Correlation of Inflammatory Parameters with the Development of Cerebral Vasospasm, Takotsubo Cardiomyopathy, and Functional Outcome after Spontaneous Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
- -
- Transient left/right ventricle wall motion abnormalities, usually extending beyond a single epicardial coronary artery distribution;
- -
- Evidence of myocardial involvement by biomarker elevation: cardiac troponin I, creatine kinase, brain natriuretic peptide, N-terminal prohormone of brain natriuretic peptides;
- -
- ECG abnormalities: ST elevation/depression, negative T-waves, new bundle branch blocks;
- -
- Potential coronary artery culprit ruled out [17].
3. Results
3.1. The Relationship between Inflammatory Markers and SAH Severity at Admission
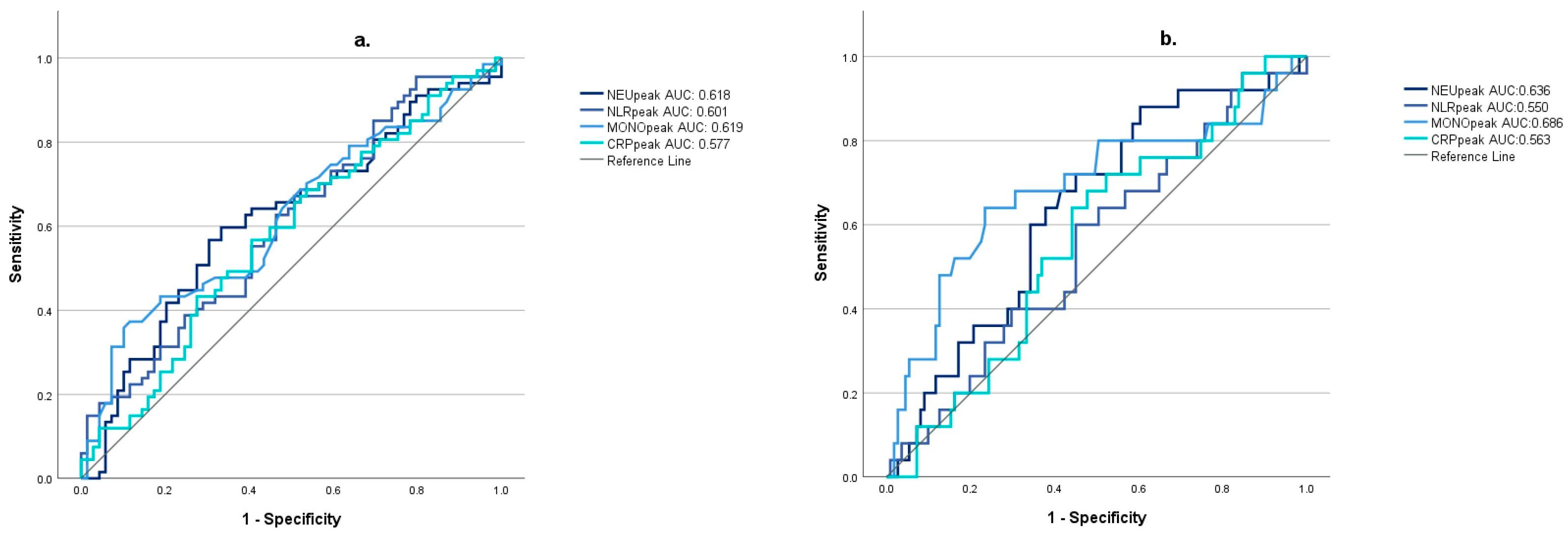
3.2. Relationship between Inflammatory Markers and the Development of Cerebral Vasospasm
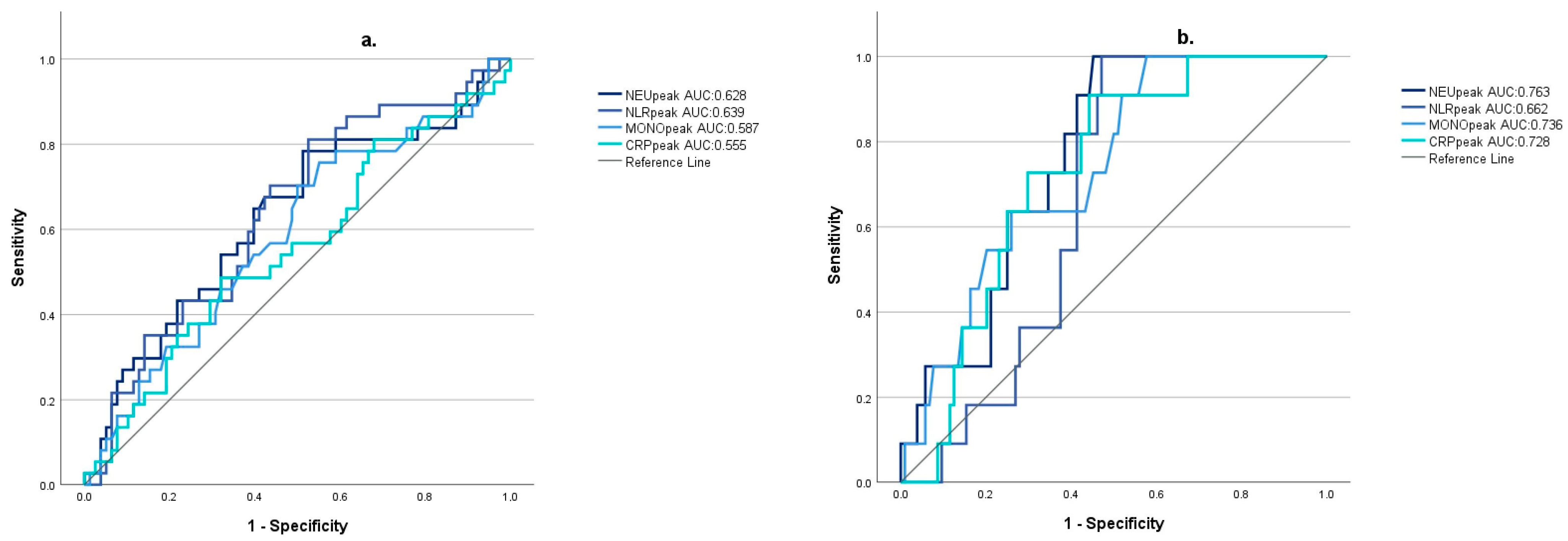
3.3. Correlation of Inflammatory Markers with the Severity of Takotsubo Cardiomyopathy
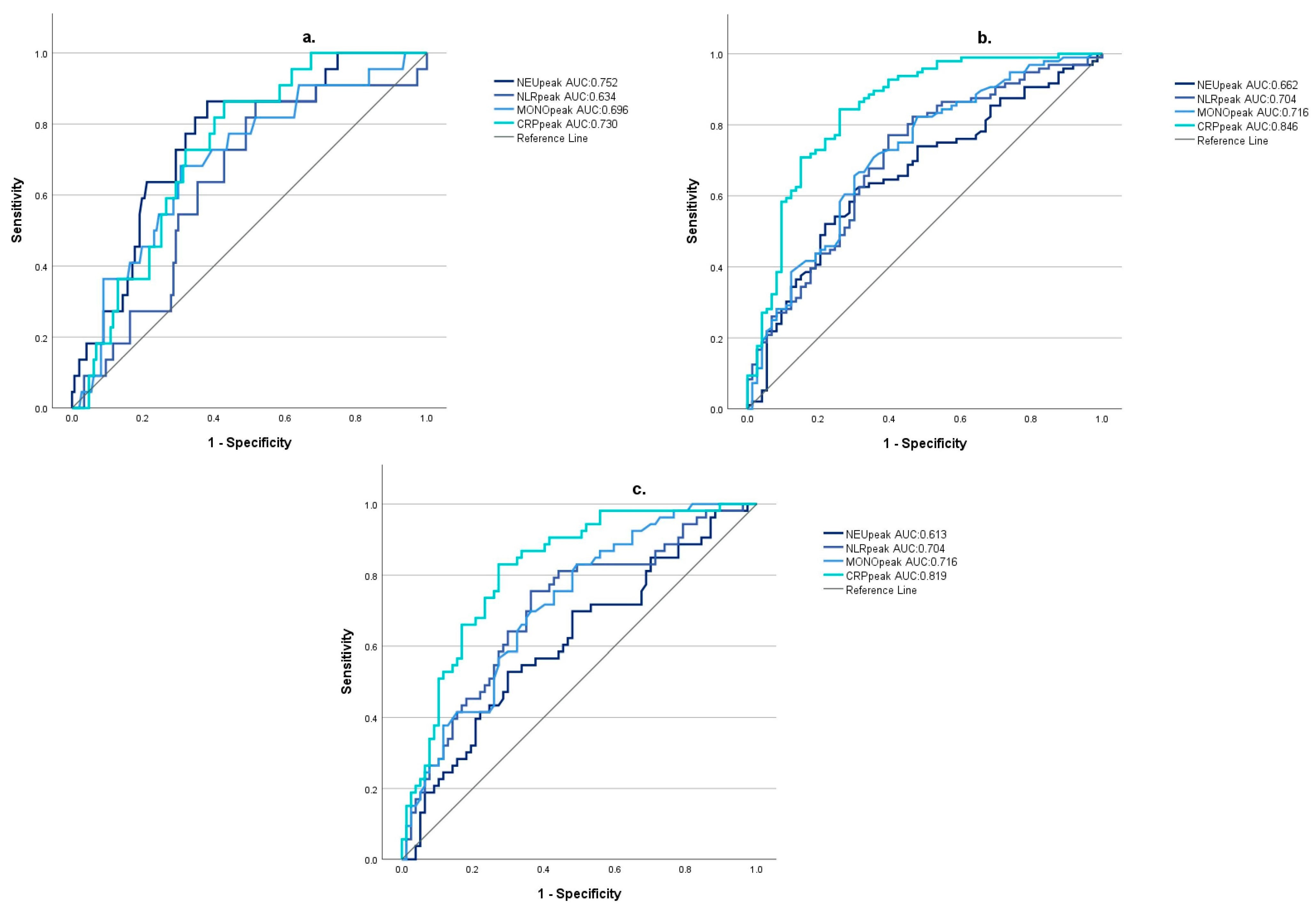
3.4. The Predictive Value of Inflammatory Markers on Patient Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wach, J.; Vychopen, M.; Güresir, A.; Güresir, E. Anti-Inflammatory Drug Therapy in Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis of Prospective Randomized and Placebo-Controlled Trials. J. Clin. Med. 2023, 12, 4165. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.R.; Presseau, J.; Saigle, V.; Etminan, N.; Vergouwen, M.D.I.; English, S.W. Outcomes in Subarachnoid Haemorrhage Working Group. Core outcomes for subarachnoid haemorrhage. Lancet Neurol. 2019, 18, 1075–1076. [Google Scholar] [CrossRef]
- Goursaud, S.; Martinez de Lizarrondo, S.; Grolleau, F.; Chagnot, A.; Agin, V.; Maubert, E.; Gauberti, M.; Vivien, D.; Ali, C.; Gakuba, C. Delayed Cerebral Ischemia After Subarachnoid Hemorrhage: Is There a Relevant Experimental Model? A Systematic Review of Preclinical Literature. Front. Cardiovasc. Med. 2021, 8, 752769. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.P.; Provencio, J.J. Aneurysmal Subarachnoid Hemorrhage: An Overview of Inflammation-Induced Cellular Changes. Neurotherapeutics 2020, 17, 436–445. [Google Scholar] [CrossRef]
