Morphological Variability of the Thigh Muscle Traps in an Ultrasound That Awaits Clinicians
Abstract
1. Introduction
2. Methods and Review Design
- Manuscripts written in any language other than English.
- Lack of information regarding the description of the typical anatomy/morphological variability in a structure covered by the topic of this review/clinical significance/application of imaging studies/the use of US in diagnosis.
- Articles focused mainly on methods of visualization other than US imaging.
- Type of the article, including expert opinion/letter to the editor/conference report.
- Publication after July 2024.
3. Discussion
3.1. Anterior Thigh
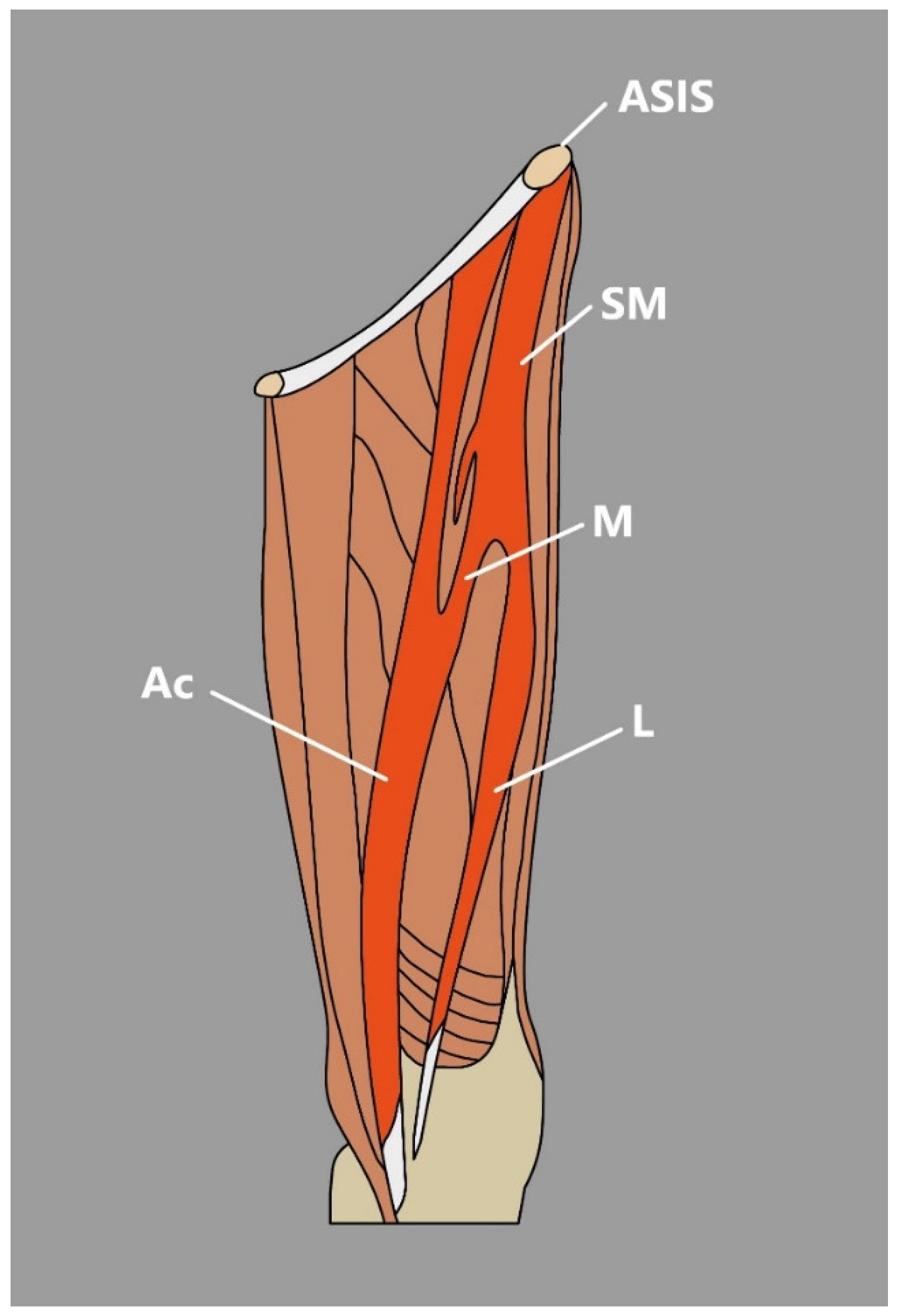
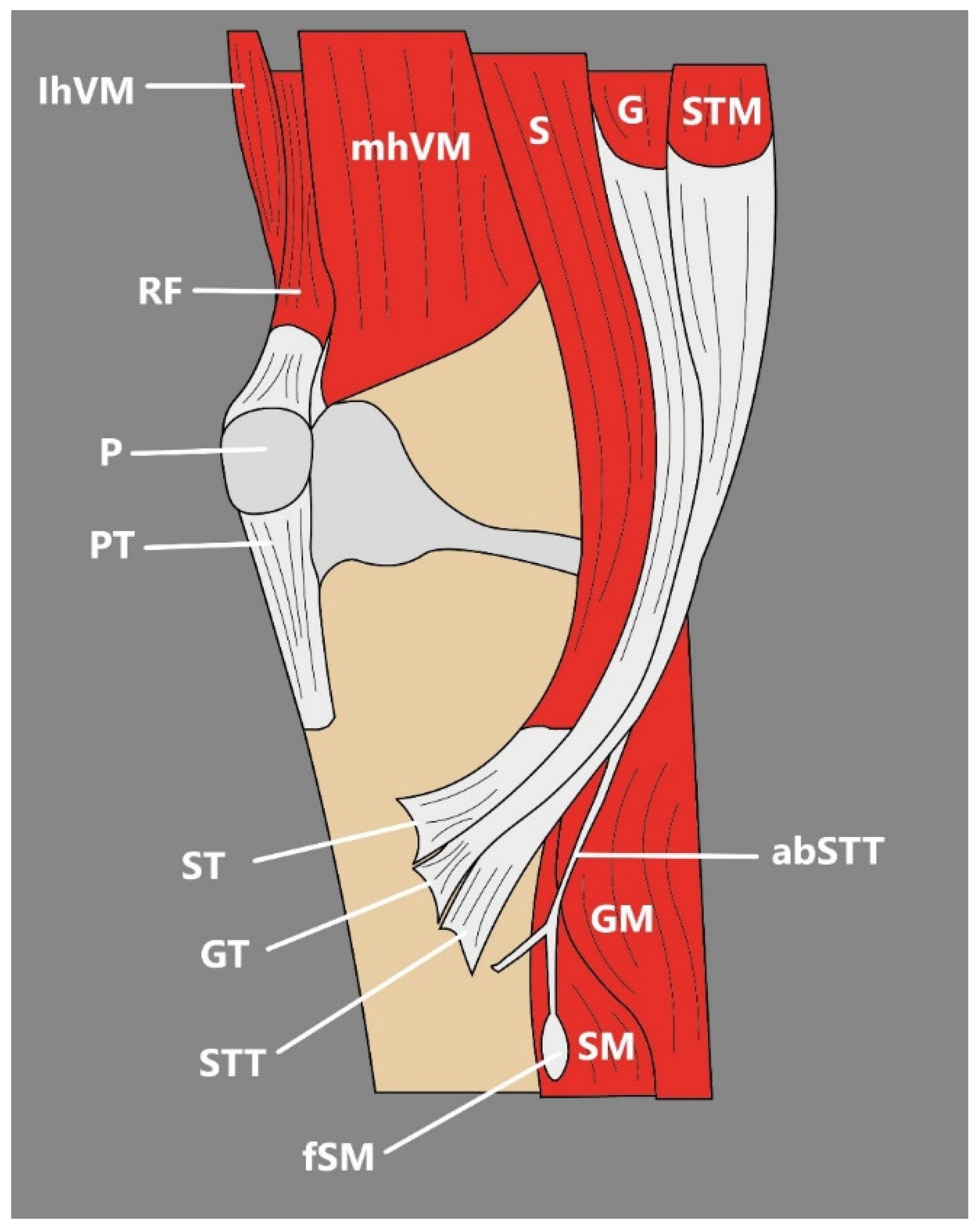
3.2. Sartorius Muscle
- The SM might be observed as a cone-shaped or rectangular structure [3]. Occasionally, an extra tendon that starts muscle duplication in the form of a hollow occurs, sometimes partial duplication or the distal muscular fragment can be noted, or discontinuous SM that consists of three muscular parts and two connecting tendinous parts [3].
