Association Between Systemic Symptoms and Recovery in Acute Low Back Pain: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Association Between Demographic Characteristics and Pain Intensity
3.3. Association Between Past Medical History and Pain Intensity
3.4. Association Between Low Back Pain Characteristics and Pain Intensity
3.5. Association Between Systemic Symptoms and Pain Intensity
3.6. Association Between Systemic Symptoms and Pain Characteristics
3.7. Multiple Regression Analysis
3.7.1. Pain at Discharge
3.7.2. Absolute Pain Change
3.7.3. Relative Pain Change
3.7.4. Sensitive and Subgroup Analysis
3.7.5. Association Between Binary Variables Included in Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBP | Low Back Pain |
| ALBP | Acute Low Back Pain |
| CLBP | Chronic Low Back Pain |
| GI | Gastrointestinal |
| TKM | Traditional Korean Medicine |
| IRB | Institutional Review Board |
| HTN | Hypertension |
| DM | Diabetes Mellitus |
| PSY | Psychological history |
| ROM | Range of Motion |
| NRS | Numeric Rating Scale |
| NRS AD | Numeric Rating Scale at Admission |
| NRS DC | Numeric Rating Scale at Discharge |
| GAM | Generalized Additive Model |
| VIF | Variance Inflation Factors |
| IAP | Intra-abdominal Pressure |
| ACC | Anterior Cingulate Cortex |
References
- Burton, A.K.; Balague, F.; Cardon, G.; Eriksen, H.R.; Henrotin, Y.; Lahad, A.; Leclerc, A.; Müller, G.; van der Beek, A.J. Chapter 2 European guidelines for prevention in low back pain. Eur. Spine J. 2006, 15, S136–S168. [Google Scholar] [CrossRef]
- Chien, J.J.; Bajwa, Z.H. What is mechanical back pain and how best to treat it? Curr. Pain Headache Rep. 2008, 12, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G. Treatment of acute lower back pain. In Non-Operative Treatment of the Lumbar Spine; Bono, C.M., Schoenfeld, A.J., Vaccaro, A.R., Eds.; Springer: Cham, Switzerland, 2015; pp. 15–18. [Google Scholar]
- Apkarian, A.V.; Baliki, M.N.; Geha, P.Y. Towards a theory of chronic pain. Prog. Neurobiol. 2009, 87, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.G.; Dana, B.; Anna, W.S.; Glenn, P. Psychosocial differences between high-risk acute vs chronic low back pain patients. Pain Pract. 2008, 8, 91–97. [Google Scholar]
- Marin, R.; Cyhan, T.; Miklos, W. Sleep disturbance in patients with chronic low back pain. Am. J. Phys. Med. Rehabil. 2006, 85, 430–435. [Google Scholar] [CrossRef]
- Wang, H.Y.; Fu, T.S.; Hsu, S.C.; Hung, C.I. Association of depression with sleep quality might be greater than that of pain intensity among outpatients with chronic low back pain. Neuropsychiatr. Dis. Treat. 2016, 12, 1993–1998. [Google Scholar] [CrossRef]
- Montgomery, W.; Sato, M.; Nagasaka, Y.; Vietri, J. The economic and humanistic costs of chronic lower back pain in Japan. Clin. Outcomes Res. 2017, 9, 361–371. [Google Scholar] [CrossRef]
- Moradi, B.; Hagmann, S.; Zahlten-Hinguranage, A.; Caldeira, F.; Putz, C.; Rosshirt, N.; Schönit, E.; Mesrian, A.; Schiltenwolf, M.; Neubauer, E. Efficacy of multidisciplinary treatment for patients with chronic low back pain: A prospective clinical study in 395 patients. J. Clin. Rheumatol. 2012, 18, 76–82. [Google Scholar] [CrossRef]
- Valat, J.P.; Goupille, P.; Védère, V. Low back pain: Risk factors for chronicity. Rev. Rhum. Engl. Ed. 1997, 64, 189–194. [Google Scholar]
- Waddell, G. 1987 Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine 1987, 12, 632–644. [Google Scholar] [CrossRef]
- Nieminen, L.K.; Pyysalo, L.M.; Kankaanpää, M.J. Prognostic factors for pain chronicity in low back pain: A systematic review. Pain Rep. 2021, 6, e919. [Google Scholar] [CrossRef]
- da Silva, T.; Macaskill, P.; Mills, K.; Maher, C.; Williams, C.; Lin, C.; Hancock, M.J. Predicting recovery in patients with acute low back pain: A clinical prediction model. Eur. J. Pain 2017, 21, 716–726. [Google Scholar] [CrossRef]
