Frozen Shoulder as a Systemic Immunometabolic Disorder: The Roles of Estrogen, Thyroid Dysfunction, Endothelial Health, Lifestyle, and Clinical Implications
Abstract
1. Introduction
2. Methods
2.1. Sources and Time Frame
2.2. Eligibility Criteria
2.2.1. Inclusion
2.2.2. Exclusion
2.3. Study Selection and Data Handling
2.4. Evidence Appraisal and Synthesis
3. Current Knowledge and Latest Hypotheses on Frozen Shoulder Pathophysiology
4. The Female Sex Hormonal Axis: Neuroendocrine, Immunologic, and Metabolic Dimensions
4.1. Neuroendocrine Influence
4.2. Immunologic Modulation
4.3. Metabolic Regulation
5. Estrogen Deficiency, Resistance, and Metabolic Disruption in FS
5.1. Estrogen Resistance and Chronobiological Disruption
5.2. Hyperglycemia and AGEs
5.3. Dyslipidemia and Cholesterol Overload
5.4. Adipose Tissue Dysfunction and Endocrine Crosstalk in FS
5.5. Clinical and Molecular Implications in FS
5.6. Estrogen Deficiency in Menopause: A Missing Link in FS
5.7. Receptor-Level Interference by Metabolic and Environmental Factors
5.7.1. Environmental Estrogen Disruptors and Receptor Occupancy
5.7.2. Chronic Low-Grade Inflammation (LGI) and Receptor Dysfunction
5.7.3. Metabolic Disruptors: Diet, Obesity, and Mitochondrial Stress
5.7.4. Hormonal Crosstalk and Receptor Interference
6. A Paradigm Shift in FS Pathophysiology
6.1. Sleep, Circadian Rhythms, and Hormonal Crosstalk in FS
6.2. Thyroid Dysfunction in FS: A Key but Overlooked Axis
6.3. The Lifestyle Hypothesis: LGI as the Root Cause
The Lifestyle Hypothesis: Endothelial Health
7. Future Research Directions
7.1. Short-Term, Feasible Directions (12–24 Months)
- (A)
- Longitudinal endocrine monitoring in routine care
- (B)
- Endothelial–vascular assessments in FS
- (C)
- Sleep and circadian alignment pilots
- (D)
- Diet-first pragmatic trials
- (E)
- Focused micronutrient add-on trials (women-centric)
- (F)
- Integrative biomarker panels for stratification
7.2. Long-Term, Exploratory Programs (24–60+ Months)
- (G)
- Estrogen deficiency/resistance and receptor-level interference
- (H)
- Systems mapping of the vascular–immune–fibrotic axis
- (I)
- Chronobiology and sleep as disease modifiers
- (J)
- Gut microbiota and the gut–vascular–joint axis
- (K)
- Multi-omics and precision medicine frameworks
- (L)
- Scalable interventional platforms and implementation science
8. Clinical Implications
- Hormonal, metabolic, and vascular evaluation. Routine assessment of sex hormones (estradiol, progesterone, testosterone), thyroid function, cortisol rhythms, HbA1c, lipid profile, inflammatory mediators (e.g., IL-6, TNF-α), and vascular health (NO metabolites, ADMA, ICAM-1/VCAM-1) should be considered, particularly in women over 40 or in patients with metabolic risk factors.
- Lifestyle assessment and modification. Screening for sleep quality, circadian rhythm disruption, stress levels, physical activity, and dietary habits must become part of FS evaluation. Personalized interventions may include stress reduction strategies (e.g., mindfulness, CBT), structured physical activity programs, and dietary approaches such as anti-inflammatory, low-AGE, or phytoestrogen-rich diets to support estrogen signaling, endothelial health, and systemic metabolic balance.
- Targeting environmental and metabolic disruptors. Reducing exposure to endocrine-disrupting chemicals (e.g., BPA, phthalates, heavy metals) and addressing insulin and leptin resistance are essential for restoring endocrine sensitivity. Equally important is supporting mitochondrial function and reducing oxidative stress through antioxidant therapies, which may help normalize estrogen receptor activity and endothelial nitric oxide signaling.
- Gut–vascular–immune axis modulation. Given growing evidence of the role of dysbiosis and intestinal permeability in systemic inflammation, therapeutic approaches including prebiotics, probiotics, and microbiome-targeted nutrition could be explored as adjunctive strategies to mitigate endothelial dysfunction, reduce ADMA levels, and restore immune tolerance.
- Interdisciplinary management. Optimal care requires collaboration between orthopedic specialists, endocrinologists, physiotherapists, and nutritionists. Selected patients may benefit from hormonal support (e.g., bioidentical hormone therapy), correction of subclinical hypothyroidism, or integrative metabolic therapies following individualized risk–benefit evaluation.
- Patient education. Empowering patients with knowledge about how nutrition, sleep, stress, hormonal balance, and environmental exposures influence their condition can foster adherence to lifestyle changes, strengthen self-management, and improve long-term outcomes.
9. Limitations
10. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Sophie, A.; Franck, K.; Sebastian, P.; Joe, C.-H.C.; Patrick, S.; Pierre, H.; Alexandre, L. Frozen Shoulder Is Ill-Defined. How Can It Be Described Better? EFORT Open Rev. 2020, 5, 145–155. [Google Scholar] [CrossRef]
- Brindisino, F.; Turgut, E.; Struyf, F. Frozen Shoulder: Exploration of Terminology and Classification. Front. Rehabil. Sci. 2024, 5, 1498263. [Google Scholar] [CrossRef] [PubMed]
- Brindisino, F.; Silvestri, E.; Gallo, C.; Venturin, D.; Di Giacomo, G.; Peebles, A.M.; Provencher, M.T.; Innocenti, T. Depression and Anxiety Are Associated With Worse Subjective and Functional Baseline Scores in Patients With Frozen Shoulder Contracture Syndrome: A Systematic Review. Arthrosc. Sports Med. Rehabil. 2022, 4, e1219–e1234. [Google Scholar] [CrossRef]
- Lewis, J. Frozen Shoulder Contracture Syndrome—Aetiology, Diagnosis and Management. Man. Ther. 2015, 20, 2–9. [Google Scholar] [CrossRef]
- de la Serna, D.; Navarro-Ledesma, S.; Alayón, F.; López, E.; Pruimboom, L. A Comprehensive View of Frozen Shoulder: A Mystery Syndrome. Front. Med. 2021, 8, 663703. [Google Scholar] [CrossRef]
- Sarasua, S.M.; Floyd, S.; Bridges, W.C.; Pill, S.G. The Epidemiology and Etiology of Adhesive Capsulitis in the U.S. Medicare Population. BMC Musculoskelet. Disord. 2021, 22, 828. [Google Scholar] [CrossRef]
- Whelton, C.; Peach, C.A. Review of Diabetic Frozen Shoulder. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 363–371. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S.; Hamed-Hamed, D.; Pruimboom, L. A New Perspective of Frozen Shoulder Pathology; the Interplay between the Brain and the Immune System. Front. Physiol. 2024, 15, 1248612. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhu, Z.H.; Li, Q.; Zuo, Z.C.; Zhou, K.L. Causal Associations of Hypothyroidism with Frozen Shoulder: A Two-Sample Bidirectional Mendelian Randomization Study. BMC Musculoskelet. Disord. 2024, 25, 693. [Google Scholar] [CrossRef]
- Kean Ann Phua, S.; Si Ning Loh, R.; Tan, B.Y.; Wei Loong Ho, S. Does Concomitant Thyroid Disorder Lead to Worse Outcomes in Frozen Shoulder? A Systematic Review. J. Shoulder Elb. Surg. 2025, 34, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Date, A.; Rahman, L. Frozen Shoulder: Overview of Clinical Presentation and Review of the Current Evidence Base for Management Strategies. Future Sci. OA 2020, 6, FSO647. [Google Scholar] [CrossRef]
- Le, H.V.; Lee, S.J.; Nazarian, A.; Rodriguez, E.K. Adhesive Capsulitis of the Shoulder: Review of Pathophysiology and Current Clinical Treatments. Shoulder Elb. 2017, 9, 75–84. [Google Scholar] [CrossRef]
- Page, M.J.; Green, S.; Kramer, S.; Johnston, R.V.; Mcbain, B.; Chau, M.; Buchbinder, R. Manual Therapy and Exercise for Adhesive Capsulitis (Frozen Shoulder). Cochrane Database Syst. Rev. 2014, 2014. [Google Scholar] [CrossRef]
- Navarro-Ledesma, S. Frozen Shoulder as a Metabolic and Immune Disorder: Potential Roles of Leptin Resistance, JAK-STAT Dysregulation, and Fibrosis. J. Clin. Med. 2025, 14, 1780. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, S.; Thakur, R.; Ranjan, R. Prevalence of Frozen Shoulder in Post-Menopausal Women: A Cross-Sectional Study. J. Pharm. Negat. Results 2022, 13, 1124–1130. Available online: https://www.pnrjournal.com/index.php/home/article/view/10667 (accessed on 14 October 2025).
