Evidence on Non-Invasive Respiratory Support During Flexible Bronchoscopy: A Narrative Review
Abstract
1. Introduction
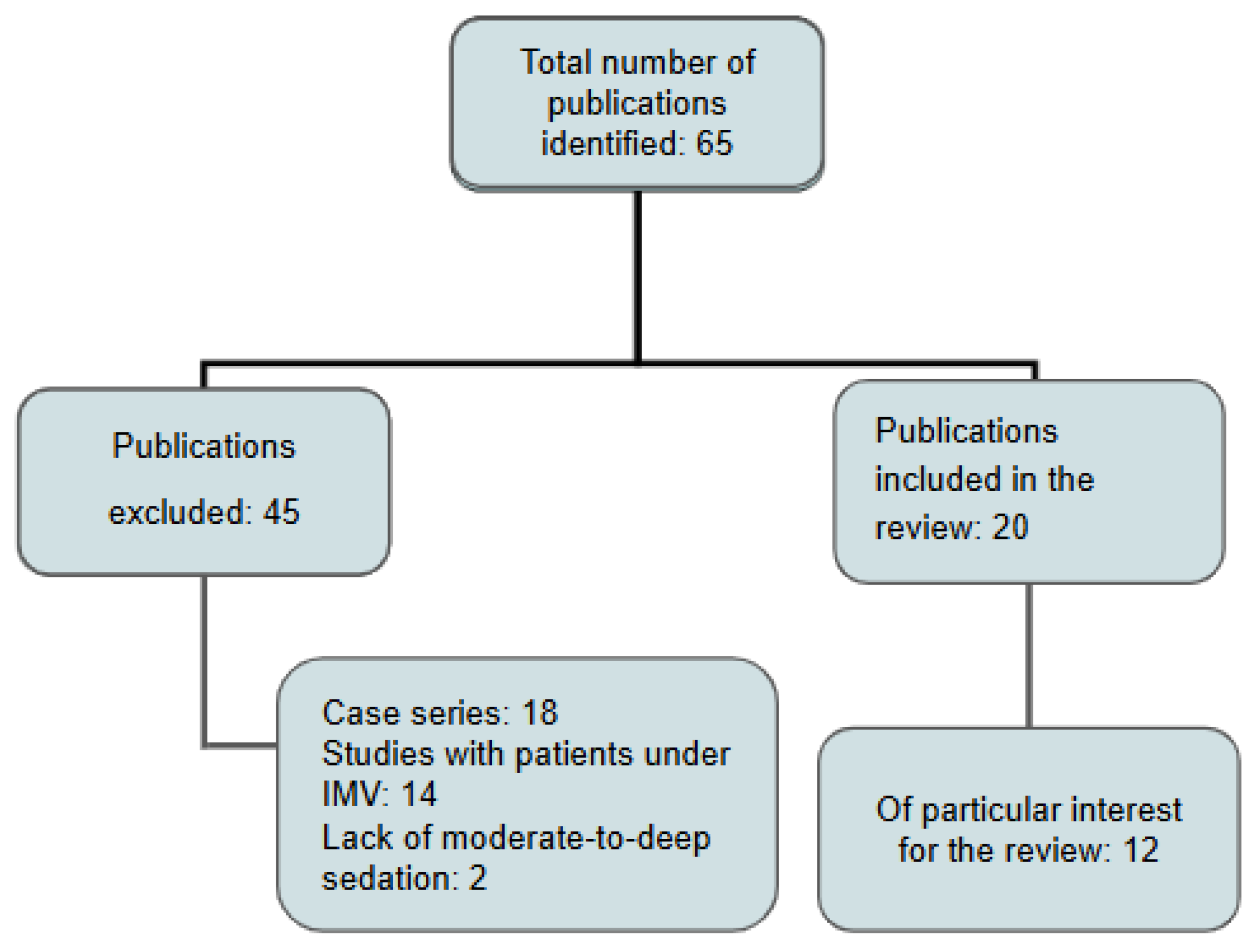
2. Materials and Methods
3. Results
3.1. Modalities of Respiratory Support During Flexible Bronchoscopy
3.1.1. Conventional Oxygen Therapy
3.1.2. High-Flow Oxygen Therapy
3.1.3. Continuous Positive Airway Pressure
3.1.4. Non-Invasive Mechanical Ventilation
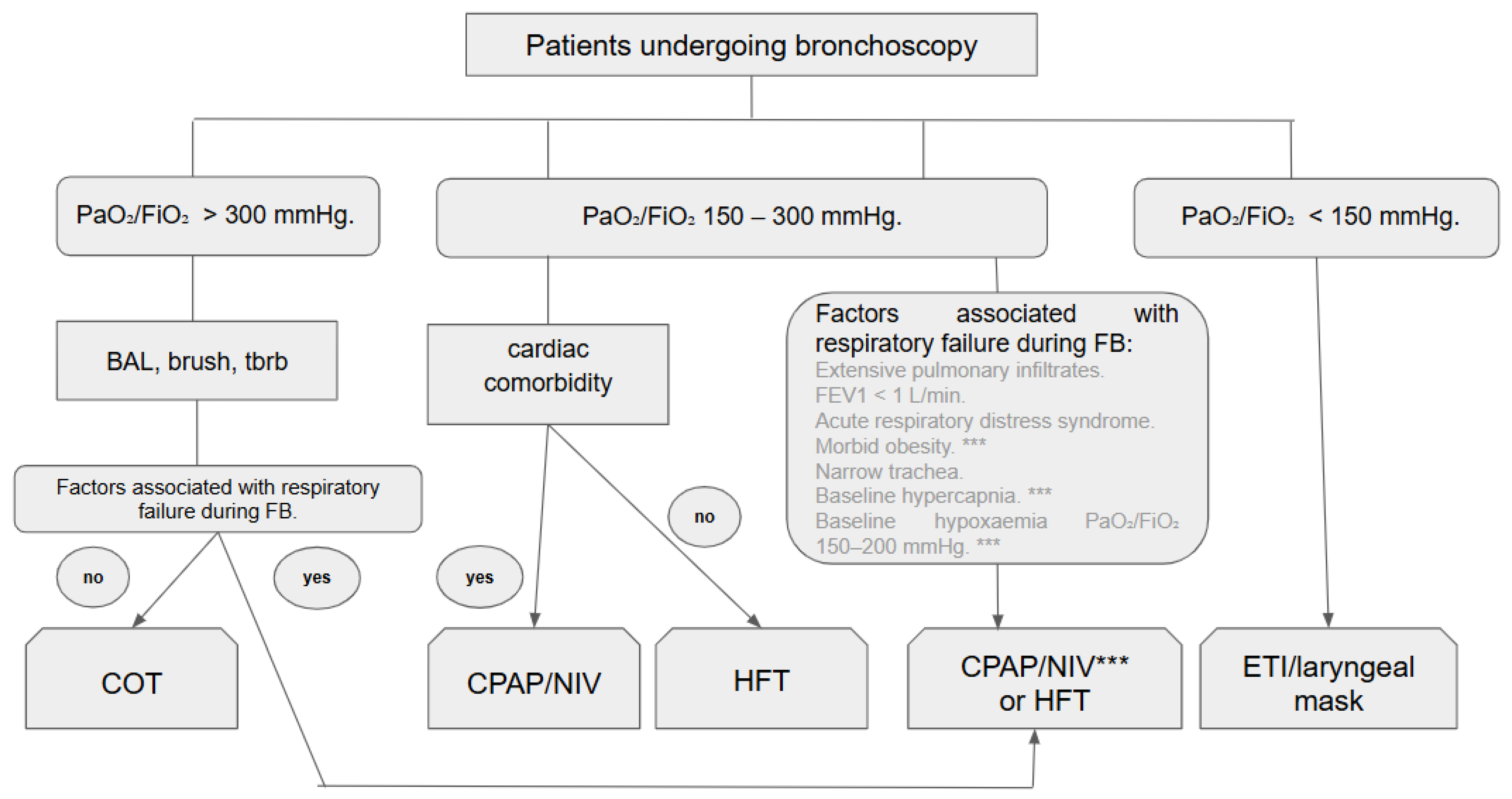
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | Acute respiratory distress syndrome |
| ARF | Acute respiratory failure |
| BAL | Bronchoalveolar lavage |
| BP | Blood pressure |
| COPD | Chronic obstructive pulmonary disease |
| COT | Conventional oxygen therapy |
| CPAP | Continuous positive airway pressure |
| EBUS | Endobronchial ultrasound |
| ETI | Endotracheal intubation |
| FB | Flexible bronchoscopy |
| FEV1 | Forced expiratory volume in one second |
| FiO2 | Fraction of inspired oxygen |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| HFT | High-flow therapy |
| HR | Heart rate |
| ICU | Intensive care unit |
| IMV | Invasive mechanical ventilation |
| IPAP | Inspiratory positive airway pressure |
| LOS | Length of stay (hospital/ICU) |
| ND | Not defined |
| NIV | Non-invasive ventilation |
| NIPPV | Non-invasive positive pressure ventilation |
| OSA | Obstructive sleep apnoea |
| PaCO2 | Partial pressure of carbon dioxide in arterial blood |
| PaO2 | Partial pressure of oxygen in arterial blood |
| PaO2/FiO2 | Ratio of arterial oxygen partial pressure to inspired oxygen fraction |
| PEEP | Positive end-expiratory pressure |
| RR | Respiratory rate |
| SpO2 | Peripheral capillary oxygen saturation |
References
- Matsushima, Y.; Jones, R.L.; King, E.G.; Moysa, G.; Alton, J.D. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest 1984, 86, 184–188. [Google Scholar] [CrossRef]
- Dubrawsky, C.; Awe, R.J.; Jenkins, D.E. The effect of bronchofiberscopic examination on oxygenation status. Chest 1975, 67, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Odenstedt, H.; Erlandsson, K.; Grivans, C.; Lundin, S.; Stenqvist, O. Bronchoscopic suctioning may cause lung collapse: A lung model and clinical evaluation. Acta Anaesthesiol. Scand. 2008, 52, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Brach, B.B.; Escano, G.G.; Harrell, J.H.; Moser, K.M. Ventilation-perfusion alterations induced by fiberoptic bronchoscopy. Chest 1976, 69, 335–337. [Google Scholar] [CrossRef]