- Friedman, B.W.; O’Mahony, S.; Mulvey, L.; Davitt, M.; Choi, H.; Xia, S.; Esses, D.; Bijur, P.E.; Gallagher, E.J. One-week and 3-month outcomes after an emergency department visit for undifferentiated musculoskeletal low back pain. Ann. Emerg. Med. 2012, 59, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, H. The review of systems: An important part of a comprehensive examination. Postgrad. Med. 1982, 71, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Russell, A.; Hodges, P.W. How common is back pain in women with gastrointestinal problems? Clin. J. Pain 2008, 24, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Russell, A.; Hodges, P.W. The relationship between incontinence, breathing disorders, gastrointestinal symptoms, and back pain in women: A longitudinal cohort study. Clin. J. Pain 2014, 30, 162–167. [Google Scholar] [CrossRef]
- Yu, H.Y.; Jeong, Y.W. Influence of constipation in women in their twenties on low back pain. J. Korean Orthop. Manip. Phys. Ther. 2018, 24, 43–49. [Google Scholar]
- Alsaadi, S.M.; McAuley, J.H.; Hush, J.M.; Lo, S.; Bartlett, D.J.; Grunstein, R.R.; Maher, C.G. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin. J. Pain 2014, 30, 755–765. [Google Scholar] [CrossRef]
- The Society of Korean Rehabilitation Medicine. Korean Rehabilitation Medicine, 5th ed.; Kunja Publisher: Seoul, Republic of Korea, 2020; pp. 6–10, 16–20, 87–88. [Google Scholar]
- Park, S.H.; Keum, D.H. Systemic symptoms as potential predictors of chronic neck pain on initial examination: Can systemic symptoms act as a predictor of neck pain? J. Pers. Med. 2024, 14, 688. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi. J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Wong, A.Y.L.; Karppinen, J.; Samartzis, D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis Spinal Disord. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Rundell, S.D.; Sherman, K.J.; Heagerty, P.J.; Mock, C.N.; Dettori, N.J.; Comstock, B.A.; Avins, A.L.; Nedeljkovic, S.S.; Nerenz, D.R.; Jarvik, J.G. Predictors of persistent disability and back pain in older adults with a new episode of care for back pain. Pain Med. 2017, 18, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; Downie, A.; Popal, N.; Maher, C.; Koes, B.W. Red flags presented in current low back pain guidelines: A review. Eur. Spine J. 2016, 25, 2788–2802. [Google Scholar] [CrossRef] [PubMed]
- Kuris, E.O.; McDonald, C.L.; Palumbo, M.A.; Daniels, A.H. Evaluation and management of cauda equina syndrome. Am. J. Med. 2021, 134, 1483–1489. [Google Scholar] [CrossRef]
- Donnally, C.J., III; Margetis, K.; Varacallo, M.A. Vertebral Compression Fractures. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Suzuki, N.; Ogikubo, O.; Hansson, T. The course of the acute vertebral body fragility fracture: Its effect on pain, disability and quality of life during 12 months. Eur. Spine J. 2008, 17, 1380–1390. [Google Scholar] [CrossRef]
- Sahbaie, P.; Irvine, K.A.; Liang, D.Y.; Shi, X.; Clark, J.D. Mild traumatic brain injury causes nociceptive sensitization through spinal chemokine upregulation. Sci. Rep. 2019, 9, 19500. [Google Scholar] [CrossRef]
- Danielli, E.; Simard, N.; DeMatteo, C.A.; Kumbhare, D.; Ulmer, S.; Noseworthy, M.D. A review of brain regions and associated post-concussion symptoms. Front. Neurol. 2023, 14, 1136367. [Google Scholar] [CrossRef]
- Strate, L.L.; Ayanian, J.Z.; Kotler, G.; Syngal, S. Risk factors for mortality in lower intestinal bleeding. Clin. Gastroenterol. Hepatol. 2008, 6, 1004–1010. [Google Scholar] [CrossRef]
- Center for Behavioral Health Statistics and Quality. Additional Disorders for Consideration. In Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2016; pp. 151–153. [Google Scholar]
- Rome Foundation, Rome IV Criteria. Available online: https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed on 4 February 2025).
- International Continence Society Committees, Nocturia—Terminology Discussion. Available online: https://www.ics.org/committees/standardisation/terminologydiscussions/nocturia (accessed on 4 February 2025).