- Geraghty, J.R.; Lung, T.J.; Hirsch, Y.; Katz, E.A.; Cheng, T.; Saini, N.S.; Pandey, D.K.; Testai, F.D. Systemic Immune-Inflammation Index Predicts Delayed Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2021, 89, 1071–1079. [Google Scholar] [CrossRef]
- Buce-Satoba, I.; Rozkalne, D.; Mamaja, B.; Krumina, G.; Ozolina, A. Leukocytosis and C-Reactive Protein May Predict Development of Secondary Cerebral Vasospasm in Patients with Aneurysmal Subarachnoid Hemorrhage. Medicina 2022, 58, 323. [Google Scholar] [CrossRef] [PubMed]
- Kula, O.; Günay, B.; Kayabaş, M.Y.; Aktürk, Y.; Kula, E.; Tütüncüler, B.; Süt, N.; Solak, S. Neutrophil to Lymphocyte Ratio and Serum Biomarkers: A Potential Tool for Prediction of Clinically Relevant Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage. J. Korean Neurosurg. Soc. 2023, 66, 681–689. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, J.; Zeng, H.; Cai, L.; Wang, T.; Wu, X.; Yu, K.; Zheng, Y.; Chen, H.; Peng, Y.; et al. Neutrophil to lymphocyte ratio predicting poor outcome after aneurysmal subarachnoid hemorrhage: A retrospective study and updated meta-analysis. Front. Immunol. 2022, 13, 962760. [Google Scholar] [CrossRef]
- Giede-Jeppe, A.; Reichl, J.; Sprügel, M.I.; Lücking, H.; Hoelter, P.; Eyüpoglu, I.Y.; Kuramatsu, J.B.; Huttner, H.B.; Gerner, S.T. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2019, 132, 400–407. [Google Scholar] [CrossRef]
- Alessandro, O.; Rene, W.; Stefan, W.; Miodrag, F.; Martin, S.; Oliver, B.; Urs, P. C-reactive protein elevation predicts in-hospital deterioration after aneurysmal subarachnoid hemorrhage: A retrospective observational study. Acta Neurochir. 2022, 164, 1805–1814. [Google Scholar] [CrossRef]
- Krzyżewski, R.M.; Kliś, K.M.; Kwinta, B.M.; Stachura, K.; Guzik, T.J.; Gąsowski, J. High Leukocyte Count and Risk of Poor Outcome After Subarachnoid Hemorrhage: A Meta-Analysis. World Neurosurg. 2020, 135, 541–547. [Google Scholar]
- Peng, L.; Li, X.; Li, H.; Zhong, Y.; Lian, J.; Gao, H.; Chen, G. Relationship between Peripheral Blood Inflammatory Factors and Prognosis of Subarachnoid Hemorrhage: A Meta-Analysis. Eur. Neurol. 2023, 86, 193–206. [Google Scholar] [CrossRef]