- Randazzo, G.P.; Wilson, A.R. Cardiopulmonary changes during flexible fiberoptic bronchoscopy. Respiration 1976, 33, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Shrader, D.L.; Lakshminarayan, S. The effect of fiberoptic bronchoscopy on cardiac rhythm. Chest 1978, 73, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Mister, R.; Spence, D.P.; Calverley, P.M.; Earis, J.E.; Pearson, M.G. Cardiovascular consequences of fiberoptic bronchoscopy. Eur. Respir. J. 1997, 10, 695–698. [Google Scholar] [CrossRef]
- Golpe, R.; Mateos, A. Supplemental oxygen during flexible bronchoscopy. Chest 2002, 121, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Longhini, F.; Pelaia, C.; Garofalo, E.; Bruni, A.; Placida, R.; Iaquinta, C.; Arrighi, E.; Perri, G.; Procopio, G.; Cancelliere, A.; et al. High-flow nasal cannula oxygen therapy for outpatients undergoing flexible bronchoscopy: A randomised controlled trial. Thorax 2022, 77, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.L.; Fu, S.; Zhou, J.M.; Zhu, Y.J.; Cai, S.N.; Fang, J.; Xie, K.-J.; Chen, X.-Z. Incidence of oxygen desaturation using a high-flow nasal cannula versus a facemask during flexible bronchoscopy in patients at risk of hypoxemia: A randomised controlled trial. BMC Pulm. Med. 2022, 22, 389, Erratum in BMC Pulm. Med. 2022, 22, 451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arias-Sanchez, P.P.; Ledesma, G.; Cobos, J.; Tirape, H.; Jaramillo, B.; Ruiz, J.; Pacheco, L.; Martinez, J.; Maldonado, R.; Andrade, L.; et al. Changes in Oxygen Saturation During Fiberoptic Bronchoscopy: High-Flow Nasal Cannula versus Standard Oxygen Therapy. Respir. Care 2023, 68, 727–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, H.; Li, J.; Wang, J.; Yang, Y.G.; Jing, G.Q.; Chen, R.Z.; Tan, W.; Zhang, Y.-Q.; Li, T.; Yang, J.-C.; et al. Comparison of High-Flow Nasal Cannula and Conventional Oxygen Therapy for High-Risk Patients During Bronchoscopy Examination: A Multi-Center Randomized Controlled Trial. Ann. Am. Thorac. Soc. 2025, 22, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; McKenzie, J.; O’Sullivan, C.; Havryk, A.P. High-flow nasal oxygen versus standard oxygen during flexible bronchoscopy in lung transplant patients: A randomized controlled trial. J. Bronchol. Interv. Pulmonol. 2020, 27, 259–265. [Google Scholar] [CrossRef]