- Choi, E.P.H.; Wan, E.Y.F.; Kwok, J.Y.Y.; Chin, W.Y.; Lam, C.L.K. The mediating role of sleep quality in the association between nocturia and health-related quality of life. Health. Qual. Life Outcomes. 2019, 17, 181. [Google Scholar] [CrossRef]
- Dijkers, M. Comparing quantification of pain severity by verbal rating and numeric rating scales. J. Spinal Cord Med. 2010, 33, 232–242. [Google Scholar] [CrossRef]
- Haase, I.; Kladny, B. Clinical relevance of changes in pain intensity in patients with specific back pain. Z. Orthop. Unfall. 2022, 160, 213–221. [Google Scholar] [CrossRef]
- Gupta, A.; Stead, T.S.; Ganti, L. Determining a meaningful R-squared value in clinical medicine. Acad. Med. Surg. 2024. [Google Scholar] [CrossRef]
- Marras, W.S.; Davis, K.G.; Ferguson, S.A.; Lucas, B.R.; Gupta, P. Spine loading characteristics of patients with low back pain compared with asymptomatic individuals. Spine 2001, 26, 2566–2574. [Google Scholar] [CrossRef]
- Hodges, P.W.; Eriksson, A.E.; Shirley, D.; Gandevia, S.C. Intra-abdominal pressure increases stiffness of the lumbar spine. J. Biomech. 2005, 38, 1873–1880. [Google Scholar] [CrossRef]
- Stokes, I.A.F.; Gardner-Morse, M.G.; Henry, S.M. Intra-abdominal pressure and abdominal wall muscular function: Spinal unloading mechanism. Clin. Biomech. 2010, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, F.F.; Gislason, H.G.; Dicko, A.; Horn, A.; Viste, A.; Grong, K.; Svanes, K. Effects of prolonged increased intra-abdominal pressure on gastrointestinal blood flow in pigs. Surg. Endosc. 2001, 15, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.D. Visceral inputs to sensory pathways in the spinal cord. In Progress in Brain Research; Cervero, F., Morrison, J.F.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 67, pp. 207–225. [Google Scholar]
- Beal, M.C. Viscerosomatic reflexes: A review. J. Am. Osteopath. Assoc. 1985, 85, 53–68. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between deformation of the thoracolumbar fascia and activation of the erector spinae and multifidus muscle in patients with acute low back pain and healthy controls: A matched pair case-control study. Life 2022, 12, 1735. [Google Scholar] [CrossRef]
- Liu, P.; Wang, G.; Zeng, F.; Liu, Y.; Fan, Y.; Wei, Y.; Qin, W.; Calhoun, V.D. Abnormal brain structure implicated in patients with functional dyspepsia. Brain Imaging Behav. 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Yan, N.; Cao, B.; Xu, J.; Hao, C.; Zhang, X.; Li, Y. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol. Learn. Mem. 2012, 97, 156–164. [Google Scholar] [CrossRef]