- Xu, J.-W.; Gong, J.; Chang, X.-M.; Luo, J.-Y.; Dong, L.; Hao, Z.-M.; Jia, A.; Xu, G.-P. Estrogen Reduces CCL 4-Induced Liver Fibrosis in Rats. World J. Gastroenterol. 2002, 8, 883. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Umar, S.; Ruffenach, G.; Aryan, L.; Li, J.; Sharma, S.; Motayagheni, N.; Nadadur, R.D.; Bopassa, J.C.; Eghbali, M. Estrogen Rescues Heart Failure through Estrogen Receptor Beta Activation. Biol. Sex. Differ. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, F.; Di, Z.; Xiong, Y.; Zhang, S.; Ma, Q.; Bian, X.; Lang, Z.; Ye, Q.; Wang, Y. Estradiol Ameliorates Acute Kidney Ischemia-Reperfusion Injury by Inhibiting the TGF-ΒRI-SMAD Pathway. Front. Immunol. 2022, 13, 822604. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.P. Idiopathic Frozen Shoulder. Aust. J. Gen. Pr. 2019, 48, 757–761. [Google Scholar] [CrossRef]
- Cho, C.H.; Song, K.S.; Kim, B.S.; Kim, D.H.; Lho, Y.M. Biological Aspect of Pathophysiology for Frozen Shoulder. Biomed. Res. Int. 2018, 2018, 7274517. [Google Scholar] [CrossRef]
- Akbar, M.; McLean, M.; Garcia-Melchor, E.; Crowe, L.A.N.; McMillan, P.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; McInnes, I.B.; et al. Fibroblast Activation and Inflammation in Frozen Shoulder. PLoS ONE 2019, 14, e0215301. [Google Scholar] [CrossRef]
- Ng, M.T.H.; Borst, R.; Gacaferi, H.; Davidson, S.; Ackerman, J.E.; Johnson, P.A.; Machado, C.C.; Reekie, I.; Attar, M.; Windell, D.; et al. A Single Cell Atlas of Frozen Shoulder Capsule Identifies Features Associated with Inflammatory Fibrosis Resolution. Nat. Commun. 2024, 15, 1394. [Google Scholar] [CrossRef]
- Struyf, F.; Mertens, M.G.C.A.M.; Navarro-Ledesma, S. Causes of Shoulder Dysfunction in Diabetic Patients: A Review of Literature. Int. J. Environ. Res. Public Health 2022, 19, 6228. [Google Scholar] [CrossRef]
- Hwang, K.R.; Murrell, G.A.C.; Millar, N.L.; Bonar, F.; Lam, P.; Walton, J.R. Advanced Glycation End Products in Idiopathic Frozen Shoulders. J. Shoulder Elb. Surg. 2016, 25, 981–988. [Google Scholar] [CrossRef]
- Kraal, T.; Lübbers, J.; van den Bekerom, M.P.J.; Alessie, J.; van Kooyk, Y.; Eygendaal, D.; Koorevaar, R.C.T. The Puzzling Pathophysiology of Frozen Shoulders—A Scoping Review. J. Exp. Orthop. 2020, 7, 91. [Google Scholar] [CrossRef]
- Bunker, T.D.; Reilly, J.; Baird, K.S.; Hamblen, D.L. Expression of Growth Factors, Cytokines and Matrix Metalloproteinases in Frozen Shoulder. J. Bone Jt. Surg.—Ser. B 2000, 82, 768–773. [Google Scholar] [CrossRef]
- Lho, Y.M.; Ha, E.; Cho, C.H.; Song, K.S.; Min, B.W.; Bae, K.C.; Lee, K.J.; Hwang, I.; Park, H. Bin Inflammatory Cytokines Are Overexpressed in the Subacromial Bursa of Frozen Shoulder. J. Shoulder Elb. Surg. 2013, 22, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Cher, J.Z.B.; Akbar, M.; Kitson, S.; Crowe, L.A.N.; Garcia-Melchor, E.; Hannah, S.C.; McLean, M.; Fazzi, U.G.; Kerr, S.C.; Murrell, G.A.C.; et al. Alarmins in Frozen Shoulder: A Molecular Association Between Inflammation and Pain. Am. J. Sports Med. 2018, 46, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Ledesma, S.; Hamed-Hamed, D.; Gonzalez-Muñoz, A.; Pruimboom, L. Impact of Physical Therapy Techniques and Common Interventions on Sleep Quality in Patients with Chronic Pain: A Systematic Review. Sleep Med. Rev. 2024, 76, 101937. [Google Scholar] [CrossRef]
- Brindisino, F.; Rizzo, V.; Dejaco, B.; Andriesse, A.; Verardo, A.; Turolla, A. Association between Pain, Stiffness, Mood, Catastrophizing, and Perceived Social Support, in Subjects with Frozen Shoulder: A Cross-Sectional Study. Shoulder Elb. 2025; ahead of print. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Liu, X.; Yang, Y.; Yan, Y.; Cui, D.; Meng, C.; Ali, M.I.; Zhang, J.; Yao, Z.; et al. Mechanistic Insights into the Anti-Fibrotic Effects of Estrogen via the PI3K-Akt Pathway in Frozen Shoulder. J. Steroid Biochem. Mol. Biol. 2025, 249, 106701. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, F.; Silveira, G.; Maccoll, G.S.; Bouloux, P.M.G. The hypothalamic-pituitary-gonadal axis: Immune function and autoimmunity. J. Endocrinol. 2003, 176, 293–304. [Google Scholar] [CrossRef]
- Athnaiel, O.; Cantillo, S.; Paredes, S.; Knezevic, N.N. The Role of Sex Hormones in Pain-Related Conditions. Int. J. Mol. Sci. 2023, 24, 1866. [Google Scholar] [CrossRef]
- Vegeto, E.; Benedusi, V.; Maggi, A. Estrogen Anti-Inflammatory Activity in Brain: A Therapeutic Opportunity for Menopause and Neurodegenerative Diseases. Front. Neuroendocr. 2008, 29, 507–519. [Google Scholar] [CrossRef]
- Ansar Ahmed, S.; Penhale, W.J.; Talal, N. Sex Hormones, Immune Responses, and Autoimmune Diseases Mechanisms of Sex Hormone Action. Am. J. Pathol. 1985, 121, 531. [Google Scholar]
- Maurer, A.J.; Lissounov, A.; Knezevic, I.; Candido, K.D.; Knezevic, N.N. Pain and Sex Hormones: A Review of Current Understanding. Pain. Manag. 2016, 6, 258–296. [Google Scholar] [CrossRef]
- Aloisi, A.M. Gonadal Hormones and Sex Differences in Pain Reactivity. Clin. J. Pain. 2003, 19, 168–174. [Google Scholar] [CrossRef]
- Arjmand, S.; Ilaghi, M.; Sisakht, A.K.; Guldager, M.B.; Wegener, G.; Landau, A.M.; Gjedde, A. Regulation of Mitochondrial Dysfunction by Estrogens and Estrogen Receptors in Alzheimer’s Disease: A Focused Review. Basic. Clin. Pharmacol. Toxicol. 2024, 135, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Korszun, A. Sex, Trauma, Stress Hormones and Depression. Mol. Psychiatry 2010, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.J.; Mani, S.K.; Uht, R.M. Estrogen Receptors and the Regulation of Neural Stress Responses. Neuroendocrinology 2012, 96, 111–118. [Google Scholar] [CrossRef]
- Rebecca, M. Modulation of Pain by Estrogens. Pain 2007, 132, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lan, H.Q.; Liao, Z.H.; Huang, J.W.; Jian, X.T.; Hu, J.; Liao, H. Regulatory T Cells-Centered Regulatory Networks of Skeletal Muscle Inflammation and Regeneration. Cell Biosci 2022, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Enright, S.; Werstuck, G.H. Investigating the Effects of Sex Hormones on Macrophage Polarization. Int. J. Mol. Sci. 2024, 25, 951. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhao, X.; Sun, S.C. NF-ΚB in Inflammation and Cancer. Cell Mol. Immunol. 2025, 22, 811–839. [Google Scholar] [CrossRef]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen Regulation of Adiposity and Fuel Partitioning: Evidence of Genomic and Non-Genomic Regulation of Lipogenic and Oxidative Pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef]