- There are also reports about accessory heads of origin that usually arise from below the anterior superior iliac spine, pubic bone, iliopectineal line, or inguinal ligament [5]. Natsis et al. [6] reported a case of biceps-bicaudatus SM, where both heads (lateral and medial) originated from the anterior superior iliac spine and where the lateral head was additionally split into a lateral and medial bundle.
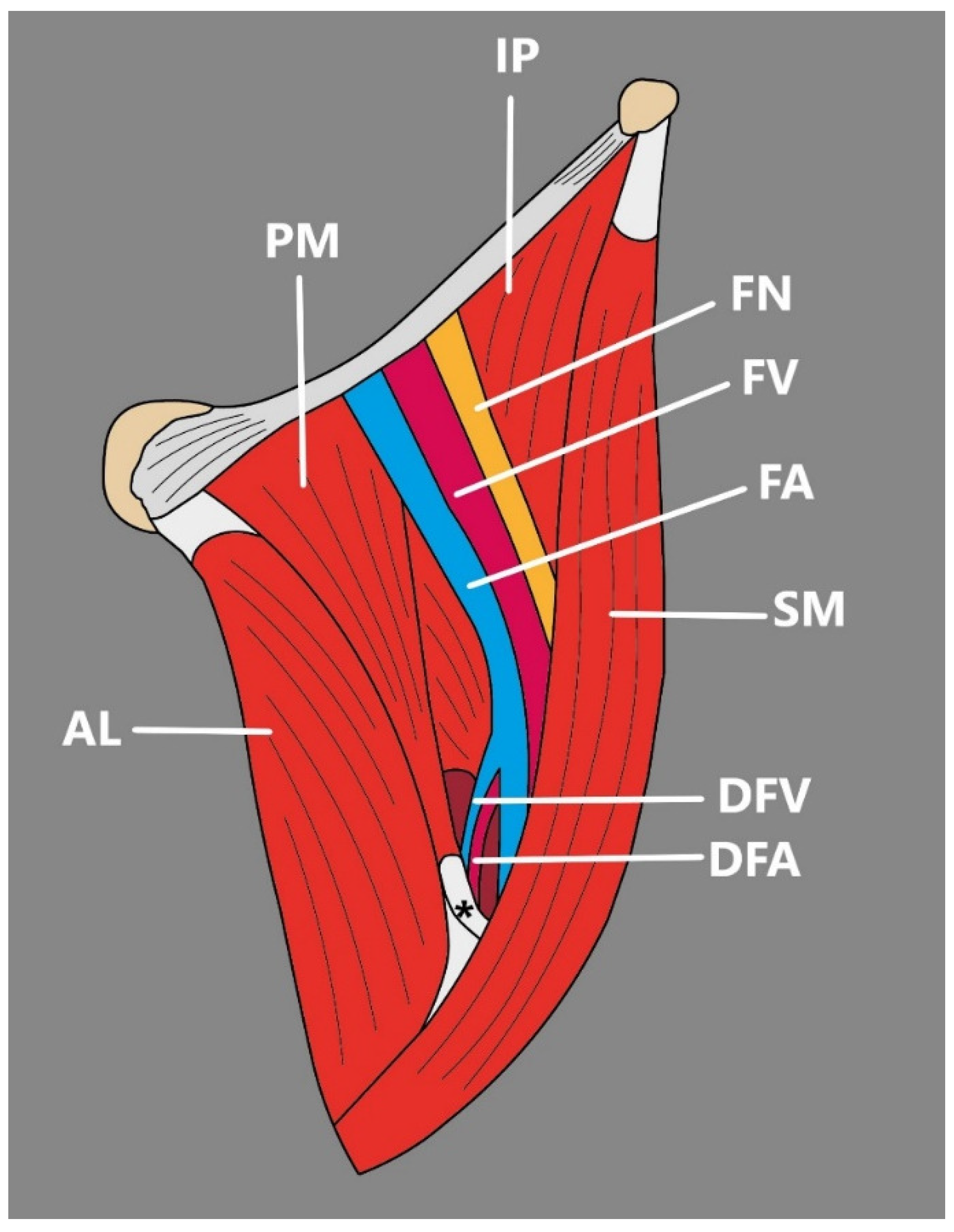
- Kim et al. [7] Presented an interesting case of a bifurcated SM connected with the presence of a unique morphological variation—an accessory SM found during dissection. In the mentioned study, SM originated from the anterior superior iliac spine and then divided into medial and lateral heads of the SM [7]. The lateral head traveled to its site of insertion—the medial aspect of the patella; however, the medial head formed a muscular belly to the vastus medialis and later united with accessory SM, which originated from the inguinal ligament, merged with the medial head of the SM and inserted onto the PA [7] (Figure 2).