- Sengupta, J.N. Visceral pain: The neurophysiological mechanism. Handb. Exp. Pharmacol. 2009, 194, 31–74. [Google Scholar]
- Arai, Y.C.; Shiro, Y.; Funak, Y.; Kasugaii, K.; Omichi, Y.; Sakurai, H.; Matsubara, T.; Inoue, M.; Shimo, K.; Saisu, H.; et al. The association between constipation or stool consistency and pain severity in patients with chronic pain. Anesth. Pain Med. 2018, 8, e69275. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Mallah, N.; Nedjat, S.; Beasley, M.J.; Takkouche, B. Association between alcohol consumption and chronic pain: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 129, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.R.; Hassett, A.L.; Schrepf, A.D.; Brummett, C.M.; Harris, R.E.; Clauw, D.J.; Harte, S.E. Moderate alcohol consumption is associated with reduced pain and fibromyalgia symptoms in chronic pain patients. Pain Med. 2018, 19, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- DeWall, C.N.; Giancola, P.R.; Bushman, B.J. Too insensitive to care: Alcohol increases human aggression by increasing pain threshold. J. Stud. Alcohol. Drugs. 2024, 86, 755–760. [Google Scholar] [CrossRef]
- Henschke, N.; Maher, C.G.; Refshauge, K.M.; Herbert, R.D.; Cumming, R.G.; Bleasel, J.; York, J.; Das, A.; McAuley, J.H. Prognosis in patients with recent onset low back pain in Australian primary care: Inception cohort study. BMJ 2008, 337, a171. [Google Scholar] [CrossRef]
- Hüllemann, P.; Keller, T.; Kabelitz, M.; Gierthmühlen, J.; Freynhagen, R.; Tölle, T.; Forstenpointner, J.; Baron, R. Clinical manifestation of acute, subacute, and chronic low back pain in different age groups: Low back pain in 35,446 patients. Pain Pract. 2018, 18, 1011–1023. [Google Scholar] [CrossRef]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 22, 316–329. [Google Scholar]
- Jacob, L.; Rathmann, W.; Koyanagi, A.; Haro, J.M.; Kostev, K. Association between type 2 diabetes and chronic low back pain in general practices in Germany. BMJ Open Diabetes Res. Care 2021, 9, e002426. [Google Scholar] [CrossRef]
- Zhou, W.B.S.; Meng, J.; Zhang, J. Does Low Grade Systemic Inflammation Have a Role in Chronic Pain? Front. Mol. Neurosci. 2021, 14, 785214. [Google Scholar] [CrossRef]