- Maitre, B.; Jaber, S.; Maggiore, S.M.; Bergot, E.; Richard, J.C.; Bakthiari, H.; Housset, B.; Boussignac, G.; Brochard, L. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am. J. Respir. Crit. Care Med. 2000, 162 Pt 1, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Conti, G.; Rocco, M.; Arcangeli, A.; Cavaliere, F.; Proietti, R.; Meduri, G.U. Noninvasive positive-pressure ventilation vs. conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002, 121, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Da Conceiçao, M.; Genco, G.; Favier, J.C.; Bidallier, I.; Pitti, R. Fiberoptic bronchoscopy during noninvasive positive-pressure ventilation in patients with chronic obstructive lung disease with hypoxemia and hypercapnia. Ann. Fr. Anesth. Reanim. 2000, 19, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Braune, S.; Frings, D.; Wiontzek, A.-K.; Klose, H.; Kluge, S. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy-a prospective randomised trial. Crit. Care 2014, 18, 712. [Google Scholar] [CrossRef] [PubMed]
- Saksitthichok, B.; Petnak, T.; So-Ngern, A.; Boonsarngsuk, V. A prospective randomized comparative study of high-flow nasal cannula oxygen and non-invasive ventilation in hypoxemic patients undergoing diagnostic flexible bronchoscopy. J. Thorac. Dis. 2019, 11, 1929–1939. [Google Scholar] [CrossRef]
- Tan, D.; Wang, B.; Cao, P.; Wang, Y.; Sun, J.; Geng, P.; Walline, J.H.; Wang, Y.; Wang, C. High flow nasal cannula oxygen therapy versus non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: A randomized controlled non-inferiority trial. Crit. Care 2024, 28, 250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bazuaye, E.A.; Stone, T.N.; Corris, P.A.; Gibson, G.J. Variability of inspired oxygen concentration with nasal cannulas. Thorax 1992, 47, 609–611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duprez, F.; Dupriez, F.; De Greef, J.; Gabriel, J.; Bruyneel, A.; Reychler, G.; De Terwangne, C.; Poncin, W. Performance of Different Low-Flow Oxygen Delivery Systems. Respir. Care 2022, 67, 322–330. [Google Scholar] [CrossRef] [PubMed]
- McCain, T.W.; Dunagan, D.P.; Adair, N.E.; Chin, R., Jr. Prospective randomized trial comparing oxygen administration during nasal flexible bronchoscopy: Oral vs. nasal delivery. Chest 2001, 120, 1671–1674. [Google Scholar] [CrossRef] [PubMed]
- Yserbyt, J.; De Maeyer, N.; Dooms, C.; Testelmans, D.; Muylle, I.; Bruyneel, M.; Ninane, V. The Feasibility of Tracheal Oxygen Supplementation during Flexible Bronchoscopy. Respiration 2016, 92, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.R.; Chevret, S.; Yadav, H.; Mehta, S.; Pickkers, P.; Bukan, R.B.; Rello, J.; van de Louw, A.; Klouche, K.; Meert, A.-P.; et al. Diagnosis and outcome of acute respiratory failure in immunocompromised patients after bronchoscopy. Eur. Respir. J. 2019, 54, 1802442. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Bruni, A.; Garofalo, E.; Rovida, S.; Arrighi, E.; Cammarota, G.; Navalesi, P.; Pelaia, G.; Longhini, F. Oxygenation strategies during flexible bronchoscopy: A review of the literature. Respir. Res. 2021, 22, 253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society Bronchoscopy Guideline Group. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax 2013, 68 (Suppl. 1), 1–44. [Google Scholar] [CrossRef] [PubMed]
- Corley, A.; Caruana, L.R.; Barnett, A.G.; Tronstad, O.; Fraser, J.F. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br. J. Anaesth. 2011, 107, 998–1004. [Google Scholar] [CrossRef]
- Maggiore, S.M.; Idone, F.A.; Vaschetto, R.; Festa, R.; Cataldo, A.; Antonicelli, F.; Montini, L.; De Gaetano, A.; Navalesi, P.; Antonelli, M. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am. J. Respir. Crit. Care Med. 2014, 190, 282–288. [Google Scholar] [CrossRef]
- Moller, W.; Celik, G.; Feng, S.; Bartenstein, P.; Meyer, G.; Eickelberg, O.; Schmid, O.; Tatkov, S. Nasal high flow clears anatomical dead space in upper airway models. J. Appl. Physiol. 2015, 118, 1525–1532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rochwerg, B.; Einav, S.; Chaudhuri, D.; Mancebo, J.; Mauri, T.; Helviz, Y.; Goligher, E.C.; Jaber, S.; Ricard, J.-D.; Rittayamai, N.; et al. The role for high flow nasal cannula as a respiratory support strategy in adults: A clinical practice guideline. Intensive Care Med. 2020, 46, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Lucangelo, U.; Vassallo, F.G.; Marras, E.; Ferluga, M.; Beziza, E.; Comuzzi, L.; Berlot, G.; Zin, W.A. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit. Care Res. Pract. 2012, 2012, 506382. [Google Scholar] [CrossRef]
- Irfan, M.; Ahmed, M.; Breen, D. Assessment of high flow nasal cannula oxygenation in endobronchial ultrasound bronchoscopy: A randomized controlled trial. J. Bronchol. Interv. Pulmonol. 2021, 28, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jung, C.Y.; Kim, K.C. Effectiveness and safety of high-flow nasal cannula oxygen delivery during bronchoalveolar lavage in acute respiratory failure patients. Tuberc. Respir. Dis. 2018, 81, 319–329. [Google Scholar] [CrossRef]
- Verra, F.; Hmouda, H.; Rauss, A.; Lebargy, F.; Cordonnier, C.; Bignon, J.; Lemaire, F.; Brochard, L. Bronchoalveolar lavage in immunocompromised patients. Clinical and functional consequences. Chest 1992, 101, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khanna, P.; Chowdhury, S.R.; Haritha, D.; Sarkar, S. The Impact of High-flow Nasal Cannula vs. Other Oxygen Delivery Devices during Bronchoscopy under Sedation: A Systematic Review and Meta-analyses. Indian J. Crit. Care Med. 2022, 26, 1131–1140. [Google Scholar] [CrossRef]