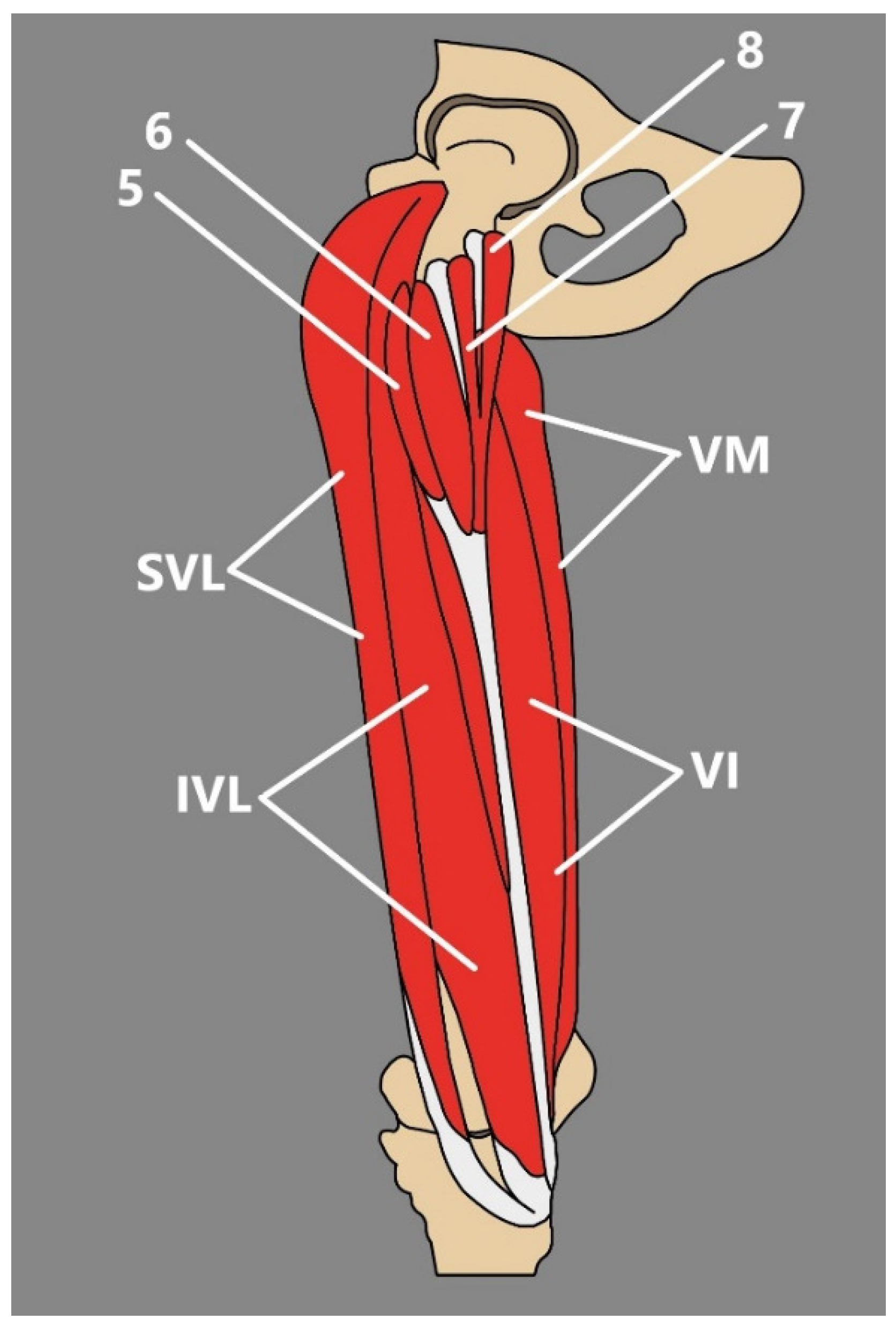
3.3. Quadriceps Femoris
- According to MacAlister [20], the acetabular origin can be absent, the origin from the anterior inferior iliac spine can be doubled, and acetabular and spinous heads can be continuous.
- Moore et al. [22] presented an interesting report of three additional muscular heads related to the RF. The more medial traveled from the distal part of the proximal tendon of RF origin and coursed distally to attach to a second, laterally placed accessory head that arose from the deep layer of fascia lata [22]. A most lateral variant head arose from the deep surface of the deep layer of the fascia lata via a broad tendon. Additional heads fused and formed a chiasmatic structure [22].