- Wang, R.; Peng, M.S.; Wang, Y.Z.; Chen, P.J.; Wang, X.Q. Influence of Depression on Pain and Disability in Patients with Chronic Low Back Pain after Physical Therapy: A Secondary Analysis of a Randomized Controlled Trial. Depress. Anxiety 2024, 2024, 9065325. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Szpalski, M.; Hayez, J.P. How many days of bed rest for acute low back pain? Objective assessment of trunk function. Eur. Spine J. 1992, 1, 29–31. [Google Scholar] [CrossRef]
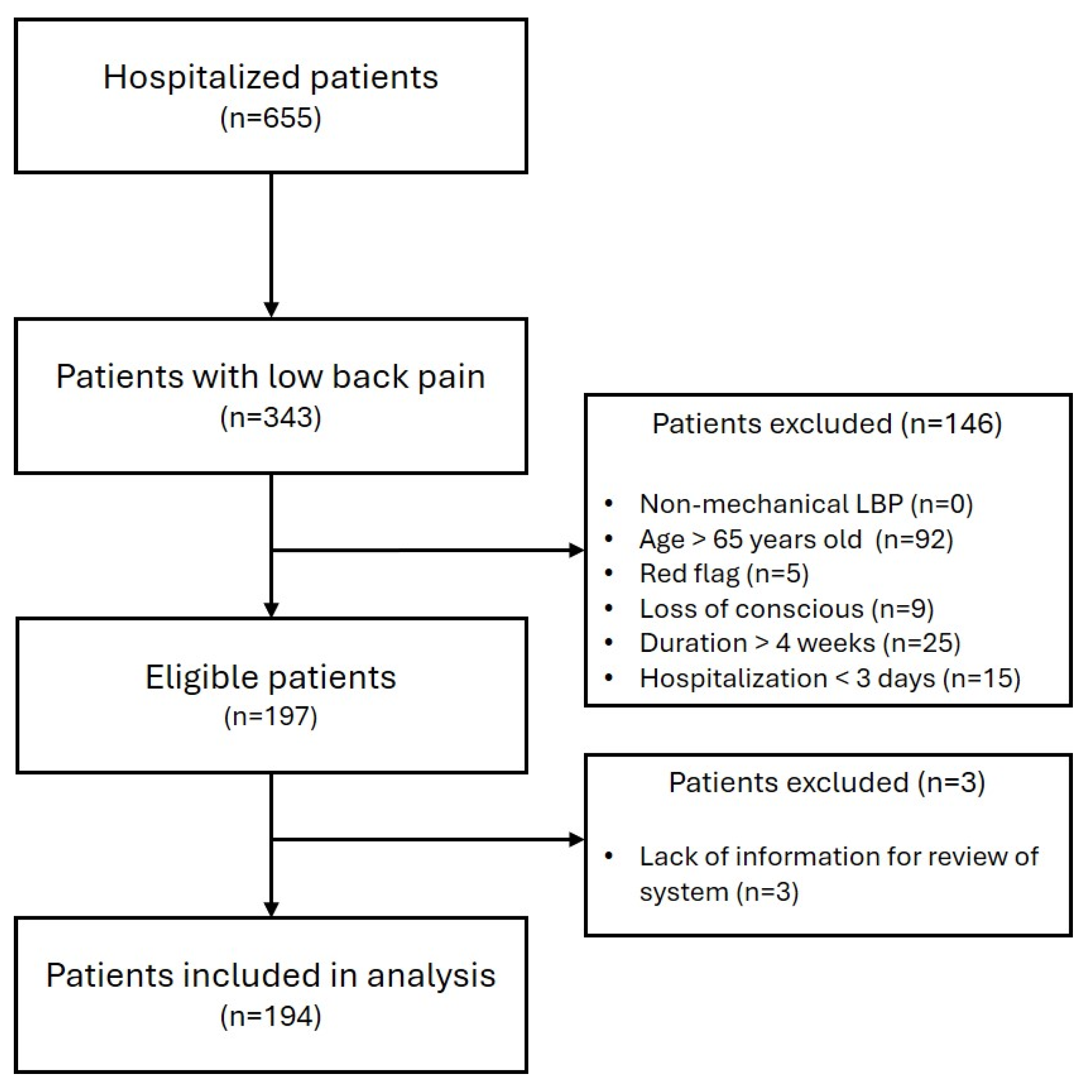
| Definition of LBP | KCD Codes S30–S39 (Injuries to the Abdomen, Lower Back, Lumbar Spine and Pelvis) or M50–M54 (Other Dorsopathies) Related to LBP |
| Inclusion Criteria | Age ≤ 65 years old Duration of LBP ≤ 4 weeks Hospitalization period ≥ 3 days |
| Exclusion Criteria | Age > 65 years old Duration of LBP > 4 weeks Hospitalization period < 3 days Non-mechanical LBP Red flags (Cauda equina, Compression fracture, etc.) Loss of conscious at onset Lack of sufficient information regarding systemic symptoms |
| Systemic Symptoms | Referenced Diagnostic Criteria | Adapted Inclusion Criteria |
|---|---|---|
| Sleep disturbance | DSM-5 - Difficulty initiating or maintaining sleep, or early-morning awakening, occurring ≥3 nights per week for ≥3 months - Symptoms not attributable to mental disorder, substance use, or medical condition | - Frequency of DSM-5-defined sleep disturbance symptoms ≥3 nights per week during hospitalization - Excludes cases attributable to mental disorder, medical condition (ex. nocturia) or substance use (caffeine intake) |
| Anorexia | None | - Patient’s dietary intake decreased to ≤50% of baseline during hospitalization |
| Dyspepsia | Rome IV Criteria - Presence of at least one of the following: postprandial fullness, early satiation, epigastric pain, or epigastric burning - Symptom presentation for the last 3 months, with onset ≥6 months before diagnosis - No evidence of structural disease or identifiable gastrointestinal disorder | - Occurrence of at least one Rome IV-defined symptom during hospitalization - No evidence of structural disease or identifiable gastrointestinal disorder based on past history |
| Constipation | Rome IV Criteria - Presence of at least two of the following: bowel movements ≤ 3 times per week, straining, hard stools, sensation of incomplete evacuation, anorectal obstruction, or manual maneuvers - Symptom presentation for the last 3 months, with onset ≥6 months before diagnosis | - Occurrence of at least two Rome IV-defined symptoms during hospitalization |