- Barros, R.P.A.; Gustafsson, J.Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A Master Regulator of Bioenergetic Systems in the Brain and Body. Front. Neuroendocr. 2014, 35, 8–30. [Google Scholar] [CrossRef]
- Ryan, V.; Brown, H.; Minns Lowe, C.J.; Lewis, J.S. The Pathophysiology Associated with Primary (Idiopathic) Frozen Shoulder: A Systematic Review. BMC Musculoskelet. Disord. 2016, 17, 340. [Google Scholar] [CrossRef]
- Konarski, W.; Poboży, T.; Hordowicz, M.; Poboży, K.; Domańska, J. Current Concepts of Natural Course and in Management of Frozen Shoulder: A Clinical Overview. Orthop. Rev. 2020, 12, 186–190. [Google Scholar] [CrossRef]
- Ryu, J.D.; Kirpalani, P.A.; Kim, J.M.; Nam, K.H.; Han, C.W.; Han, S.H. Expression of Vascular Endothelial Growth Factor and Angiogenesis in the Diabetic Frozen Shoulder. J. Shoulder Elb. Surg. 2006, 15, 679–685. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. How Cells Handle Cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef]
- Stocco, D.M. The Role of the StAR Protein in Steroidogenesis: Challenges for the Future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroid Hormone Synthesis in Mitochondria. Mol. Cell Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F. All Sex Steroids Are Made Intracellularly in Peripheral Tissues by the Mechanisms of Intracrinology after Menopause. J. Steroid Biochem. Mol. Biol. 2015, 145, 133–138. [Google Scholar] [CrossRef]
- Hamed-Hamed, D.; Rodríguez-Pérez, C.; Pruimboom, L.; Navarro-Ledesma, S. Influence of the Metabolic and Inflammatory Profile in Patients with Frozen Shoulder—Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2025, 26, 475. [Google Scholar]
- Hamed Hamed, D.; Rodríguez-Pérez, C.; Pruimboom, L.; Navarro-Ledesma, S. Relationship Between Metabolic Profile, Pain, and Functionality in Patients with Frozen Shoulder: A Cross-Sectional Study. Healthcare 2024, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Virella, M.F.; Klein, R.L.; Lyons, T.J.; Stevenson, H.C.; Witztum, J.L. Glycosylation of Low-Density Lipoprotein Enhances Cholesteryl Ester Synthesis in Human Monocyte-Derived Macrophages. Diabetes 1988, 37, 550–557. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-Specific Epitopes Are Danger-Associated Molecular Patterns Recognized by Pattern Recognition Receptors of Innate Immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, A.N.; Sivagurunathan, B.; Monaco, C. Toll-like Receptors and Macrophage Activation in Atherosclerosis. Clin. Chim. Acta 2012, 413, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Lubis, A.M.T.; Lubis, V.K. Matrix Metalloproteinase, Tissue Inhibitor of Metalloproteinase and Transforming Growth Factor-Beta 1 in Frozen Shoulder, and Their Changes as Response to Intensive Stretching and Supervised Neglect Exercise. J. Orthop. Sci. 2013, 18, 519–527. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of Estrogen and Their Importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Nilsson, S.; Gustafsson, J.Å. Estrogen Receptors: Therapies Targeted to Receptor Subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, Inflammation and Cognition. Front. Neuroendocr. 2016, 40, 87–100. [Google Scholar] [CrossRef]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Estrogen and Androgen Receptors: Regulators of Fuel Homeostasis and Emerging Targets for Diabetes and Obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Manson, J.A.E.; Stevenson, J.C.; Fonseca, V.A. Menopausal Hormone Therapy and Type 2 Diabetes Prevention: Evidence, Mechanisms, and Clinical Implications. Endocr. Rev. 2017, 38, 173–188. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex Differences in Obesity, Lipid Metabolism, and Inflammation—A Role for the Sex Chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef]
- Nilsen, J.; Diaz Brinton, R. Impact of Progestins on Estrogen-Induced Neuroprotection: Synergy by Progesterone and 19-Norprogesterone and Antagonism by Medroxyprogesterone Acetate. Endocrinology 2002, 143, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.M.; Liu, R.Y.; van Someren, E.J.W.; Hofman, M.A.; Cao, Y.X.; Zhou, J.N. Diurnal Rhythm of Free Estradiol during the Menstrual Cycle. Eur. J. Endocrinol. 2003, 148, 227–232. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex Differences in Sleep, Circadian Rhythms, and Metabolism: Implications for Precision Medicine. Sleep Med. Rev. 2024, 75, 101926. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Silver, R. Sex Differences in Circadian Timing Systems: Implications for Disease. Front. Neuroendocr. 2014, 35, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.L.; Resuehr, D.; Johnson, C.H. Shift Work and Circadian Dysregulation of Reproduction. Front. Endocrinol. 2013, 4, 92. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of Life: Circadian Disruption and Brain Disorders across the Lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Hale, G.E.; Zhao, X.; Hughes, C.L.; Burger, H.G.; Robertson, D.M.; Fraser, I.S. Endocrine Features of Menstrual Cycles in Middle and Late Reproductive Age and the Menopausal Transition Classified According to the Staging of Reproductive Aging Workshop (STRAW) Staging System. J. Clin. Endocrinol. Metab. 2007, 92, 3060–3067. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Yano, T.; Hagiwara, Y.; Ando, A.; Kanazawa, K.; Koide, M.; Sekiguchi, T.; Itaya, N.; Onoki, T.; Suzuki, K.; Tsuchiya, M.; et al. RAGE-Dependent NF-KB Inflammation Processes in the Capsule of Frozen Shoulders. J. Shoulder Elb. Surg. 2020, 29, 1884–1891. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.R.; Li, P.C.; Feng, B. Advanced Glycation End Products Increase Lipids Accumulation in Macrophages through Upregulation of Receptor of Advanced Glycation End Products: Increasing Uptake, Esterification and Decreasing Efflux of Cholesterol. Lipids Health Dis. 2016, 15, 161. [Google Scholar] [CrossRef]