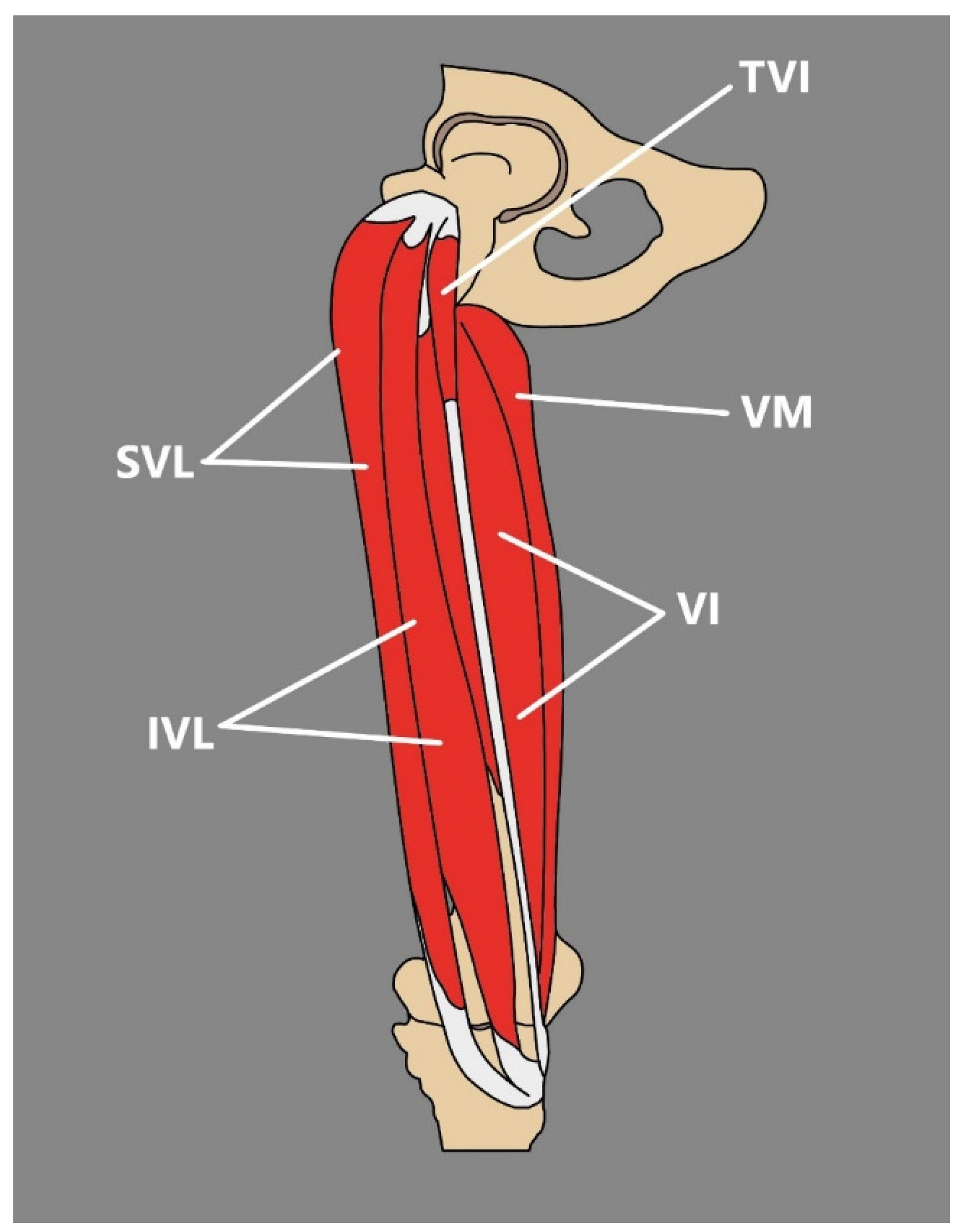
3.4. Tensor of the Vastus Intermedius
| Type | Description | Occurrence | |
|---|---|---|---|
| I | Independent fifth head of QF-TVI, with independent muscle belly that originates from: | ||
| Subtype IA | The upper level of the anterior surface of the greater trochanter joins the intertrochanteric and gluteus medius ridge; muscle belly runs laterally to the VI, and its tendon passes medially | 29.4% | |
| Subtype IB | The upper level of the anterior surface of the greater trochanter joins the intertrochanteric and gluteus medius ridge; muscle belly runs medial to the VI | 14.7% | |
| II | TVI that originates from other muscles: | ||
| Subtype IIA | From the VL | 23.5% | |
| Subtype IIB | From the VI | 4.5% | |
| Subtype IIC | From the gluteus minimus | 2.9% | |
| III | Multiple supplementary heads: | ||
| Subtype IIIA | Two heads with common tendon: lateral head that originates from the upper level of the greater trochanter’s anterior surface and joins the intertrochanteric and gluteus medius ridge; and medial head, that originates from the femur’s anterior surface just above the VI muscle’s proximal attachment | 5.9% | |
| Subtype IIIB | Two heads with two separate tendons: lateral that originates from the upper level of the greater trochanter’s anterior surface where it joins the intertrochanteric and gluteus medius ridge; medial with origin from the femur’s anteromedial surface just above the VI muscle proximal attachment | 14.7% | |
| Subtype IIIC | Three heads: lateral and intermediate that originate from the VL and form a common tendon, and medial with origin from the upper level of the greater trochanter’s anterior surface and joins the intertrochanteric and gluteus medius ridge | 2.9% | |
| Subtype IIID | Four heads (bifurcated lateral and medial): bifurcated medial, that consists of medial and lateral heads—medial that originates from the femur’s innominate tubercle and lateral from m the inferior level of the greater trochanter’s anterior surface; and bifurcated lateral that consists of medial and lateral heads—medial from the inferior level of the greater trochanter’s anterior surface and from the intermediate part of the VL and lateral that originates from the intermediate part of the VL and from the antero-lateral surface of the shaft of the femur (Figure 4) | 1.5% | |
4. Medial Thigh
4.1. Gracilis Muscle
4.2. Pectineus Muscle
4.3. Adductor Magnus
4.4. Adductor Longus
4.5. Adductor Brevis Muscle
5. Posterior Thigh
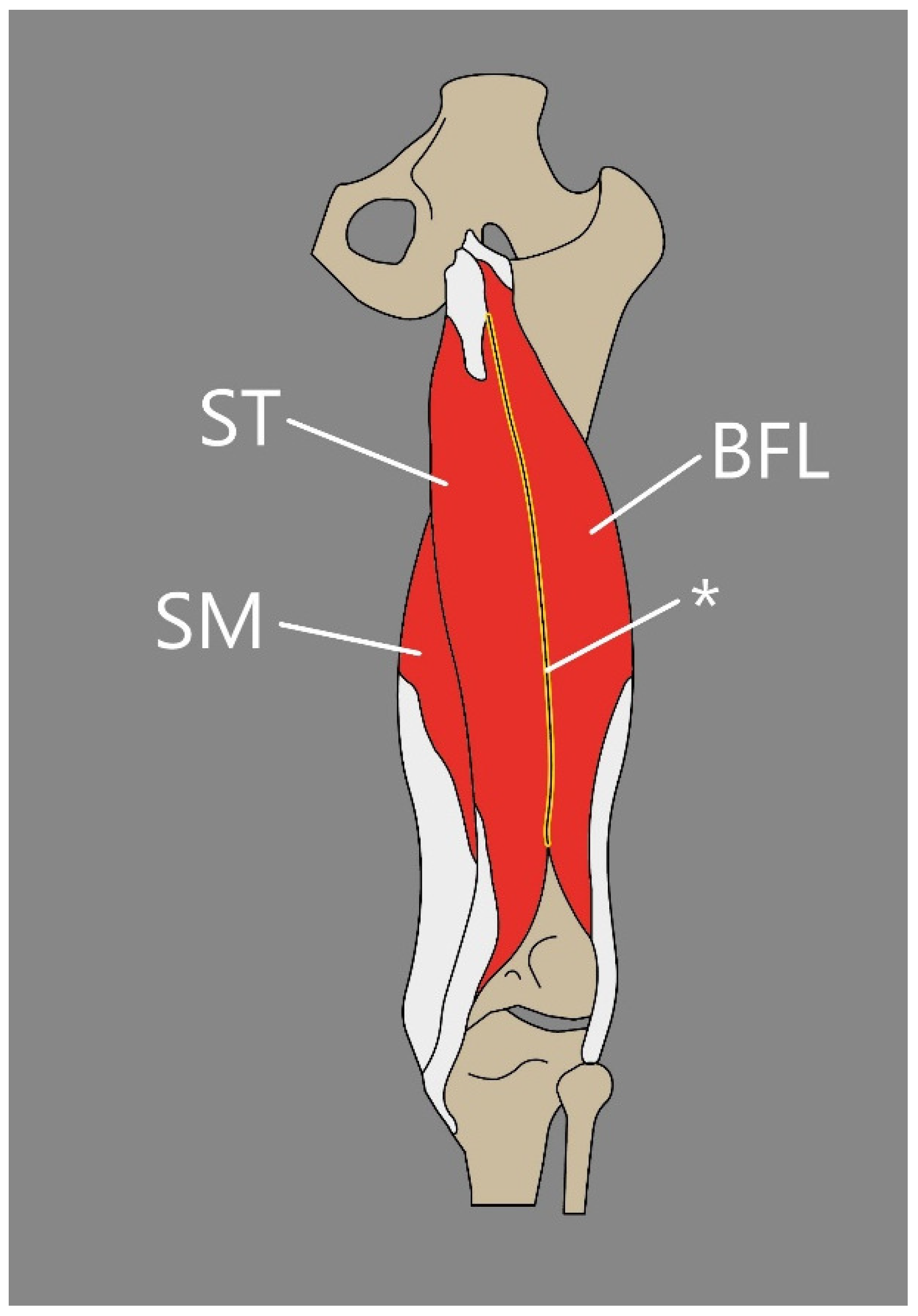
5.1. Semitendinosus Muscle
5.2. Semimembranosus Muscle
5.3. Biceps Femoris
5.4. Tensor Fasciae Suralis
6. Conclusions
Funding
Conflicts of Interest
References
- Hur, M.S.; Won, H.S.; Chung, I.H. A New Morphological Classification for the Fibularis Quartus Muscle. Surg. Radiol. Anat. 2015, 37, 27–32. [Google Scholar] [CrossRef]
- Olewnik, Ł.; Gonera, B.; Kurtys, K.; Tubbs, R.S.; Polguj, M. “Popliteofascial Muscle” or Rare Variant of the Tensor Fasciae Suralis? Folia Morphol. 2021, 80, 1037–1042. [Google Scholar] [CrossRef]
- Kędzia, A.; Wałek, E.; Podleśny, K.; Dudek, K. Musculus Sartorius Metrology in the Fetal Period. Adv. Clin. Exp. Med. 2011, 20, 567–574. [Google Scholar]
- Tsakotos, G.; Olewnik, Ł.; Triantafyllou, G.; Georgiev, G.P.; Zielinska, N.; Piagkou, M. A Bicaudatus Sartorius Muscle: A Rare Variant with Potential Clinical Implications. Folia Morphol. 2024. [Google Scholar] [CrossRef]
- Plessis, M.d.; Loukas, M. Thigh Muscles. In Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 410–420. [Google Scholar]
- Natsis, K.; Koutserimpas, C.; Totlis, T.; Triantafyllou, G.; Tsakotos, G.; Nasraoui, K.A.; Karageorgos, F.; Piagkou, M. A Biceps-Bicaudatus Sartorius Muscle: Dissection of a Variant with Possible Clinical Implications. Anat. Cell Biol. 2024, 57, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.H. A Unique Case of an Accessory Sartorius Muscle. Surg. Radiol. Anat. 2019, 41, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Morris, S.F.; Sigurdson, L. The sartorius muscle: Anatomic considerations for reconstructive surgeons. Surg. Radiol. Anat. 1998, 20, 307–310. [Google Scholar] [CrossRef]