- Molnár, C.; Gál, J.; Szántó, D.; Fülöp, L.; Szegedi, A.; Siró, P.; Nagy, E.V.; Lengyel, S.; Kappelmayer, J.; Fülesdi, B. Takotsubo cardiomyopathy in patients suffering from acute non-traumatic subarachnoid hemorrhage-A single center follow-up study. PLoS ONE 2022, 17, e0268525. [Google Scholar] [CrossRef] [PubMed]
- Szántó, D.; Luterán, P.; Gál, J.; V Nagy, E.; Fülesdi, B.; Molnár, C. Diagnosis and Management of Takotsubo Syndrome in Acute Aneurysmal Subarachnoid Hemorrhage: A Comprehensive Review. Rev. Cardiovasc. Med. 2023, 24, 177. [Google Scholar] [CrossRef]
- Yalta, K.; Yetkin, E.; Yalta, T. Systemic inflammation in patients with Takotsubo syndrome: A review of mechanistic and clinical implications. Monaldi Arch. Chest Dis. 2021, 91, 1718. [Google Scholar] [CrossRef]
- D’Andrea, A.; Conte, M.; Scarafile, R.; Riegler, L.; Cocchia, R.; Pezzullo, E.; Cavallaro, M.; Carbone, A.; Natale, F.; Russo, M.G.; et al. Transcranial Doppler Ultrasound: Physical Principles and Principal Applications in Neurocritical Care Unit. J. Cardiovasc. Echogr. 2016, 26, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- van Donkelaar, C.E.; Bakker, N.A.; Birks, J.; Veeger, N.J.G.M.; Metzemaekers, J.D.M.; Molyneux, A.J.; Groen, R.J.M.; van Dijk, J.M.C. Prediction of Outcome After Aneurysmal Subarachnoid Hemorrhage. Stroke 2019, 50, 837–844. [Google Scholar] [CrossRef]
- Chai, C.Z.; Ho, U.C.; Kuo, L.T. Systemic Inflammation after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2023, 24, 10943. [Google Scholar] [CrossRef]
- Schneider, U.C.; Xu, R.; Vajkoczy, P. Inflammatory Events Following Subarachnoid Hemorrhage (SAH). Curr. Neuropharmacol. 2018, 16, 1385–1395. [Google Scholar] [CrossRef]
- Zheng, V.Z.; Wong, G.K.C. Neuroinflammation responses after subarachnoid hemorrhage: A review. J. Clin. Neurosci. 2017, 42, 7–11. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Li, G.; Zhou, L.; Huang, K.; Wu, Z.; Zhan, R.; Shen, J. Inflammation and Oxidative Stress: Potential Targets for Improving Prognosis After Subarachnoid Hemorrhage. Front. Cell Neurosci. 2021, 15, 739506. [Google Scholar] [CrossRef]
- Hou, Y.; Fan, J.; Yuan, H.; Zheng, H.; Yang, H.; Li, H.; Chen, R.; Yu, J. Prognostic capacity of the systemic inflammation response index for functional outcome in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 2023, 14, 1054315. [Google Scholar] [CrossRef]