- Miller, W.L.; Bose, H.S. Early Steps in Steroidogenesis: Intracellular Cholesterol Trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc Med. 2022, 9, 925923. [Google Scholar] [CrossRef] [PubMed]
- Kilic, G.; Alvarez-Mercado, A.I.; Zarrouki, B.; Opland, D.; Liew, C.W.; Alonso, L.C.; Myers, M.G.; Jonas, J.C.; Poitout, V.; Kulkarni, R.N.; et al. The Islet Estrogen Receptor-α Is Induced by Hyperglycemia and Protects against Oxidative Stress-Induced Insulin-Deficient Diabetes. PLoS ONE 2014, 9, e87941. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Davidge, S.T. High Glucose-Induced Oxidative Stress Alters Estrogen Effects on ERα and ERβ in Human Endothelial Cells: Reversal by AMPK Activator. J. Steroid Biochem. Mol. Biol. 2009, 117, 99–106. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Shen, W.J.; Azhar, S. Cellular Cholesterol Delivery, Intracellular Processing and Utilization for Biosynthesis of Steroid Hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef]
- Pérez-Montilla, J.J.; Guzmán-García, R.; Pruimboom, L.; Navarro-Ledesma, S. Does Leptin and Insulin Levels Influence Pain and Disability in Subjects With Frozen Shoulder? A Cross-Sectional Study. Eur. J. Pain. 2025, 29, e70007. [Google Scholar] [CrossRef]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered Lipid Metabolism and the Pathogenesis of Insulin Resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid Med. Cell Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Nelson, E.R. 27-Hydroxycholesterol, an Endogenous Selective Estrogen Receptor Modulator. Maturitas 2017, 104, 29–35. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Austin, D.C.; Gans, I.; Park, M.J.; Carey, J.L.; Kelly, J.D. The Association of Metabolic Syndrome Markers with Adhesive Capsulitis. J. Shoulder Elb. Surg. 2014, 23, 1043–1051. [Google Scholar] [CrossRef]
- Raut, P.K.; Choi, D.Y.; Kim, S.H.; Hong, J.T.; Kwon, T.K.; Jeong, J.H.; Park, P.-H. Estrogen Receptor Signaling Mediates Leptin-Induced Growth of Breast Cancer Cells via Autophagy Induction. Oncotarget 2017, 8, 109417. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Dama, A.; Baggio, C.; Boscaro, C.; Albiero, M.; Cignarella, A. Estrogen Receptor Functions and Pathways at the Vascular Immune Interface. Int. J. Mol. Sci. 2021, 22, 4254. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.; Zampieri, T.T.; Pedroso, J.A.B.; Nagaishi, V.S.; Ramos-Lobo, A.M.; Furigo, I.C.; Câmara, N.O.; Frazão, R.; Donato, J. Leptin Resistance Is Not the Primary Cause of Weight Gain Associated with Reduced Sex Hormone Levels in Female Mice. Endocrinology 2014, 155, 4226–4236. [Google Scholar] [CrossRef]
- Zheng, J.; Du, M.; Ye, W.; Xie, J.; Zhang, P.; Huang, C.; Lin, H. Editorial: Molecular Mechanisms and Therapeutic Strategies in Inflammation. Front. Immunol. 2025, 16. [Google Scholar] [CrossRef]
- Hetemäki, N.; Robciuc, A.; Vihma, V.; Haanpää, M.; Hämäläinen, E.; Tikkanen, M.J.; Mikkola, T.S.; Savolainen-Peltonen, H. Adipose Tissue Sex Steroids in Postmenopausal Women With and Without Menopausal Hormone Therapy. J. Clin. Endocrinol. Metab. 2025, 110, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Yuxin, L.; Chen, L.; Xiaoxia, L.; Yue, L.; Junjie, L.; Youzhu, L.; Huiliang, Z.; Qicai, L. Research Progress on the Relationship between Obesity-Inflammation-Aromatase Axis and Male Infertility. Oxid Med. Cell Longev. 2021, 2021, 6612796. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.M.; Gaw, R.; MacLean, M.R. Obesity, Estrogens and Adipose Tissue Dysfunction—Implications for Pulmonary Arterial Hypertension. Pulm. Circ. 2020, 10, 2045894020952019. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Hernández-Ledesma, B.; Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Blüher, M. Importance of Estrogen Receptors in Adipose Tissue Function. Mol. Metab. 2013, 2, 130–132. [Google Scholar] [CrossRef]
- Eaton, S.A.; Sethi, J.K. Immunometabolic Links between Estrogen, Adipose Tissue and Female Reproductive Metabolism. Biology 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.M.; Berry, D.C. The Regulation of Adipose Tissue Health by Estrogens. Front. Endocrinol. 2022, 13, 889923. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-Stimulated MSC-Derived Exosomes Improve Diabetic Wound Healing through Regulating Macrophage M1 and M2 Polarization by Targeting the PTEN/AKT Pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and Alarmins: All We Need to Know about Danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. The Antioxidant Neuroprotective Effects of Estrogens and Phenolic Compounds Are Independent from Their Estrogenic Properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Sadana, N.; Chen, X. Estrogen Receptors in Pain Modulation: Cellular Signaling. Biol. Sex Differ. 2021, 12, 22. [Google Scholar] [CrossRef]
- Longo, U.G.; Mazzola, A.; Carotti, S.; Francesconi, M.; Catapano, S.; Magrì, F.; Perrone, G.; Morini, S.; De Salvatore, S.; Denaro, V. The Role of Estrogen and Progesterone Receptors in the Rotator Cuff Disease: A Retrospective Cohort Study. BMC Musculoskelet. Disord. 2021, 22, 891. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Li, Y.; Huang, Y.; Li, P.; Zhang, Y.; Xu, G.; Sun, K. Estrogen Inhibits TGF-Β1-Stimulated Cardiac Fibroblast Differentiation and Collagen Synthesis by Promoting Cdc42. Mol. Med. Rep. 2024, 30, 123. [Google Scholar] [CrossRef]
- Batty, M.J.; Chabrier, G.; Sheridan, A.; Gage, M.C. Metabolic Hormones Modulate Macrophage Inflammatory Responses. Cancers 2021, 13, 4661. [Google Scholar] [CrossRef] [PubMed]
- Belboul, A.; Ashworth, J.; Fadel, A.; Mcloughlin, J.; Mahmoud, A.; El Mohtadi, M. Estrogen Induces the Alternative Activation of Macrophages through Binding to Estrogen Receptor-Alpha. Exp. Mol. Pathol. 2025, 143, 104971. [Google Scholar] [CrossRef] [PubMed]