- Gitschlag, K.F.; Sandier, M.A.; Madraz, B.L. Disease in the Femoral Triangle: Sonographic Appearance. Am. J. Roentgenol. 1981, 139, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Franchi, T. Tensor Vastus Intermedius: A Review of Its Discovery, Morphology and Clinical Importance. Folia Morphol. 2020, 80, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Willan, P.L.T.; Ransome, J.A.; Mahon, M. Variability in Human Quadriceps Muscles: Quantitative Study and Review of Clinical Literature. Clin. Anat. 2002, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Ruzik, K.; Olewnik, L.; Westrych, K.; Zielinska, N.; Szewczyk, B.; Tubbs, R.S.; Polguj, M. Anatomical Variation of Co-Existing Bilaminar Tensor of the Vastus Intermedius Muscle and New Type of Sixth Head of the Quadriceps Femoris. Folia Morphol. 2021, 81, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Ruzik, K.; Waśniewska, A.; Olewnik, Ł.; Tubbs, R.S.; Karauda, P.; Polguj, M. Unusual Case Report of Seven-Headed Quadriceps Femoris Muscle. Surg. Radiol. Anat. 2020, 42, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Grob, K.; Ackland, T.; Kuster, M.S.; Manestar, M.; Filgueira, L. A Newly Discovered Muscle: The Tensor of the Vastus Intermedius. Clin. Anat. 2016, 29, 256–263. [Google Scholar] [CrossRef]
- Grob, K.; Manestar, M.; Filgueira, L.; Kuster, M.S.; Gilbey, H.; Ackland, T. The Interaction between the Vastus Medialis and Vastus Intermedius and Its Influence on the Extensor Apparatus of the Knee Joint. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Angullo-Gómez, P.; Jiménez-Luna, C.; Perazzoli, G.; Prados, J.; Ortiz, R.; Cabeza, L. Quadriceps Femoris Muscle: Anatomical Variations, Population Frequencies and Clinical Implications. Folia Morphol. 2023, 83, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Testus, L. Les Anomalies Musculaires Chez l ’homme: Expliquées par l ’anatomie Comparée leur Importance en Anthropologie; G. Masson: Paris, France, 1884. [Google Scholar]
- Olewnik, Ł.; Ruzik, K.; Szewczyk, B.; Podgórski, M.; Aragonés, P.; Karauda, P.; Tubbs, R.S.; Sanudo, J.R.; Pires, M.B.; Polguj, M. The Relationship between Additional Heads of the Quadriceps Femoris, the Vasti Muscles, and the Patellar Ligament. Biomed Res. Int. 2022, 2022, 9569101. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Stetler, W.; Savage, A.J.; Shoja, M.M.; Shakeri, A.B.; Loukas, M.; Salter, E.G.; Oakes, W.J. Does a Third Head of the Rectus Femoris Muscle Exist? Folia Morphol. 2006, 65, 377–380. [Google Scholar]
- Macalister, A. Additional Observations on Muscular Anomalies in Human Anatomy (Third Series): With a Catalogue of the Principal Muscular Variations Hitherto Published. Trans. R. Ir. Acad. 1875, 25, 126–200. [Google Scholar]
- Tubbs, R.S.; Salter, G.; Oakes, W.J. Femoral Head of the Rectus Femoris Muscle. Clin. Anat. 2004, 17, 276–278. [Google Scholar] [CrossRef]
- Moore, V.A.; Xu, L.; Olewnik; Georgiev, G.P.; Iwanaga, J.; Tubbs, R.S. Previously Unreported Variant of the Rectus Femoris Muscle. Folia Morphol. 2023, 82, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Olewnik, Ł.; Zielinska, N.; Ruzik, K.; Karauda, P.; Borowski, A.; LaPrade, R.F. A New Look at Quadriceps Tendon-Is It Really Composed of Three Layers? Knee 2023, 40, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Golland, J.A.; Mahon, M.; Willan, P.L. Anatomical Variations in Human Quadriceps Femoris Muscles. J. Anat. 1986, 146, 263–264. [Google Scholar]
- Olewnik, Ł.; Tubbs, R.S.; Ruzik, K.; Podgórski, M.; Aragonés, P.; Waśniewska, A.; Karauda, P.; Szewczyk, B.; Sanudo, J.R.; Polguj, M. Quadriceps or Multiceps Femoris?—Cadaveric Study. Clin. Anat. 2021, 34, 71–81. [Google Scholar] [CrossRef]
- Veeramani, R.; Gnanasekaran, D. Morphometric Study of Tensor of Vastus Intermedius in South Indian Population. Anat. Cell Biol. 2017, 50, 7–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajasekaran, S.; Hall, M.M. Sonographic Appearance of the Tensor of the Vastus Intermedius. PM R. 2016, 8, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, D.; Gurses, K.M.; Taydas, O.; Poker, A.; Ozer, N.; Hazirolan, T.; Tokgozoglu, L. Role of Femoral Artery Ultrasound Imaging in Cardiovascular Event Risk Prediction in a Primary Prevention Cohort at a Medium-Term Follow-Up. J. Cardiol. 2020, 75, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Aparisi Gómez, M.P.; Errani, C.; Lalam, R.; Vasilevska Nikodinovska, V.; Fanti, S.; Tagliafico, A.S.; Sconfienza, L.M.; Bazzocchi, A. The Role of Ultrasound in the Diagnosis of Soft Tissue Tumors. Semin. Musculoskelet. Radiol. 2020, 24, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Moretti, L.; Cocca, M.P.; Martucci, A.; Orsini, U.; Moretti, B. Low-Grade Fibromyxoid Sarcoma of the Medial Vastus: A Case Report. Musculoskelet. Surg. 2010, 94, 109–112. [Google Scholar] [CrossRef]