- Saand, A.R.; Yu, F.; Chen, J.; Chou, S.H. Systemic inflammation in hemorrhagic strokes—A novel neurological sign and therapeutic target? J. Cereb. Blood Flow. Metab. 2019, 39, 959–988. [Google Scholar] [CrossRef]
- Han, W.; Yi, H.J.; Shin, D.S.; Kim, B.T. Pan-immune-inflammation value predict delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 2024, 121, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, Y.C. Monocyte Count and Systemic Immune-Inflammation Index Score as Predictors of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. J. Korean Neurosurg. Soc. 2024, 67, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pandey, S.; Shen, R.; Xu, Y.; Zhang, Q. Increased Systemic Immune-Inflammation Index Is Associated with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage Patients. Front. Neurol. 2021, 12, 745175. [Google Scholar] [CrossRef] [PubMed]
- Chamling, B.; Gross, S.; Stoffel-Wagner, B.; Schubert, G.A.; Clusmann, H.; Coburn, M.; Höllig, A. Early Diagnosis of Delayed Cerebral Ischemia: Possible Relevance for Inflammatory Biomarkers in Routine Clinical Practice? World Neurosurg. 2017, 104, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Zhang, Q.; Hou, F.; Wang, L.; Zheng, Z.; Guo, Y.; Chen, Z.; Hernesniemi, J.; Feng, G.; et al. Prognostic significance of white blood cell to platelet ratio in delayed cerebral ischemia and long-term clinical outcome after aneurysmal subarachnoid hemorrhage. Front. Neurol. 2023, 14, 1180178. [Google Scholar] [CrossRef] [PubMed]
- Unda, S.R.; Birnbaum, J.; Labagnara, K.; Wong, M.; Vaishnav, D.P.; Altschul, D.J. Peripheral Monocytosis at Admission to Predict Cerebral Infarct and Poor Functional Outcomes in Subarachnoid Hemorrhage Patients. World Neurosurg. 2020, 138, e523–e529. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, J.R.; Saini, N.S.; Deshpande, A.; Cheng, T.; Nazir, N.; Testai, F.D. The Role of Serum Monocytes and Tissue Macrophages in Driving Left Ventricular Systolic Dysfunction and Cardiac Inflammation Following Subarachnoid Hemorrhage. Neurocrit Care 2023. [Google Scholar] [CrossRef]