- Sampsonas, F.; Karamouzos, V.; Karampitsakos, T.; Papaioannou, O.; Katsaras, M.; Lagadinou, M.; Zarkadi, E.; Malakounidou, E.; Velissaris, D.; Stratakos, G.; et al. High-Flow vs. Low-Flow Nasal Cannula in Reducing Hypoxemic Events During Bronchoscopic Procedures: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 815799. [Google Scholar] [CrossRef]
- Thiruvenkatarajan, V.; Sekhar, V.; Wong, D.T.; Currie, J.; Van Wijk, R.; Ludbrook, G.L. Effect of high-flow nasal oxygen on hypoxaemia during procedural sedation: A systematic review and meta-analysis. Anaesthesia 2023, 78, 81–92. [Google Scholar] [CrossRef]
- Corral-Blanco, M.; Sayas-Catalán, J.; Hernández-Voth, A.; Rey-Terrón, L.; Villena-Garrido, V. High-Flow Nasal Cannula Therapy as an Adjuvant Therapy for Respiratory Support during Endoscopic Techniques: A Narrative Review. J. Clin. Med. 2023, 13, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leone, M.; Einav, S.; Chiumello, D.; Constantin, J.M.; De Robertis, E.; De Abreu, M.G.; Gregoretti, C.; Jaber, S.; Maggiore, S.M.; Pelosi, P.; et al. Guideline contributors. Noninvasive respiratory support in the hypoxaemic peri-operative/periprocedural patient: A joint ESA/ESICM guideline. Intensiv. Care Med. 2020, 46, 697–713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabrini, L.; Nobile, L.; Cama, E.; Borghi, G.; Pieri, M.; Bocchino, S.; Zangrillo, A. Non-invasive ventilation during upper endoscopies in adult patients. A systematic review. Minerva Anestesiol. 2013, 79, 683–694. [Google Scholar] [PubMed]
- Agarwal, R.; Khan, A.; Aggarwal, A.N.; Gupta, D. Bronchoscopic lung biopsy using noninvasive ventilatory support: Case series and review of literature of NIV-assisted bronchoscopy. Respir. Care 2012, 57, 1927–1936. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, H.; Peng, Y.; Xia, L.; Liu, N.; Lin, H. Feasibility analysis of flexible bronchoscopy in conjunction with noninvasive ventilation for therapy of hypoxemic patients with Central Airway Obstruction: A retrospective study. PeerJ 2020, 8, e8687. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, P.K.; Govindagoudar, M.B.; Thulasi, A.; Chaudhry, D.; Shriram, C.P.; Lalwani, L.K.; Ahuja, A. Efficacy of different respiratory supports to prevent hypoxia during flexible bronchoscopy in patients of COPD: A triple-arm, randomised controlled trial. BMJ Open Respir. Res. 2023, 10, e001524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrer, M.; Torres, A. Noninvasive Ventilation and High-Flow Nasal Therapy Administration in Chronic Obstructive Pulmonary Disease Exacerbations. Semin. Respir. Crit. Care Med. 2020, 41, 786–797. [Google Scholar] [CrossRef]