| Nocturia | ICS - At least one episode of awakening during the night to void Article - Clinically significant, occurring at least twice per night - Regardless of voiding frequency, clinical significance could be determined by the degree of sleep disruption | - Occurrence of voiding at least twice per night during hospitalization or - Frequency of sleep disturbance due to nocturia ≥3 times per week during hospitalization |
| Thirst | None | - Patient-reported thirst or increased water intake during hospitalization |
| Total number of cohorts | 194 | |
| Gender (Male/Female) | 79/115 | |
| Age (Year) | 47.06 ± 12.11 | |
| Complaint duration (Day) | 2.65 ± 4.18 | |
| Hospitalization period (Day) | 6.57 ± 3.84 | |
| Diagnosis | Sprain and strain of lumbar spine | 181 (93.3%) |
| Sprain and strain of other and unspecified parts of lumbar spine and pelvis | 8 (4.1%) | |
| Lumbar and other intervertebral disc disorders with radiculopathy | 5 (2.6%) | |
| Spinal stenosis, lumbar region | 2 (1.0%) | |
| Other spondylosis, lumbar region | 1 (0.5%) | |
| Low back pain, lumbar region | 1 (0.5%) | |
| Lumbago due to displacement of intervertebral disc | 1 (0.5%) | |
| Spondylolysis, lumbar region | 1 (0.5%) | |
| NRS AD | 6.09 ± 1.46 | |
| NRS DC | 4.77 ± 2.01 | |
| ΔNRS | −1.32 ± 1.75 | |
| ΔNRS (%) | −21.61 ± 27.93 | |
| Variables | NRS AD | NRS DC | ΔNRS | ΔNRS (%) | ||
|---|---|---|---|---|---|---|
| (A) Demographic characteristics | Gender | Male (79) | 6.03 ± 1.41 | 4.71 ± 2.02 | −1.32 ± 1.82 | −21.08 ± 28.87 |
| Female (115) | 6.14 ± 1.49 | 4.82 ± 2.02 | −1.32 ± 1.70 | −21.96 ± 27.39 | ||
| p-value | 0.801 | 0.693 | 0.819 | 0.759 | ||
| Age | Correlation coefficient | 0.013 | 0.050 | 0.035 | 0.041 | |
| p-value | 0.858 | 0.491 | 0.630 | 0.634 | ||
| Smoking | Yes (28) | 5.96 ± 1.53 | 3.93 ± 1.90 | −2.04 ± 2.13 | −31.99 ± 32.05 | |
| No (166) | 6.11 ± 1.45 | 4.92 ± 2.00 | −1.20 ± 1.65 | −19.86 ± 26.89 | ||
| p-value | 0.546 | 0.017 * | 0.051 † | 0.047 * | ||
| Alcohol | Yes (57) | 6.04 ± 1.49 | 4.25 ± 2.17 | −1.79 ± 1.81 | −30.00 ± 27.78 | |
| No (137) | 6.12 ± 1.45 | 4.99 ± 1.91 | −1.12 ± 1.69 | −18.11 ± 27.34 | ||
| p-value | 0.549 | 0.007 ** | 0.018 * | 0.009 ** | ||
| (B) Pain characteristics | NRS AD | Correlation coefficient | - | 0.506 | −0.145 | −0.012 |
| p-value | - | 0.000 *** | 0.044 * | 0.863 | ||