- Mafu, T.S.; September, A.V.; Shamley, D. The Potential Role of Angiogenesis in the Development of Shoulder Pain, Shoulder Dysfunction, and Lymphedema after Breast Cancer Treatment. Cancer Manag. Res. 2018, 10, 81–90. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, P.; Fahmi, N.; Garg, B.; Dutta, S.; Sachar, S.; Matharu, A.S.; Vimaleswaran, K.S. Endocrine Disruption and Obesity: A Current Review on Environmental Obesogens. Curr. Res. Green Sustain. Chem. 2020, 3, 100009. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by Stealth—Interference of Endocrine Disrupting Chemicals on Hormonal Crosstalk with Thyroid Axis Function in Humans and Other Animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Tamai, K.; Hamada, J.; Nagase, Y.; Morishige, M.; Naito, M.; Asai, H.; Tanaka, S. Frozen Shoulder. An Overview of Pathology and Biology with Hopes to Novel Drug Therapies. Mod. Rheumatol. 2024, 34, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Capellino, S.; Sulli, A.; Serioli, B.; Secchi, M.E.; Villaggio, B.; Straub, R.H. Estrogens and autoimmune diseases. Ann. N Y Acad. Sci. 2006, 1089, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Pruimboom, L.; Alayon Rocio, F.; Navarro-Ledesma, S. Psychoneuroimmunology in the Daily Clinic Is Only Possible Within a Contextual Frame. In PsychoNeuroImmunology: Integration of Psychology, Neurology, and Immunology; Springer Nature: Berlin/Heidelberg, Germany, 2025; Volume 1, pp. 515–563. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- De Punder, K.; Pruimboom, L. Stress Induces Endotoxemia and Low-Grade Inflammation by Increasing Barrier Permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Bosma-Den Boer, M.M.; Van Wetten, M.L.; Pruimboom, L. Chronic Inflammatory Diseases Are Stimulated by Current Lifestyle: How Diet, Stress Levels and Medication Prevent Our Body from Recovering. Nutr. Metab. 2012, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis—No Longer a Theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef]
- Dai, M.; Mei, B.; Zheng, F.; Ballestar, E. Sex Hormones and Epigenetic Dysregulation in Autoimmune Disease. Curr. Opin. Immunol. 2025, 95, 102595. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed Food and Chronic Noncommunicable Diseases: A Systematic Review and Meta-Analysis of 43 Observational Studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Glycemic Index Physiological Mechanisms Relating to Obesity, Diabetes, and Cardiovascular Disease. Jama 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, T.; Liu, H. Leptin Signaling and Its Central Role in Energy Homeostasis. Front. Neurosci. 2023, 17, 1238528. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It Is All about Energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Shafarin, J.; Taneera, J.; Hamad, M. Estrogen Signaling Induces Mitochondrial Dysfunction-Associated Autophagy and Senescence in Breast Cancer Cells. Biology 2020, 9, 68. [Google Scholar] [CrossRef]
- Vianello, E.; Beltrami, A.P.; Aleksova, A.; Janjusevic, M.; Fluca, A.L.; Corsi Romanelli, M.M.; La Sala, L.; Dozio, E. The Advanced Glycation End-Products (AGE)–Receptor for AGE System (RAGE): An Inflammatory Pathway Linking Obesity and Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 3707. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 1043, pp. 227–256. [Google Scholar]
- Xiao, Z.; Liu, H. The Estrogen Receptor and Metabolism. Women’s Health 2024, 20, 17455057241227362. [Google Scholar] [CrossRef]
- Mahboobifard, F.; Pourgholami, M.H.; Jorjani, M.; Dargahi, L.; Amiri, M.; Sadeghi, S.; Tehrani, F.R. Estrogen as a Key Regulator of Energy Homeostasis and Metabolic Health. Biomed. Pharmacother. 2022, 156, 113808. [Google Scholar] [CrossRef]
- Safe, S.; Kim, K. Non-Classical Genomic Estrogen Receptor (ER)/Specificity Protein and ER/Activating Protein-1 Signaling Pathways. J. Mol. Endocrinol. 2008, 41, 263–275. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Bulun, S.E.; Simpson, E.R. Regulation of Aromatase Expression in Human Tissues. Breast Cancer Res. Treat. 1994, 30, 19–29. [Google Scholar] [CrossRef]
- Fattahi, R.; Chamkhorami, F.M.; Taghipour, N.; Keshel, S.H. The Effect of Extracellular Matrix Remodeling on Material-Based Strategies for Bone Regeneration: Review Article. Tissue Cell 2022, 76, 101748. [Google Scholar] [CrossRef]
- Zomer, H.D.; Cooke, P.S. Targeting Estrogen Signaling and Biosynthesis for Aged Skin Repair. Front. Physiol. 2023, 14, 1281071. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Dong, W.; Peng, Q.; Liu, Z.; Xie, Z.; Guo, X.; Li, Y.; Chen, C. Estrogen Plays an Important Role by Influencing the NLRP3 Inflammasome. Biomed. Pharmacother. 2023, 167, 115554. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Chong, H.C.; Tan, E.H.P.; Tan, N.S. Getting “Smad” about Obesity and Diabetes. Nutr. Diabetes 2012, 2, e29. [Google Scholar] [CrossRef] [PubMed]
- Atasoy-Zeybek, A.; Showel, K.K.; Nagelli, C.V.; Westendorf, J.J.; Evans, C.H. The Intersection of Aging and Estrogen in Osteoarthritis. npj Women’s Health 2025, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, L.N.; Weljie, A.M. Cross-Species Physiological Interactions of Endocrine Disrupting Chemicals with the Circadian Clock. Gen. Comp. Endocrinol. 2021, 301, 113650. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune System: An Emerging Player in Mediating Effects of Endocrine Disruptors on Metabolic Health. Endocrinology 2018, 159, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Koop, S.; Oster, H. Eat, Sleep, Repeat—Endocrine Regulation of Behavioural Circadian Rhythms. FEBS J. 2022, 289, 6543–6558. [Google Scholar] [CrossRef]
- Alvord, V.M.; Kantra, E.J.; Pendergast, J.S. Estrogens and the Circadian System. Semin. Cell Dev. Biol. 2022, 126, 56–65. [Google Scholar] [CrossRef]
- Guzmán-García, R.; Pérez-Montalbán, M.; Pruimboom, L.; Navarro-Ledesma, S. Impact of a Multimodal Intervention Combining Manual Therapy, Exercise, Reduced Methylxanthine Intake, and Nocturnal Light Avoidance on Inflammatory and Metabolic Profiles, Pain, Functionality, and Sleep Quality in Patients with Frozen Shoulder: A Single-Blind Randomized Controlled Trial. J. Clin. Med. 2025, 14, 4539. [Google Scholar] [CrossRef]
- Beroukhim, G.; Esencan, E.; Seifer, D.B. Impact of Sleep Patterns upon female neuroendocrinology and Reproductive Outcomes: A Comprehensive Review. Reprod. Biol. Endocrinol. 2022, 20, 16. [Google Scholar] [CrossRef]
- Kim, T.W.; Jeong, J.H.; Hong, S.C. The Impact of Sleep and Circadian Disturbance on Hormones and Metabolism. Int. J. Endocrinol. 2015, 2015, 591729. [Google Scholar] [CrossRef]
- Zhou, P.; Li, H.; Li, H.; Chen, Y.; Lv, Y. A Possible Important Regulatory Role of Estrogen in Obstructive Sleep Apnea Hypoventilation Syndrome. Front. Med. 2025, 12, 1369393. [Google Scholar] [CrossRef]