- Zhang, X.L.; Schaedel, J.F.; Ahmad, A.; Wagener, B.M. Inflammation as a Source of Cardiac Injury After Subarachnoid Hemorrhage. Neurocrit Care 2023. [Google Scholar] [CrossRef] [PubMed]
- Gaastra, B.; Barron, P.; Newitt, L.; Chhugani, S.; Turner, C.; Kirkpatrick, P.; MacArthur, B.; Galea, I.; Bulters, D. CRP (C-Reactive Protein) in Outcome Prediction After Subarachnoid Hemorrhage and the Role of Machine Learning. Stroke 2021, 52, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| Age (years, mean ± SD) | 55.34 ± 10.97 |
| Gender (F/M) | 69/106 |
| Location of the aneurysm | |
| Internal carotid | 23 |
| Middle cerebral | 40 |
| Anterior cerebral and anterior communicating | 57 |
| Posterior cerebral and posterior communicating | 8 |
| Basilar and vertebral | 13 |
| Other | 9 |
| Without identified aneurysm | 25 |
| Modified Fisher grade at admission | |
| I–II | 56 |
| III–IV | 119 |
| Hunt–Hess score at admission | |
| I–III | 109 |
| IV–V | 66 |
| Vasospasm | |
| Moderate | 43 |
| Severe | 25 |
| Without vasospasm | 72 |
| No data available | 35 |
| Takotsubo cardiomyopathy (TTC) | |
| TTC with moderate left ventricular dysfunction | 27 |
| TTC with severe left ventricular dysfunction | 14 |
| Without TTC | 86 |
| No data available | 48 |
| Mortality | |
| 1-week mortality | 22 |
| 30-day mortality | 39 |
| 30-day Glasgow outcome scale score | |
| 1–3 | 99 |
| 4–5 | 74 |
| No data available | 2 |
| 30-day Barthel Index (survivors) | |
| <50 | 53 |
| 50≤ | 81 |
| No data available | 2 |
| Modified Fisher’s Grade at Admission | |||
| Inflammatory Markers | Modified Fisher I–II | Modified Fisher III–IV | Mann–Whitney U-Test |
| NEUpeak | 10.29 (IQR 5.87) | 12.87 (IQR 6.37) | p = 0.005 * |
| NLRpeak | 8.29 (IQR 9.94) | 13.28 (IQR 11.49) | p < 0.001 * |
| MONOpeak | 0.85 (IQR 0.74) | 1.1 (IQR 0.6) | p = 0.003 * |
| CRPpeak | 30.41 (IQR 74.25) | 155.1 (IQR 171.91) | p < 0.001 * |
| Hunt–Hess grading at admission | |||
| Inflammatory markers | Hunt–Hess I–III | Hunt–Hess IV–V | Mann–Whitney U-test |
| NEUpeak | 10.81 (IQR 5.31) | 14.9 (IQR 6.45) | p < 0.001 * |
| NLRpeak | 9.48 (IQR 10.56) | 14.27 (IQR 9.63) | p < 0.001 * |
| MONOpeak | 0.95 (IQR 0.61) | 1.27 (IQR 0.57 | p < 0.001 * |
| CRPpeak | 48.85 (IQR 138.95) | 195.3 (IQR 153.56) | p < 0.001 * |
