef]
- Motwani, K.; Dodd, W.S.; Laurent, D.; Lucke-Wold, B.; Chalouhi, N. Delayed cerebral ischemia: A look at the role of endothelial dysfunction, emerging endovascular management, and glymphatic clearance. Clin. Neurol. Neurosurg. 2022, 218, 107273. [Google Scholar] [CrossRef]
- Robba, C.; Busl, K.M.; Claassen, J.; Diringer, M.N.; Helbok, R.; Park, S.; Rabinstein, A.; Treggiari, M.; Vergouwen, M.D.I.; Citerio, G. Contemporary management of aneurysmal subarachnoid haemorrhage. An update for the intensivist. Intensive Care Med. 2024, 50, 646–664. [Google Scholar] [CrossRef]
- D’Andrea, A.; Conte, M.; Cavallaro, M.; Scarafile, R.; Riegler, L.; Cocchia, R.; Pezzullo, E.; Carbone, A.; Natale, F.; Santoro, G.; et al. Transcranial Doppler ultrasonography: From methodology to major clinical applications. World J. Cardiol. 2016, 8, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Kohama, M.; Sugiyama, S.; Sato, K.; Endo, H.; Niizuma, K.; Endo, T.; Ohta, M.; Matsumoto, Y.; Fujimura, M.; Tominaga, T. Difference in Transcranial Doppler Velocity and Patient Age between Proximal and Distal Middle Cerebral Artery Vasospasms after Aneurysmal Subarachnoid Hemorrhage. Cerebrovasc. Dis. Extra 2016, 6, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Piotin, M.; Gailloud, P.; Bidaut, L.; Mandai, S.; Muster, M.; Moret, J.; Rüfenacht, D.A. CT angiography, MR angiography and rotational digital subtraction angiography for volumetric assessment of intracranial aneurysms. An experimental study. Neuroradiology 2003, 45, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.M.; Nowicki, K.W.; Mantena, R.; Cao, C.; Rochlin, E.K.; Dembinski, R.; Lang, M.J.; Gross, B.A.; Friedlander, R.M. Advances in biomarkers for vasospasm—Towards a future blood-based diagnostic test. World Neurosurg. X 2024, 22, 100343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Meng, J.; Xu, F.; Wang, Q.; Tian, X.; Li, M.; Zeng, X.; Hu, C.; Zheng, Y. IgG Glycosylation Profiling of Peripheral Artery Diseases with Lectin Microarray. J. Clin. Med. 2022, 11, 5727. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Y.; Zhang, C.; Wang, H.; Bi, R.; Wei, Y.; Li, Y.; Hu, B. The role of protein glycosylation in the occurrence and outcome of acute ischemic stroke. Pharmacol. Res. 2023, 191, 106726. [Google Scholar] [CrossRef]
- Pradeep, P.; Kang, H.; Lee, B. Glycosylation and behavioral symptoms in neurological disorders. Transl. Psychiatry 2023, 13, 154. [Google Scholar] [CrossRef]
- Harvey, D.J.; Merry, A.H.; Royle, L.; Campbell, M.P.; Rudd, P.M. Symbol nomenclature for representing glycan structures: Extension to cover different carbohydrate types. Proteomics 2011, 11, 4291–4295. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.; Wang, A.; Ge, S.; Wang, H.; Zhang, X.; Sun, Q.; Cao, W.; Sun, M.; Wu, L.; et al. Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflamm. 2018, 15, 123. [Google Scholar] [CrossRef]
- Rocco, A.; Heuschmann, P.U.; Schellinger, P.D.; Köhrmann, M.; Diedler, J.; Sykora, M.; Nolte, C.H.; Ringleb, P.; Hacke, W.; Jüttler, E. Glycosylated Hemoglobin A1 Predicts Risk for Symptomatic Hemorrhage After Thrombolysis for Acute Stroke. Stroke 2013, 44, 2134–2138. [Google Scholar] [CrossRef]
- Nanetti, L.; Vignini, A.; Raffaelli, F.; Taffi, R.; Silvestrini, M.; Provinciali, L.; Mazzanti, L. Sialic acid and sialidase activity in acute stroke. Dis. Markers 2008, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.P.; Edgell, R.C.; Alshekhlee, A.; Borhani Haghighi, A.; Sweeny, J.; Felton, J.; Kitchener, J.; Vora, N.; Bieneman, B.K.; Cruz-Flores, S.; et al. Age-associated vasospasm in aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2013, 22, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, Y.; Hamada, H.; Kurimoto, M.; Origasa, H.; Endo, S. Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2005, 102, 882–887. [Google Scholar] [CrossRef]
- Rieß, C.; Darkwah Oppong, M.; Dinger, T.-F.; Rodemerk, J.; Rauschenbach, L.; Gümüs, M.; Frank, B.; Dammann, P.; Wrede, K.H.; Sure, U.; et al. Baseline and average platelet count can predict the outcome of patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. X 2024, 22, 100302. [Google Scholar] [CrossRef]
- Ohkuma, H.; Suzuki, S.; Kimura, M.; Sobata, E. Role of platelet function in symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1991, 22, 854–859. [Google Scholar] [CrossRef]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. Platelet adhesion under flow. Microcirculation 2009, 16, 58–83. [Google Scholar] [CrossRef]
- Ludwig, N.; Hilger, A.; Zarbock, A.; Rossaint, J. Platelets at the Crossroads of Pro-Inflammatory and Resolution Pathways during Inflammation. Cells 2022, 11, 1957. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis-From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 2772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czabajszki, M.; Garami, A.; Molnár, T.; Csécsei, P.; Viskolcz, B.; Oláh, C.; Váradi, C. Altered Pattern of Serum N-Glycome in Subarachnoid Hemorrhage and Cerebral Vasospasm. J. Clin. Med. 2025, 14, 465. https://doi.org/10.3390/jcm14020465
Czabajszki M, Garami A, Molnár T, Csécsei P, Viskolcz B, Oláh C, Váradi C. Altered Pattern of Serum N-Glycome in Subarachnoid Hemorrhage and Cerebral Vasospasm. Journal of Clinical Medicine. 2025; 14(2):465. https://doi.org/10.3390/jcm14020465
Chicago/Turabian StyleCzabajszki, Máté, Attila Garami, Tihamér Molnár, Péter Csécsei, Béla Viskolcz, Csaba Oláh, and Csaba Váradi. 2025. "Altered Pattern of Serum N-Glycome in Subarachnoid Hemorrhage and Cerebral Vasospasm" Journal of Clinical Medicine 14, no. 2: 465. https://doi.org/10.3390/jcm14020465
APA StyleCzabajszki, M., Garami, A., Molnár, T., Csécsei, P., Viskolcz, B., Oláh, C., & Váradi, C. (2025). Altered Pattern of Serum N-Glycome in Subarachnoid Hemorrhage and Cerebral Vasospasm. Journal of Clinical Medicine, 14(2), 465. https://doi.org/10.3390/jcm14020465