- Carter, E. Examining the Effects of Cortisol on Insulin. Endocrinol. Diabetes Res. 2024, 10, 5. [Google Scholar]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of Insulin and Insulin Resistance in Androgen Excess Disorders. World J. Diabetes 2021, 12, 616–629. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules 2018, 23, 336. [Google Scholar] [CrossRef]
- Huang, W.; Zheng, J.; Wang, M.; Du, L.Y.; Bai, L.; Tang, H. The Potential Therapeutic Role of Melatonin in Organ Fibrosis: A Comprehensive Review. Front. Med. 2024, 11, 1502368. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening Use of Light-Emitting EReaders Negatively Affects Sleep, Circadian Timing, and next-Morning Alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Sleep and Inflammation: Partners in Sickness and in Health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Schiefer, M.; Teixeira, P.F.S.; Fontenelle, C.; Carminatti, T.; Santos, D.A.; Righi, L.D.; Conceição, F.L. Prevalence of Hypothyroidism in Patients with Frozen Shoulder. J. Shoulder Elb. Surg. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Tran, M.; Stovitz, S.D. Prevalence of Diabetes Mellitus and Thyroid Disease in Patients Diagnosed with Adhesive Capsulitis (Frozen Shoulder). Ann. Sports Med. Res. 2021, 8, 1178. [Google Scholar]
- Chuang, S.H.; Chen, Y.P.; Huang, S.W.; Kuo, Y.J. Association between Adhesive Capsulitis and Thyroid Disease: A Meta-Analysis. J. Shoulder Elb. Surg. 2023, 32, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Merino, E.; Orozco, R.M.; Ruíz-Llorente, L.; Martínez-Iglesias, O.A.; Velasco-Martín, J.P.; Montero-Pedrazuela, A.; Fanjul-Rodríguez, L.; Contreras-Jurado, C.; Regadera, J.; Aranda, A. Thyroid Hormones Inhibit TGF-β Signaling and Attenuate Fibrotic Responses. Proc. Natl. Acad. Sci. USA 2016, 113, E3451–E3460. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Salah, S. Insulin Resistance, Leptin and Adiponectin in Lean and Hypothyroid Children and Adolescents with Obesity. BMC Pediatr. 2022, 22, 245. [Google Scholar] [CrossRef]
- Trotta, M.C.; Esposito, D.; Carotenuto, R.; di Fraia, R.; Selvaggio, L.D.; Allosso, F.; Russo, M.; Accardo, G.; Alfano, R.; D’Amico, M.; et al. Thyroid Dysfunction in Hashimoto’s Thyroiditis: A Pilot Study on the Putative Role of MiR-29a and TGFβ1. Endocrine 2024, 86, 1090–1096. [Google Scholar] [CrossRef]
- Brown, E.D.L.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The Thyroid Hormone Axis and Female Reproduction. Int. J. Mol. Sci. 2023, 24, 9815. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Marouli, E.; Papadopoulou, A.; Deloukas, P.; Kuś, A.; Sterenborg, R.; Teumer, A.; Burgess, S.; Åsvold, B.O.; Chasman, D.I.; et al. Thyroid Function, Sex Hormones and Sexual Function: A Mendelian Randomization Study. Eur. J. Epidemiol. 2021, 36, 335–344. [Google Scholar] [CrossRef]
- Sutkowska, E.; Kisiel, M.; Zubkiewicz-Kucharska, A. When Should the Treatment of Obesity in Thyroid Disease Begin? Biomedicines 2025, 13, 157. [Google Scholar] [CrossRef]
- Song, C.H.; Kim, N.; Kim, D.H.; Lee, H.N.; Surh, Y.J. 17-β Estradiol Exerts Anti-Inflammatory Effects through Activation of Nrf2 in Mouse Embryonic Fibroblasts. PLoS ONE 2019, 14, e0221650. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease, and Therapeutics. Signal Transduct. Target Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Böhm, M.; Nickenig, G. Modulation of Antioxidant Enzyme Expression and Function by Estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef]
- Sun, J.X.; Xu, X.H.; Jin, L. Effects of Metabolism on Macrophage Polarization Under Different Disease Backgrounds. Front. Immunol. 2022, 13, 880286. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Russart, K.L.G.; Nelson, R.J. Light at Night as an Environmental Endocrine Disruptor. Physiol. Behav. 2018, 190, 82–89. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Monk, J.M.; Robinson, L.E.; Mourtzakis, M. The Integrative Role of Leptin, Oestrogen and the Insulin Family in Obesity-Associated Breast Cancer: Potential Effects of Exercise. Obes. Rev. 2015, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of Leptin on the Skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Haskologlu, I.C.; Erdag, E. Molecular Links Between Circadian Rhythm Disruption, Melatonin, and Neurodegenerative Diseases: An Updated Review. Molecules 2025, 30, 1888. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Inflammation—Story of a Double-Edged Blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Huang, X.; Pan, C.H.; Yin, F.; Peng, J.; Yang, L. The Role of Estrogen in Mitochondrial Disease. Cell Mol. Neurobiol. 2025, 45, 68. [Google Scholar] [CrossRef]
- Sánchez-Robalino, A.; Sinchi-Sinchi, H.; Ramírez, A. Effectiveness of Pain Neuroscience Education in Physical Therapy: A Systematic Review and Meta-Analysis. Brain Sci. 2025, 15, 658. [Google Scholar] [CrossRef]
- Watson, J.A.; Ryan, C.G.; Cooper, L.; Ellington, D.; Whittle, R.; Lavender, M.; Dixon, J.; Atkinson, G.; Cooper, K.; Martin, D.J. Pain Neuroscience Education for Adults With Chronic Musculoskeletal Pain: A Mixed-Methods Systematic Review and Meta-Analysis. J. Pain. 2019, 20, e1–e1140. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Martínez, F.; Suso-Martí, L.; Calatayud, J.; Ferrer-Sargues, F.J.; Muñoz-Alarcos, V.; Alba-Quesada, P.; Biviá-Roig, G. Pain Neuroscience Education in Patients with Chronic Musculoskeletal Pain: An Umbrella Review. Front. Neurosci. 2023, 17, 1272068. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Maharana, K.C.; Meenakshi, S.; Singh, S. Endothelial Dysfunction and Its Relation in Different Disorders: Recent Update. Health Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Liang, F.; Yang, Y.; Lu, X.; Gu, D. Air Pollution Exposure and Vascular Endothelial Function: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. 2023, 30, 28525–28549. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient Air Pollution and Cardiovascular Diseases: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Böger, R.H. The Emerging Role of Asymmetric Dimethylarginine as a Novel Cardiovascular Risk Factor. Cardiovasc. Res. 2003, 59, 824–833. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The Microbiome and Innate Immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The Intestinal Microbiota Fuelling Metabolic Inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Böger, R.H.; Maas, R.; Schulze, F.; Schwedhelm, E. Asymmetric Dimethylarginine (ADMA) as a Prospective Marker of Cardiovascular Disease and Mortality-An Update on Patient Populations with a Wide Range of Cardiovascular Risk. Pharmacol. Res. 2009, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Mustata, G.T.; Biemel, K.L.; Reihl, O.; Lederer, M.O.; Zhenyu, D.; Sell, D.R. Cross-Linking of the Extracellular Matrix by the Maillard Reaction in Aging and Diabetes: An Update on “a Puzzle Nearing Resolution”. In Annals of the New York Academy of Sciences; New York Academy of Sciences: New York, NY, USA, 2005; Volume 1043, pp. 533–544. [Google Scholar]