| Symptom duration | Correlation coefficient | –0.072 | –0.266 | −0.280 | −0.302 | |
| p-value | 0.320 | 0.000 *** | 0.000 *** | 0.000 *** | ||
| Hospitalization length | Correlation coefficient | 0.022 | –0.181 | −0.215 | −0.199 | |
| p-value | 0.761 | 0.011 * | 0.003 ** | 0.005 ** | ||
| Distribution of LBP | Widespread (131) | 6.24 ± 1.46 | 4.80 ± 1.97 | −1.44 ± 1.73 | −22.91 ± 26.36 | |
| Localized (63) | 5.79 ± 1.43 | 4.71 ± 2.11 | −1.08 ± 1.76 | −18.90 ± 31.00 | ||
| p-value | 0.067 † | 0.725 | 0.156 | 0.255 | ||
| ROM | Normal (81) | 5.72 ± 1.39 | 4.48 ± 2.09 | −1.23 ± 1.62 | −22.85 ± 29.34 | |
| Abnormal (113) | 6.36 ± 1.45 | 4.98 ± 1.94 | −1.38 ± 1.83 | −20.71 ± 26.97 | ||
| p-value | 0.007 ** | 0.089 † | 0.781 | 0.660 | ||
| (C) Systemic symptoms | Sleep disturbance | Yes (114) | 6.20 ± 1.42 | 5.01 ± 2.09 | −1.19 ± 1.68 | −19.78 ± 26.91 |
| No (80) | 5.94 ± 1.51 | 4.44 ± 1.86 | −1.50 ± 1.83 | −24.21 ± 29.30 | ||
| p-value | 0.262 | 0.049 * | 0.215 | 0.215 | ||
| Anorexia | Yes (47) | 6.17 ± 1.36 | 4.96 ± 1.89 | −1.21 ± 1.47 | −19.95 ± 22.57 | |
| No (147) | 6.07 ± 1.49 | 4.71 ± 2.05 | −1.35 ± 1.83 | −22.13 ± 29.49 | ||
| p-value | 0.942 | 0.592 | 0.848 | 0.813 | ||
| Constipation | Yes (8) | 6.88 ± 1.46 | 6.13 ± 2.10 | −0.75 ± 1.83 | −9.97 ± 26.96 | |
| No (186) | 6.06 ± 1.45 | 4.72 ± 1.99 | −1.34 ± 1.74 | −22.11 ± 27.93 | ||
| p-value | 0.133 | 0.076 † | 0.311 | 0.213 | ||
| Dyspepsia | Yes (68) | 6.15 ± 1.56 | 5.16 ± 2.06 | −0.99 ± 1.52 | −16.34 ± 24.81 | |
| No (126) | 6.06 ± 1.41 | 4.56 ± 1.97 | −1.50 ± 1.84 | −24.45 ± 29.18 | ||
| p-value | 0.771 | 0.091 | 0.052 † | 0.054 † | ||
| Nocturia | Yes (65) | 6.22 ± 1.39 | 4.88 ± 1.89 | −1.34 ± 1.88 | −20.59 ± 26.91 | |
| No (129) | 6.03 ± 1.49 | 4.72 ± 2.08 | −1.31 ± 1.68 | −22.12 ± 28.52 | ||
| p-value | 0.581 | 0.644 | 0.822 | 0.733 | ||
| Thirst | Yes (76) | 6.09 ± 1.56 | 4.80 ± 2.03 | −1.29 ± 1.78 | −21.24 ± 28.40 | |
| No (118) | 6.09 ± 1.40 | 4.75 ± 2.01 | −1.34 ± 1.73 | −21.84 ± 27.74 | ||
| p-value | 0.680 | 0.887 | 0.744 | 0.820 |
| Variables | NRS DC | ΔNRS | ΔNRS (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parametric Terms | Estimate | p-Value | 95% CI | Estimate | p-Value | 95% CI | Estimate | p-Value | 95% CI |
| NRS AD | 0.71 | 0.000 *** | (0.56, 0.90) ‡ | - | - | - | - | - | - |
| Sleep disturbance | 0.29 | 0.223 | (−0.18, 0.77) | 0.24 | 0.315 | (−0.20, 0.76) | 2.82 | 0.465 | (−5.18, 10.27) |