- Trouillet, J.L.; Guiguet, M.; Gibert, C.; Fagon, J.Y.; Dreyfuss, D.; Blanchet, F.; Chastre, J. Fiberoptic bronchoscopy in ventilated patients. Evaluation of cardiopulmonary risk under midazolam sedation. Chest 1990, 97, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Jing, G.Q.; Tan, W.; Wang, J.; Yin, Y.; Chen, R.; Zhang, W.; Li, J. Comparison of high-flow nasal cannula and conventional oxygen therapy for high-risk patients during bronchoscopy examination: Protocol for a randomized controlled trial. Trials 2023, 24, 12. [Google Scholar] [CrossRef]
- Avari, H.; Hiebert, R.J.; Ryzynski, A.A.; Levy, A.; Nardi, J.; Kanji-Jaffer, H.; Kiiza, P.; Pinto, R.; Plenderleith, S.W.; Fowler, R.A.; et al. Quantitative Assessment of Viral Dispersion Associated with Respiratory Support Devices in a Simulated Critical Care Environment. Am. J. Respir. Crit. Care Med. 2021, 203, 1112–1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esquinas, A.; Zuil, M.; Scala, R.; Chiner, E. Bronchoscopy during non-invasive mechanical ventilation: Review of techniques and procedures. Arch. Bronconeumol. 2013, 49, 105–112. [Google Scholar] [CrossRef]
- Oraczewska, A.; Cofta, S.; Warcholiński, A.; Trejnowska, E.; Brożek, G.; Swinarew, A.; Stolz, D.; Scala, R.; Barczyk, A.; Skoczyński, S. The use of non-invasive respiratory assistance to facilitate bronchofiberoscopy performance in patients with hypoxemic (type one) respiratory failure—Study protocol. Adv. Med. Sci. 2023, 68, 474–481. [Google Scholar] [CrossRef]
- Skoczyński, S.; Ogonowski, M.; Tobiczyk, E.; Krzyżak, D.; Brożek, G.; Wierzbicka, A.; Trzaska-Sobczak, M.; Trejnowska, E.; Studnicka, A.; Swinarew, A.; et al. Risk factors of complications during noninvasive mechanical ventilation -assisted flexible bronchoscopy. Adv. Med. Sci. 2021, 66, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Munshi, L.; Mancebo, J.; Brochard, L.J.; Hardin, C.C. Noninvasive respiratory support for adults with acute respiratory failure. N. Engl. J. Med. 2022, 387, 1688–1698. [Google Scholar] [CrossRef]
| Reference | No. of Patients | Study Type | Clinical Status | Support During FB | Main Objectives |
|---|---|---|---|---|---|
| Golpe, et al. [8] | 44 | Observational | ND | COT | SpO2 |