- Werner, S. Anterior Knee Pain: An Update of Physical Therapy. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2286–2294. [Google Scholar] [CrossRef]
- Moore, K.; Dalley, A.F.; Agur, A.M.R. Clinically Oriented Anatomy, 7th ed.; Wilkins Lippincott Williams Wilkins Philadelphia: Philadelphia, PA, USA, 2014. [Google Scholar]
- Hussey, A.J.; Laing, A.J.; Regan, P.J. An Anatomical Study of the Gracilis Muscle and Its Application in Groin Wounds. Ann. Plast. Surg. 2007, 59, 404–409. [Google Scholar] [CrossRef]
- Kurtys, K.; Sanudo, J.R.; Kurtys, K.; Olewnik, Ł. A Comprehensive Anatomical Classification System of the Extramuscular Innervation of the Gracilis Muscle as Guidance for Free Functional Muscle Transfer. Ann. Anat.—Anat. Anz. 2023, 245, 152021. [Google Scholar] [CrossRef]
- Kurtys, K.; Podgórski, M.; Gonera, B.; Vazquez, T.; Olewnik, Ł. An Assessment of the Variation of the Intramuscular Innervation of the Gracilis Muscle, with the Aim of Determining Its Neuromuscular Compartments. J. Anat. 2023, 242, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, J.; Qin, B.; Gu, L.; Zheng, J. Ultrasonic Evaluation of Muscle Functional Recovery Following Free Functioning Gracilis Transfer, a Preliminary Study. Eur. J. Med. Res. 2021, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nam, Y.S. Variation of Pectineus Muscle Forming a Hiatus. Anat. Sci. Int. 2021, 96, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Jeno, S.H.; Schindler, G.S. Anatomy, Bony Pelvis and Lower Limb, Thigh Adductor Magnus Muscle; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hildebrand, R. Discovery of a Variant in the Region of the Adductor Magnus and the Short Head of the Biceps Femoris. Anat. Anz. 1978, 144, 48–50. [Google Scholar]
- Tubbs, R.S.; Zehren, S. Popliteal Vein Aneurysm Due to an Anomalous Slip of the Adductor Magnus. Clin. Anat. 2006, 19, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, D.; Björck, M.; Ljungman, C. Popliteal Venous Aneurysm—A Systematic Review. World J. Surg. 2006, 30, 273–279. [Google Scholar] [CrossRef]
- Kozioł, T.; Zarzecki, M.P.; Przybycień, W.; Twardokęs, W.; Walocha, J.A. Bilateral Accessory Head of the Adductor Longus Muscle: An Anatomical Case Study. Folia Morphol. 2022, 82, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Tuite, D.J.; Finegan, P.J.; Saliaris, A.P.; Renström, P.A.F.H.; Donne, B.; O’Brien, M. Anatomy of the Proximal Musculotendinous Junction of the Adductor Longus Muscle. Knee Surg. Sports Traumatol. Arthrosc. 1998, 6, 134–137. [Google Scholar] [CrossRef]
- Renstrom, P.; Peterson, L. Groin Injuries in Athletes. Br. J. Sports Med. 1980, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Pesquer, L.; Reboul, G.; Silvestre, A.; Poussange, N.; Meyer, P.; Dallaudière, B. Imaging of Adductor-Related Groin Pain. Diagn. Interv. Imaging 2015, 96, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Stringer, M.D.; Woodley, S.J. New Insights into the Proximal Tendons of Adductor Longus, Adductor Brevis and Gracilis. Br. J. Sports Med. 2012, 46, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Olewnik, Ł.; Podgórski, M.; Polguj, M. An Unusual Insertion of an Accessory Band of the Semitendinosus Tendon: Case Report and Review of the Literature. Folia Morphol. 2020, 79, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Iwanaga, J.; Oskouian, R.J.; Loukas, M.; Tubbs, R.S. Additional Attachment of the Semitendinosus and Gracilis Muscles to the Crural Fascia: A Review and Case Illustration. Cureus 2018, 10, e3116. [Google Scholar] [CrossRef]
- Haberfehlner, H.; Maas, H.; Harlaar, J.; Becher, J.G.; Buizer, A.I.; Jaspers, R.T. Freehand Three-Dimensional Ultrasound to Assess Semitendinosus Muscle Morphology. J. Anat. 2016, 229, 591–599. [Google Scholar] [CrossRef]
- Mpolokeng, K.; Luckrajh, J.L.; Avilova, O. Accessory Head of the Semitendinosus Muscle: An Unusual Variation. Folia Morphol. 2023, 83, 435–438. [Google Scholar] [CrossRef]