| Dyspepsia | 0.55 | 0.026 * | (0.05, 1.02) ‡ | 0.54 | 0.033 * | (0.06, 1.00) ‡ | 8.15 | 0.045 * | (0.27, 16.38) ‡ |
| Constipation | 0.41 | 0.483 | (−0.95, 1.69) | 0.21 | 0.726 | (−1.29, 1.53) | 7.54 | 0.436 | (−13.35, 26.91) |
| Lower GI | −0.23 | 0.539 | (−0.92, 0.43) | −0.05 | 0.904 | (−0.75, 0.57) | −3.07 | 0.609 | (−14.49, 7.53) |
| MSTL | −0.28 | 0.355 | (−0.95, 0.35) | −0.40 | 0.195 | (−1.07, 0.28) | −4.86 | 0.321 | (−15.59, 5.33) |
| Distribution of LBP | 0.03 | 0.908 | (−0.47, 0.56) | −0.08 | 0.748 | (−0.60, 0.41) | −0.54 | 0.899 | (−7.91, 8.98) |
| Smoking | −0.60 | 0.10 | (−1.43, 0.11) | −0.64 | 0.091 † | (−1.54, 0.13) | −5.22 | 0.384 | (−19.74, 6.67) |
| Alcohol | −0.58 | 0.037 * | (−1.06, −0.03) ‡ | −0.50 | 0.077 † | (−1.01, 0.05) | −11.57 | 0.011 * | (−19.33, −2.97) ‡ |
| ROM | 0.18 | 0.447 | (−0.31, 0.64) | 0.03 | 0.905 | (−0.46, 0.49) | 4.65 | 0.229 | (−3.67, 12.44) |
| Smooth terms | edf | F-value | p-value | edf | F-value | p-value | edf | F-value | p-value |
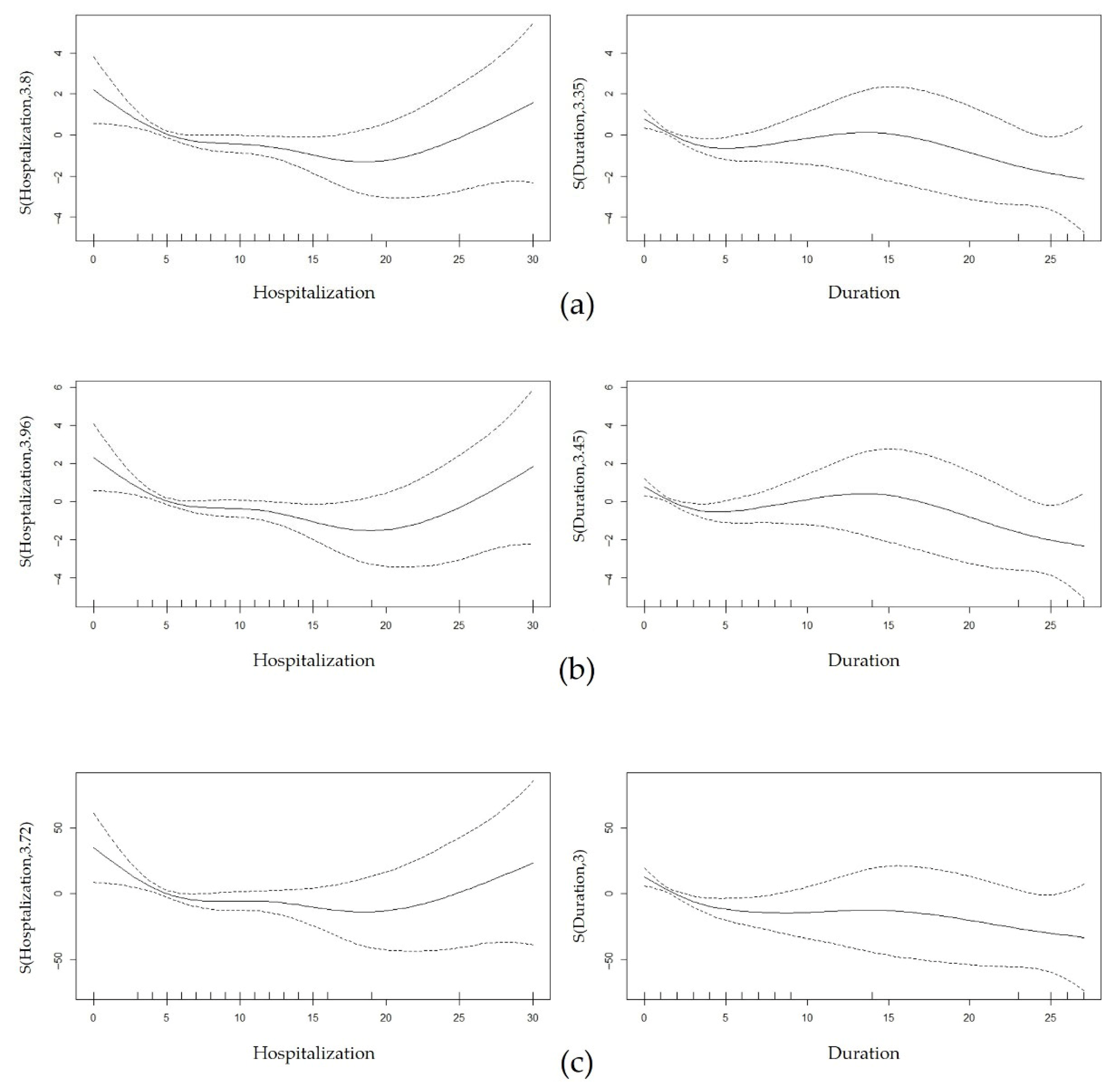
| Hospitalization length | 3.80 | 3.82 | 0.004 ** | 3.96 | 3.56 | 0.005 ** | 3.72 | 2.85 | 0.022 ** |
| Symptom duration | 3.35 | 4.24 | 0.003 ** | 3.45 | 3.75 | 0.006 ** | 3.00 | 4.98 | 0.001 ** |
| Model fit summary | n = 194 R2 (adjusted) = 0.417 Deviance explained = 46.9% | n = 194 R2 (adjusted) = 0.176 Deviance explained = 24.6% | n = 194 R2 (adjusted) = 0.168 Deviance explained = 23.6% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Han, S.-H.; Kim, M.-S.; Keum, D.-H.; Park, S.-H. Association Between Systemic Symptoms and Recovery in Acute Low Back Pain: A Retrospective Cross-Sectional Study. J. Clin. Med. 2025, 14, 6969. https://doi.org/10.3390/jcm14196969
Lee J-H, Han S-H, Kim M-S, Keum D-H, Park S-H. Association Between Systemic Symptoms and Recovery in Acute Low Back Pain: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(19):6969. https://doi.org/10.3390/jcm14196969
Chicago/Turabian StyleLee, Ji-Ho, Si-Hyun Han, Min-Su Kim, Dong-Ho Keum, and Seo-Hyun Park. 2025. "Association Between Systemic Symptoms and Recovery in Acute Low Back Pain: A Retrospective Cross-Sectional Study" Journal of Clinical Medicine 14, no. 19: 6969. https://doi.org/10.3390/jcm14196969
APA StyleLee, J.-H., Han, S.-H., Kim, M.-S., Keum, D.-H., & Park, S.-H. (2025). Association Between Systemic Symptoms and Recovery in Acute Low Back Pain: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine, 14(19), 6969. https://doi.org/10.3390/jcm14196969