| Longhini, et al. [9] | 36 | Randomised prospective | Chronic | COT vs. HFT | SpO2, PaO2 |
| Zhang, et al. [10] | 176 | Randomised prospective | Acute | COT vs. HFT | SpO2, post-procedure respiratory support |
| Arias-Sánchez, et al. [11] | 40 | Observational | Acute | COT vs. HFT | SpO2 |
| Quin H et al. [12] | 142 | Multicentre randomised prospective | Chronic | COT vs. HFT | SpO2, transcutaneous CO2, complications |
| Ben-Menachem, et al. [13] | 76 | Randomised prospective | Chronic | COT vs. HFT | SpO2 |
| Maitre, et al. [14] | 30 | Randomised prospective | Acute | COT vs. CPAP | SpO2, PaO2, post-ventilatory support |
| Antonelli, et al. [15] | 26 | Randomised prospective | Acute | COT vs. NIPPV | PaO2/FiO2, HR, BP, ETI |
| Da Conceição et al. [16] | 10 | Prospective | Acute | NIPPV | SpO2, PaO2, PaCO2, ETI, mortality |
| Simon, et al. [17] | 40 | Randomised prospective | Acute | NIV vs. HFT | SpO2, PaO2/FiO2, PaCO2, HR, BP, procedure time, ETI, mortality |
| Saksitthichok, et al. [18] | 51 | Randomised prospective | Acute | NIV vs. HFT | SpO2, PaO2, PaCO2, pH, RR, HR, BP, dyspnoea |
| Tan, et al. [19] | 225 | Randomised, open-label, non-inferiority trial | Acute hypercapnia | HFT vs. NIV | ETI, blood gases, ICU/Hospital LOS, 28-day mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo Sánchez, M.; Luján, M.; Alcolea Batres, S.; Álvarez del Vayo, J.; Mariscal-Aguilar, P.; Carpio, C.; Álvarez-Sala Walther, R. Evidence on Non-Invasive Respiratory Support During Flexible Bronchoscopy: A Narrative Review. J. Clin. Med. 2025, 14, 6658. https://doi.org/10.3390/jcm14186658
Hidalgo Sánchez M, Luján M, Alcolea Batres S, Álvarez del Vayo J, Mariscal-Aguilar P, Carpio C, Álvarez-Sala Walther R. Evidence on Non-Invasive Respiratory Support During Flexible Bronchoscopy: A Narrative Review. Journal of Clinical Medicine. 2025; 14(18):6658. https://doi.org/10.3390/jcm14186658
Chicago/Turabian StyleHidalgo Sánchez, María, Manel Luján, Sergio Alcolea Batres, Julia Álvarez del Vayo, Pablo Mariscal-Aguilar, Carlos Carpio, and Rodolfo Álvarez-Sala Walther. 2025. "Evidence on Non-Invasive Respiratory Support During Flexible Bronchoscopy: A Narrative Review" Journal of Clinical Medicine 14, no. 18: 6658. https://doi.org/10.3390/jcm14186658
APA StyleHidalgo Sánchez, M., Luján, M., Alcolea Batres, S., Álvarez del Vayo, J., Mariscal-Aguilar, P., Carpio, C., & Álvarez-Sala Walther, R. (2025). Evidence on Non-Invasive Respiratory Support During Flexible Bronchoscopy: A Narrative Review. Journal of Clinical Medicine, 14(18), 6658. https://doi.org/10.3390/jcm14186658