- Taguchi, K.; Fukami, K. RAGE Signaling Regulates the Progression of Diabetic Complications. Front. Pharmacol. 2023, 14, 1128872. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial Dysfunction and Angiogenesis Impairment in the Ageing Vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef] [PubMed]
| Domain | Study Type | Core Finding | Implication for FS |
|---|---|---|---|
| Estrogen axis | Clinical (SR/MA, cohorts) | Higher prevalence and worse outcomes in peri/postmenopausal women; metabolic comorbidity associations; ER expression in glenohumeral capsule biopsies. | Estrogen deficiency/resistance may underlie inflammation, pain, and fibrosis. |
| Estrogen axis | Experimental (in vitro/animal) | E2 inhibits NF-κB, IL-1β, IL-6, TNF-α; modulates TGF-β1 and MMP/TIMP; antioxidant and antinociceptive effects. | Supports hypothesis of receptor-level resistance in FS. |
| Thyroid axis | Clinical (SR/MA, MR, cohorts) | Overt and subclinical hypothyroidism ↑ risk of FS; causal support from Mendelian randomization; weaker evidence for hyperthyroidism. | Thyroid dysfunction contributes to fibrosis and pain; screening recommended. |
| Thyroid axis | Experimental (animal/in vitro) | Low T3/T4 → ↓MMPs, ↑TGF-β, ECM accumulation, impaired collagen turnover. | Mechanistic link between hypothyroidism and capsular fibrosis. |
| Estrogen–Thyroid crosstalk | Translational | Hypothyroidism alters SHBG, insulin and leptin resistance → reduced estrogen bioavailability/action. | Functional estrogen resistance in thyroid dysfunction. |
| Domain | Study Type | Core Finding | Implication for FS |
|---|---|---|---|
| Endothelial dysfunction (ED) | Clinical (biomarkers/imaging) | ED associated with LGI, dyslipidemia, oxidative stress; impaired microvascular perfusion. | Local hypoperfusion and abnormal angiogenesis → fibrosis and pain. |
| Nitric oxide (NO) biology | Experimental/Translational | ADMA, homocysteine, ROS/RNS reduce eNOS activity; peroxynitrite ↑ tissue damage. | Impaired NO signaling favors pro-fibrotic environment. |
| AGEs–RAGE axis | Clinical (FS biopsies) | Elevated AGEs in FS capsules; RAGE/NF-κB activation confirmed. | AGEs act as active mediators of stiffness and fibrosis. |
| Lipids/inflamed lipoproteins | Clinical (SR/MA, cohorts) | ↑ LDL and total cholesterol in FS; ICAM-1 upregulated in capsule. | Vascular inflammation and fibrosis link. |
| Domain | Study Type | Core Finding | Implication for FS |
|---|---|---|---|
| Diet & glycemic load | Clinical (SR/MA, cohorts) | HbA1c consistently elevated in FS; high-glycemic/ultra-processed diets → LGI, microbiota dysbiosis. | Diet as modifiable driver; supports anti-inflammatory strategies. |
| Physical inactivity & sleep | Clinical/Translational | Sedentarism and circadian disruption impair HPA, GH, pain modulation. | Exercise and sleep hygiene key for management. |
| Psychosocial stress | Clinical (cohorts, RCTs) | Anxiety/depression worsen FS prognosis; psychotherapies improve MSK outcomes. | Supports psychoneuroimmunology-based interventions. |
| Exposome/EDCs | Experimental/Human observational | BPA, phthalates, cadmium alter ER function and circadian release. | Exposome as overlooked contributor to FS pathogenesis. |
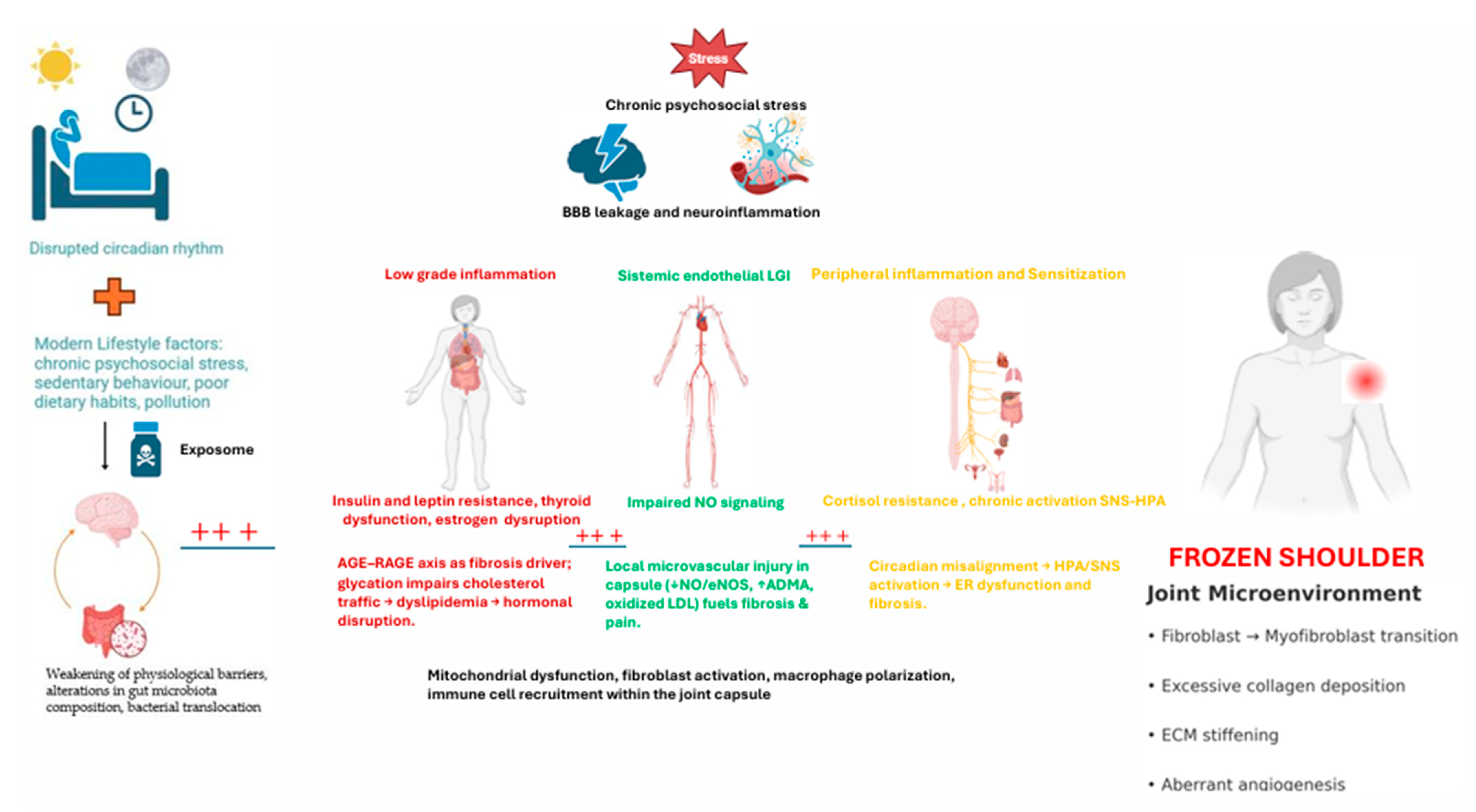
| Domain | Established Evidence (Human-Focused) | Emerging Hypotheses (Mechanistic/Early Clinical) | Notes/Level of Evidence |
|---|---|---|---|
| Epidemiology & clinical course | Female predominance; peak in perimenopause/postmenopause; painful capsular stiffness with staged course. | Sex-specific vulnerability linked to estrogen deficiency/resistance; phenotypes differing by metabolic status. | High-quality observational data; sex-hormone linkage under active study. |
| Histopathology & cytokines | Capsular fibrosis; myofibroblast proliferation; ↑ TGF-β, IL-1β, TNF-α; neoangiogenesis; mast cells. | Alarmins (HMGB1, IL-33, S100A8/9) drive nerve ingrowth and persistent fibrosis. | Multiple biopsy series; alarmin data growing (moderate). |
| Metabolic biomarkers (glycemia, lipids) | Higher HbA1c and total cholesterol in FS vs. controls (meta-analyses). | AGE–RAGE axis as fibrosis driver; glycation impairs cholesterol traffic → dyslipidemia → hormonal disruption. | Human meta-analyses (strong); mechanistic links plausible (moderate). |
| Thyroid dysfunction | Hypothyroidism (overt/subclinical) associated with FS; MR studies suggest causal link. | Autoimmune thyroiditis primes chronic inflammatory milieu, amplifying capsular fibrosis. | Systematic reviews & MR (strong); immune amplification (moderate). |
| Estrogen axis (deficiency/resistance) | Postmenopausal status correlates with worse pain/stiffness; ERs in capsule; estrogen is anti-inflammatory/antifibrotic (indirect clinical support). | Estrogen resistance from LGI/oxidative stress/EDCs; receptor-level interference in capsule tissue. | Human indirect + robust mechanistic data (moderate). |
| Endothelial dysfunction & NO biology | Endothelial impairment associates with chronic pain states; lifestyle improves endothelial function. | Local microvascular injury in capsule (↓NO/eNOS, ↑ADMA, oxidized LDL) fuels fibrosis & pain. | Human vascular data (moderate); capsule-specific vascular biology emerging (limited–moderate). |