- Olewnik, Ł.; Gonera, B.; Podgórski, M.; Polguj, M.; Jezierski, H.; Topol, M. A Proposal for a New Classification of Pes Anserinus Morphology. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, J.O.; Osinuga, T.S.; Ukwenya, V.O.; Makinde, E.O.; Adekanmbi, A.J. Pes Anserinus Structural Framework and Constituting Tendons Are Grossly Aberrant in Nigerian Population. Anat. Res. Int. 2015, 2015, 483186. [Google Scholar] [CrossRef]
- Zielinska, N.; Tubbs, R.S.; Karauda, P.; Vazquez, T.; Olewnik, Ł. Very Rare Arrangement of the Pes Anserinus: Potential Clinical Significance. Folia Morphol. 2024, 83, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Sumanont, S.; Mahaweerawat, C.; Boonrod, A.; Thammaroj, P.; Boonrod, A. Preoperative Ultrasound Evaluation of the Semitendinosus Tendon for Anterior Cruciate Ligament Reconstruction. Orthop. J. Sports Med. 2019, 7, 2325967118822318. [Google Scholar] [CrossRef]
- Zhong, S.; Wu, B.; Wang, M.; Wang, X.; Yan, Q.; Fan, X.; Hu, Y.; Han, Y.; Li, Y. The Anatomical and Imaging Study of Pes Anserinus and Its Clinical Application. Medicine 2018, 97, e0352. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, A.B.; Johnston, R.K.; Snyder, R.B.; Warburton, M.J.; Pressly Gilbert, P. Evaluation of Hamstring Strength Following Use of Semitendinosus and Gracilis Tendons to Reconstruct the Anterior Cruciate Ligament. Am. J. Sports Med. 1982, 10, 340–342. [Google Scholar] [CrossRef]
- Kittl, C.; Becker, D.K.; Raschke, M.J.; Müller, M.; Wierer, G.; Domnick, C.; Glasbrenner, J.; Michel, P.; Herbort, M. Dynamic Restraints of the Medial Side of the Knee: The Semimembranosus Corner Revisited. Am. J. Sports Med. 2019, 47, 863–869. [Google Scholar] [CrossRef]
- Beltran, L.; Ghazikhanian, V.; Padron, M.; Beltran, J. The Proximal Hamstring Muscle-Tendon-Bone Unit: A Review of the Normal Anatomy, Biomechanics, and Pathophysiology. Eur. J. Radiol. 2012, 81, 3772–3779. [Google Scholar] [CrossRef] [PubMed]
- Sussmann, A.R. Congenital Bilateral Absence of the Semimembranosus Muscles. Skelet. Radiol. 2019, 48, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Balius, R.; Pedret, C.; Iriarte, I.; Sáiz, R.; Cerezal, L. Sonographic Landmarks in Hamstring Muscles. Skelet. Radiol. 2019, 48, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.M.; Gordon, D.H. MRI of an Accessory Semimembranosus Muscle. J. Comput. Assist. Tomofr. 1995, 19, 161–162. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yoo, W.K.; Chung, I.H.; Seo, J.S.; Tanaka, S. Tendinous Insertion of Semimembranosus Muscle into the Lateral Meniscus; Springer: Berlin/Heidelberg, Germany, 1997; Volume 19. [Google Scholar]
- Tubbs, R.S.; Caycedo, F.J.; Oakes, W.J.; Salter, E.G. Descriptive Anatomy of the Insertion of the Biceps Femoris Muscle. Clin. Anat. 2006, 19, 517–521. [Google Scholar] [CrossRef]
- Tsunekawa, R.; Hirai, S.; Hatayama, N.; Yokota, H.; Naito, M.; Nakano, T. A Rare Third Head of the Biceps Femoris in the Posterior Thigh. Anat. Sci. Int. 2021, 96, 157–160. [Google Scholar] [CrossRef]
- Arakawa, T.; Kondo, T.; Tsutsumi, M.; Watanabe, Y.; Terashima, T.; Miki, A. Multiple Muscular Variations Including Tenuissimus and Tensor Fasciae Suralis Muscles in the Posterior Thigh of a Human Case. Anat. Sci. Int. 2017, 92, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Schmuter, G.; Hajtovic, S.; Guce, R.G.; Matthews, K. Rare Fusion of the Semitendinosus and the Long Head of the Biceps Femoris Muscles in a Human Cadaver. Cureus 2021, 13, e12474. [Google Scholar] [CrossRef]
- Kelch, W. Abweichung Des Biceps Femoris. Beitra¨Ge Z. Pathol. Anat. Bd 1813, 8, 36–42. [Google Scholar]
- Gruber, W. Sur Une Variante Du Muscle Tenseur de l’aponeurose Surale, Partant Du Muscle Demi-Tendinex. Bull. L’acad Imp. Sci. St. Petersbourg 1873, 18, 184–186. [Google Scholar]
- Schaeffer, J. On Two Muscle Anomalies of the Lower Extremity. Anat. Rec. 1913, 7, 1–7. [Google Scholar] [CrossRef]
- Tsifountoudis, I.; Kalaitzoglou, I.; Papacostas, E.; Malliaropoulos, N. Tensor Fasciae Suralis Muscle: Report of a Symptomatic Case With Emphasis on Imaging Findings. Clin. J. Sport. Med. 2018, 28, E79–E81. [Google Scholar] [CrossRef] [PubMed]
- Bale, L.S.W.; Herrin, S.O. Bilateral Tensor Fasciae Suralis Muscles in a Cadaver with Unilateral Accessory Flexor Digitorum Longus Muscle. Case Rep. Med. 2017, 2017, 1864272. [Google Scholar] [CrossRef]