| Inflammatory Markers | Non-Vasospasm (nVS) Group | Vasospasm (VS) Group | Mann–Whitney U-Test |
|---|---|---|---|
| NEUpeak | 11.24 (IQR 5.15) | 13.42 (IQR 7.34) | p = 0.018 * |
| NLRpeak | 9.69 (IQR 9.82) | 13.24 (IQR 12.48) | p = 0.042 * |
| MONOpeak | 0.95 (IQR 0.53) | 1.05 (IQR 0.71) | p = 0.016 * |
| CRPpeak | 74.6 (IQR 152.7) | 110.56 (IQR 183.81) | p = 0.077 |
| Inflammatory Markers | Non-Vasospasm (nVS) Group | Mild Vasospasm (mVS) Group | Severe Vasospasm (sVS) Group | Kruskal–Wallis (+ Dunn–Bonferroni’s Test) |
|---|---|---|---|---|
| NEUpeak | 11.24 (IQR 5.15) | 12.61 (IQR 7.66) | 13.79 (IQR 5.88) | p = 0.032 * (nVS vs. sVS p = 0.012 * mVS vs. sVS p = 0.262 nVS vs. mVS p = 0.125) |
| NLRpeak | 9.69 (IQR 9.82) | 13.48 (IQR 12.89) | 13.01 (IQR 11.18) | p = 0.123 |
| MONOpeak | 0.95 (IQR 0.53) | 1.0 (IQR 0.63) | 1.47 (IQR 0.72) | p = 0.008 * (nVS vs. sVS p = 0.002 * mVS vs. sVS p= 0.047 * nVS vs. mVS p = 0.251) |
| CRPpeak | 74.6 (IQR 152.7) | 103.75 (IQR 193.48) | 130.09 (IQR 164.71) | p = 0.203 |
| Inflammatory Markers | Non-TTC Group | Mild TTC Group | Severe TTC Group | Kruskal–Wallis (+ Dunn–Bonferroni’s Test) |
|---|---|---|---|---|
| NEUpeak | 12.04 (IQR 6.87) | 12.47 (IQR 8.95) | 16.28 (IQR 5.37) | p = 0.011 * (nTTC vs. sTTC p = 0.003 * mTTC vs. sTTC p = 0.031 * nTTC vs. mTTC p = 0.391) |
| NLRpeak | 9.56 (IQR 10.22) | 11.62 (IQR 14.65) | 14.93 (IQR 4.28) | p = 0.046 * (nTTC vs. sTTC p= 0.038 * mTTC vs. sTTC p = 0.427 nTTC vs. mTTC p = 0.086) |
| MONOpeak | 0.94 (IQR 0.61) | 1.05 (IQR 0.52) | 1.39 (IQR 0.65) | p = 0.035 * (nTTC vs. sTTC p = 0.01 * mTTC vs. sTTC p = 0.039 * nTTC vs. mTTC p = 0.668) |
| CRPpeak | 58.45 (IQR 191.75) | 84.1 (IQR 169.63) | 215.61 (IQR 122.15) | p = 0.036 * (nTTC vs. sTTC p= 0.012 * mTTC vs. sTTC p = 0.019 * nTTC vs. mTTC p = 0.875) |
| 1-Week Survival | |||
| Inflammatory markers | Survival > 1 week | Survival ≤ 1 week | Mann–Whitney U-test |
| NEUpeak | 11.76 (IQR 6.04) | 16.25 (IQR 4.03) | p < 0.001 * |
| NLRpeak | 10.58 (IQR 10.47) | 16.49 (IQR 8.24) | p = 0.041 * |
| MONOpeak | 1.03 (IQR 0.64) | 1.44 (IQR 0.64) | p = 0.003 * |
| CRPpeak | 84.75 (IQR 179.15) | 206.31 (IQR 141.34) | p < 0.001 * |
| Glasgow outcome scale (GOS) at day 30 | |||