| Microbiome & gut barrier | Diets high in ultra-processed foods associate with inflammation; gut barrier compromise in chronic pain cohorts. | Dysbiosis → LPS/ADMA → endothelial & ER signaling disruption; gut–joint axis in FS. | Human associative data (moderate); FS-specific data limited. |
| Psychoneuroimmunology & sleep | Stress, poor sleep linked to worse pain/function; psychoeducation/exercise benefit shoulder pain. | Circadian misalignment → HPA/SNS activation → ER dysfunction and fibrosis. | Human outcomes (moderate); circadian-ER link mechanistic (moderate). |
| Exposome/EDCs | Population exposure to BPA/phthalates/parabens widespread; endocrine effects documented in humans. | Xenoestrogens cause ER misactivation/desensitization in capsule, promoting fibrosis. | Human exposure strong; FS-targeted evidence limited (emerging). |
| Adipose tissue dysfunction | Central adiposity associates with systemic inflammation and shoulder pain risk. | Leptin resistance downregulates ERα; adipokines drive fibroblast activation in capsule. | Human association (moderate); cellular mechanisms strong. |
| Therapeutics—standard care | Exercise therapy, manual therapy, education; injections/hydrodistension as per guidelines. | Stratified care by immunometabolic/endocrine phenotype to personalize response. | High clinical evidence for core PT; precision phenotyping emerging. |
| Therapeutics—metabolic & endocrine | Diet/exercise improve pain in shoulder disorders; omega-3s analgesic in inflammatory joint pain. | Low-AGE/anti-inflammatory/phytoestrogen diets; circadian therapy; endocrine optimization (thyroid/estrogen) in selected patients. | Mixed clinical evidence (moderate); targeted trials needed. |
| Domain | Clinical Assessments (Recommended) | Interventions (Examples) | Human Clinical Evidence | Experimental/Mechanistic Support |
|---|---|---|---|---|
| Hormonal—Estrogen/Female axis | Serum estradiol, progesterone, SHBG; menopausal status; vasomotor and sleep symptom scales. (ER polymorphisms for research). | Lifestyle + nutrition to support estrogen balance; phytoestrogens (e.g., soy isoflavones) when appropriate; consider HRT in selected patients per guidelines; vitamin D repletion. | Observational links between menopausal status and FS severity; small trials on phytoestrogens and musculoskeletal symptoms; indirect evidence from shoulder pain cohorts. | Strong evidence of estrogen anti-inflammatory/antifibrotic actions; ER signaling modulates TGF-β/NF-κB; animal & cellular models. |
| Thyroid axis | TSH, free T4/T3; thyroid autoantibodies (TPOAb/TgAb) if suspected; hypothyroid symptom checklist. | Treat overt/subclinical hypothyroidism per endocrine guidelines; monitor FS outcomes after euthyroid restoration. | Epidemiology, meta-analyses, and Mendelian randomization support association between hypothyroidism and FS. | Thyroid hormones regulate collagen turnover, MMP/TIMP balance, and mitochondrial function in connective tissue. |
| Metabolic—Glucose/insulin & Lipids | HbA1c, fasting glucose/insulin, HOMA-IR; lipid profile (LDL-C, HDL-C, TG); body composition/waist circumference. | Anti-inflammatory or low-AGE diet; Mediterranean or low-glycemic patterns; weight optimization; structured exercise; omega-3 supplementation as appropriate. | Meta-analyses show elevated HbA1c and cholesterol in FS; clinical trials in shoulder pain show benefit of diet/exercise on pain/function. | AGE-RAGE activation drives fibrosis; hyperglycemia and dyslipidemia impair ECM and endothelial health (cell/animal studies). |
| Vascular/Endothelial & NO biology | CRP/IL-6 (systemic context); ADMA (research); endothelial function (flow-mediated dilation) in studies; ICAM-1/VCAM-1 (research). | Mediterranean-style diet; homocysteine lowering (B-vitamins) when deficient; antioxidant/mitochondrial support; aerobic exercise. | Emerging human data linking endothelial dysfunction with shoulder pain states; diet/exercise improve endothelial function. | Mechanistic evidence for NO/eNOS impairment, oxidized LDL, and AGE-RAGE in fibrosis and pain sensitization. |
| Lifestyle & Circadian health | Sleep quality (PSQI), actigraphy if available; chronotype; stress scales; physical activity (IPAQ/accelerometry); dietary quality (MEDAS). | Sleep/circadian hygiene (reduce light-at-night, regular schedule); stress reduction (mindfulness/CBT); graded activity programs; anti-inflammatory nutrition. | Multiple RCTs in musculoskeletal pain show benefit of exercise and psychoeducation; FS RCTs support multimodal rehab; early trials on circadian interventions. | Psychoneuroimmunology models show stress-immune-pain links; animal/experimental data on circadian disruption and inflammation. |
| Orthopedic/Physiotherapy core care | Pain (NPRS/VAS), SPADI/DASH, ROM (goniometry), strength; functional goals; imaging when indicated. | Progressive exercise therapy, manual therapy, joint mobilization; education & pacing; consider hydrodistension or injections per guidelines. | Strong evidence base for exercise/manual therapy in FS and shoulder disorders; guideline-endorsed multimodal care. | Biomechanical & neurophysiological models support tissue adaptation and central desensitization with rehab. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Ledesma, S. Frozen Shoulder as a Systemic Immunometabolic Disorder: The Roles of Estrogen, Thyroid Dysfunction, Endothelial Health, Lifestyle, and Clinical Implications. J. Clin. Med. 2025, 14, 7315. https://doi.org/10.3390/jcm14207315
Navarro-Ledesma S. Frozen Shoulder as a Systemic Immunometabolic Disorder: The Roles of Estrogen, Thyroid Dysfunction, Endothelial Health, Lifestyle, and Clinical Implications. Journal of Clinical Medicine. 2025; 14(20):7315. https://doi.org/10.3390/jcm14207315
Chicago/Turabian StyleNavarro-Ledesma, Santiago. 2025. "Frozen Shoulder as a Systemic Immunometabolic Disorder: The Roles of Estrogen, Thyroid Dysfunction, Endothelial Health, Lifestyle, and Clinical Implications" Journal of Clinical Medicine 14, no. 20: 7315. https://doi.org/10.3390/jcm14207315
APA StyleNavarro-Ledesma, S. (2025). Frozen Shoulder as a Systemic Immunometabolic Disorder: The Roles of Estrogen, Thyroid Dysfunction, Endothelial Health, Lifestyle, and Clinical Implications. Journal of Clinical Medicine, 14(20), 7315. https://doi.org/10.3390/jcm14207315