- Bale, L.S.W.; Damjanovic, M.M.; Damjanovic, I.G.; DiMaio, N.M.; Herrin, S.O. Tensor Fasciae Suralis—Prevalence Study and Literature Review. Morphologie 2024, 108, 100762. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Salter, E.G.; Oakes, W.J. Dissection of a Rare Accessory Muscle of the Leg: The Tensor Fasciae Suralis Muscle. Clin. Anat. 2006, 19, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Montet, X.; Sandoz, A.; Mauget, D.; Martinoli, C.; Bianchi, S. Sonographic and MRI Appearance of Tensor Fasciae Suralis Muscle, an Uncommon Cause of Popliteal Swelling. Skelet. Radiol. 2002, 31, 536–538. [Google Scholar] [CrossRef]
- Dickson, T.; Koulouris, G. Acute Posterior Thigh Pain in an Athlete. Skelet. Radiol. 2017, 46, 101–102. [Google Scholar] [CrossRef]
- Dickson, T.; Koulouris, G. Acute Posterior Thigh Pain in an Athlete. Skelet. Radiol. 2017, 46, 141–142. [Google Scholar] [CrossRef]
- Sookur, P.A.; Naraghi, A.M.; Bleakney, R.R.; Jalan, R.; Chan, O.; White, L.M. Accessory Muscles: Anatomy, Symptoms, and Radiologic Evaluation. Radiographics 2008, 28, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Shim, J.C.; Lee, G.J.; Lee, K.E.; Kim, H.K.; Suh, J.H. MR Imaging and Ultrasonographic Findings of Tensor Fasciae Suralis Muscle: A Case Report. J. Korean Soc. Radiol. 2015, 73, 249. [Google Scholar] [CrossRef]
- Somayaji, S.N.; Vincent, R.; Bairy, K.L. An Anomalous Muscle in the Region of the Popliteal Fossa: Case Report. J. Anat. 1998, 192, 307–308. [Google Scholar] [CrossRef] [PubMed]

| Layer | Components |
|---|---|
| Superficial (first) layer | RF tendon and fascia |
| Middle (second) layer | Superficial part of the VL and VM tendon |
| Middle-deep (third) layer | Intermediate part of the VL |
| Deep (fourth) layer | VI tendon |
| Type | Description | Occurence |
|---|---|---|
| 1-1-1 | Monotendinous ST, GT, STT | 52.9% |
| 1-1-2 | Monotendinous ST, GT, but with an additional band from STT | 31.4% |
| 1-1-3 | Monotendinous ST, GT, and two additional bands from STT | 8.8% |
| 1-2-3 | Monotendinous ST, one additional band from GT, and two additional bands from STT | 1% |
| 2-1-2 | One additional band from ST, monotendinous GT, and one additional band from STT | 2% |
| 2-2-3 | One additional band from GT and ST and two additional bands from STT | 3.9% |
| Number | Insertion |
|---|---|
| I | The posteromedial aspect of the tibia deeply to the medial collateral ligament |
| II | Direct insertion onto tibia |
| III | Oblique popliteal ligament |
| IV | Posterior oblique popliteal ligament |
| V | Popliteus aponeurosis |
| Type | Description | Number of Cases in Study by Bale et al. [72] | |
|---|---|---|---|
| I | A | One-headed muscle; origin from the long head of the biceps femoris | 16 |
| B | One-headed muscle; origin from the semitendinous muscle | 13 | |
| C | One-headed muscle; origin from the short head of the biceps femoris | 2 | |
| D | One-headed muscle; origin from the semimembranosus muscle | 0 | |
| II | Two-headed muscle; medial head arises from the semitendinosus muscle, lateral head arises from the long head of the biceps femoris muscle | 4 | |
| III | Other variations | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pośnik, M.; Zielinska, N.; Okoń, A.; Węgiel, A.; Głowacka, M.; Olewnik, Ł. Morphological Variability of the Thigh Muscle Traps in an Ultrasound That Awaits Clinicians. J. Clin. Med. 2025, 14, 464. https://doi.org/10.3390/jcm14020464
Pośnik M, Zielinska N, Okoń A, Węgiel A, Głowacka M, Olewnik Ł. Morphological Variability of the Thigh Muscle Traps in an Ultrasound That Awaits Clinicians. Journal of Clinical Medicine. 2025; 14(2):464. https://doi.org/10.3390/jcm14020464
Chicago/Turabian StylePośnik, Marta, Nicol Zielinska, Adrian Okoń, Andrzej Węgiel, Mariola Głowacka, and Łukasz Olewnik. 2025. "Morphological Variability of the Thigh Muscle Traps in an Ultrasound That Awaits Clinicians" Journal of Clinical Medicine 14, no. 2: 464. https://doi.org/10.3390/jcm14020464
APA StylePośnik, M., Zielinska, N., Okoń, A., Węgiel, A., Głowacka, M., & Olewnik, Ł. (2025). Morphological Variability of the Thigh Muscle Traps in an Ultrasound That Awaits Clinicians. Journal of Clinical Medicine, 14(2), 464. https://doi.org/10.3390/jcm14020464