| Inflammatory markers | GOS ≥ 4 | GOS < 4 | Mann–Whitney U-test |
| NEUpeak | 10.81 (IQR 5.37) | 14.1 (IQR 6.95) | p < 0.001 * |
| NLRpeak | 8.33 (IQR 8.58) | 14.27 (IQR 11.18) | p < 0.001 * |
| MONOpeak | 0.88 (IQR 0.65) | 1.2 (IQR 0.63) | p < 0.001 * |
| CRPpeak | 27.3 (IQR 72.4) | 186.5 (IQR 167.3) | p < 0.001 * |
| Barthel Index (BI) at day 30 | |||
| Inflammatory markers | BI ≥ 50 | BI < 50 | Mann–Whitney U-test |
| NEUpeak | 10.81 (IQR 5.26) | 12.45 (IQR 6.13) | p = 0.029 * |
| NLRpeak | 8.33 (IQR 8.07) | 15.12 (IQR 10.87) | p < 0.001 * |
| MONOpeak | 0.88 (IQR 0.6) | 1.2 (IQR 0.63) | p < 0.001 * |
| CRPpeak | 34.32 (IQR 74.0) | 167.55 (IQR 142.27) | p < 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szántó, D.; Luterán, P.; Kóti, N.; Siró, P.; Simon, É.; Jakab, Z.; Gál, J.; Kappelmayer, J.; Fülesdi, B.; Molnár, C. Correlation of Inflammatory Parameters with the Development of Cerebral Vasospasm, Takotsubo Cardiomyopathy, and Functional Outcome after Spontaneous Subarachnoid Hemorrhage. J. Clin. Med. 2024, 13, 1955. https://doi.org/10.3390/jcm13071955
Szántó D, Luterán P, Kóti N, Siró P, Simon É, Jakab Z, Gál J, Kappelmayer J, Fülesdi B, Molnár C. Correlation of Inflammatory Parameters with the Development of Cerebral Vasospasm, Takotsubo Cardiomyopathy, and Functional Outcome after Spontaneous Subarachnoid Hemorrhage. Journal of Clinical Medicine. 2024; 13(7):1955. https://doi.org/10.3390/jcm13071955
Chicago/Turabian StyleSzántó, Dorottya, Péter Luterán, Nikolett Kóti, Péter Siró, Éva Simon, Zsuzsa Jakab, Judit Gál, János Kappelmayer, Béla Fülesdi, and Csilla Molnár. 2024. "Correlation of Inflammatory Parameters with the Development of Cerebral Vasospasm, Takotsubo Cardiomyopathy, and Functional Outcome after Spontaneous Subarachnoid Hemorrhage" Journal of Clinical Medicine 13, no. 7: 1955. https://doi.org/10.3390/jcm13071955
APA StyleSzántó, D., Luterán, P., Kóti, N., Siró, P., Simon, É., Jakab, Z., Gál, J., Kappelmayer, J., Fülesdi, B., & Molnár, C. (2024). Correlation of Inflammatory Parameters with the Development of Cerebral Vasospasm, Takotsubo Cardiomyopathy, and Functional Outcome after Spontaneous Subarachnoid Hemorrhage. Journal of Clinical Medicine, 13(7), 1955. https://doi.org/10.3390/jcm13071955







