Spontaneous Multiple Cervical Artery Dissections Detected with High-Resolution MRI: A Prospective, Case-Series Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuroimaging Studies
2.3. Ethical Approval and Patient Consent
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yesilot Barlas, N.; Putaala, J.; Waje-Andreassen, U.; Vassilopoulou, S.; Nardi, K.; Odier, C.; Hofgart, G.; Engelter, S.; Burow, A.; Mihalka, L.; et al. Etiology of first-ever ischaemic stroke in European young adults: The 15 cities young stroke study. Eur. J. Neurol. 2013, 20, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Dittrich, T.D.; Kahles, T.; Katan, M.; Luft, A.R.; Mono, M.L.; Bolognese, M.; Arnold, M.; Heldner, M.; Michel, P.; et al. First ischemic stroke in young adults: Sex and age-related differences in stroke rates, risk factors, and etiologies. Eur. Stroke J. 2025, 10, 23969873251317347. [Google Scholar] [CrossRef]
- Engelter, S.T.; Lyrer, P.; Traenka, C. Cervical and intracranial artery dissections. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211037238. [Google Scholar] [CrossRef]
- Béjot, Y.; Aboa-Eboulé, C.; Debette, S.; Pezzini, A.; Tatlisumak, T.; Engelter, S.; Grond-Ginsbach, C.; Touzé, E.; Sessa, M.; Metso, T.; et al. Characteristics and outcomes of patients with multiple cervical artery dissection. Stroke 2014, 45, 37–41. [Google Scholar] [CrossRef]
- Hunter, M.D.; Moon, Y.P.; Miller, E.C.; Kulick, E.R.; Boehme, A.K.; Elkind, M.S. Influenza-Like Illness is Associated with Increased Short-Term Risk of Cervical Artery Dissection. J. Stroke Cerebrovasc. Dis. 2021, 30, 105490. [Google Scholar] [CrossRef]
- Trager, R.J.; Daniels, C.J.; Scott, Z.E.; Perez, J.A. Pregnancy and spontaneous cervical artery dissection: A propensity-matched retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2023, 32, 107384. [Google Scholar] [CrossRef]
- Giossi, A.; Ritelli, M.; Costa, P.; Morotti, A.; Poli, L.; Del Zotto, E.; Volonghi, I.; Chiarelli, N.; Gamba, M.; Bovi, P.; et al. Connective tissue anomalies in patients with spontaneous cervical artery dissection. Neurology 2014, 83, 2032–2037. [Google Scholar] [CrossRef]
- Bitar, A.; Almahder, D.; Jouini, A.J.; Alsaid, B. Cervical arteries tortuosity and its association with dissection: A systematic review and meta-analysis. Medicine 2025, 104, e41517. [Google Scholar] [CrossRef]
- Keser, Z.; Meschia, J.F.; Lanzino, G. Craniocervical Artery Dissections: A Concise Review for Clinicians. Mayo Clin. Proc. 2022, 97, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, E.R.; Olesen, J. Proposed general diagnostic criteria for secondary headaches. Cephalalgia 2023, 43, 3331024231213278. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Lachanis, S.; Papagiannopoulou, G.; Maili, M.; Pachi, I.; Velonakis, G.; Bakola, E.; Vassilopoulou, S.; Tsivgoulis, G. Collet-Sicard syndrome due to cervical artery dissection disclosed by high-resolution magnetic resonance imaging. Eur. J. Neurol. 2024, 31, e16398. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Palaiodimou, L.; Kokotis, P.; Papadopoulou, M.; Fradelos, S.; Voudouri, A.; Zompola, C.; Magoufis, G.; Arvaniti, C.; Bonakis, A.; et al. Teaching NeuroImages: An uncommon cause of carotid artery dissection: Fabry disease. Neurology 2020, 95, e2711–e2713. [Google Scholar] [CrossRef]
- Cuvinciuc, V.; Viallon, M.; Momjian-Mayor, I.; Sztajzel, R.; Pereira, V.M.; Lovblad, K.O.; Vargas, M.I. 3D fat-saturated T1 SPACE sequence for the diagnosis of cervical artery dissection. Neuroradiology 2013, 55, 595–602. [Google Scholar] [CrossRef]
- Edjlali, M.; Roca, P.; Rabrait, C.; Naggara, O.; Oppenheim, C. 3D fast spin-echo T1 black-blood imaging for the diagnosis of cervical artery dissection. AJNR Am. J. Neuroradiol. 2013, 34, E103–E106. [Google Scholar] [CrossRef]
- Guggenberger, K.; Krafft, A.J.; Ludwig, U.; Vogel, P.; Elsheik, S.; Raithel, E.; Forman, C.; Dovi-Akué, P.; Urbach, H.; Bley, T.; et al. High-resolution Compressed-sensing T1 Black-blood MRI: A New Multipurpose Sequence in Vascular Neuroimaging? Clin. Neuroradiol. 2021, 31, 207–216. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Papadimitropoulos, G.N.; Lachanis, S.; Palaiodimou, L.; Zompola, C.; Zervas, P.; Voumvourakis, K. Teaching NeuroImages: Vertebral artery atlas loop dissection in 3D T1 MRI multiplanar reconstruction. Neurology 2018, 91, e599–e600. [Google Scholar] [CrossRef]
- Yaghi, S.; Engelter, S.; Del Brutto, V.J.; Field, T.S.; Jadhav, A.P.; Kicielinski, K.; Madsen, T.E.; Mistry, E.A.; Salehi Omran, S.; Pandey, A.; et al. Treatment and Outcomes of Cervical Artery Dissection in Adults: A Scientific Statement from the American Heart Association. Stroke 2024, 55, e91–e106. [Google Scholar] [CrossRef]
- Markus, H.S.; Hayter, E.; Levi, C.; Feldman, A.; Venables, G.; Norris, J.; The CADISS trial investigators. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): A randomised trial. Lancet Neurol. 2015, 14, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Engelter, S.T.; Traenka, C.; Gensicke, H.; Schaedelin, S.A.; Luft, A.R.; Simonetti, B.G.; Fischer, U.; Michel, P.; Sirimarco, G.; Kägi, G.; et al. Aspirin versus anticoagulation in cervical artery dissection (TREAT-CAD): An open-label, randomised, non-inferiority trial. Lancet Neurol. 2021, 20, 341–350. [Google Scholar] [CrossRef]
- Yaghi, S.; Shu, L.; Fletcher, L.; Fayad, F.H.; Shah, A.; Herning, A.; Isho, N.; Mansour, P.; Joudi, K.; Zaidat, B.; et al. Anticoagulation Versus Antiplatelets in Spontaneous Cervical Artery Dissection: A Systematic Review and Meta-Analysis. Stroke 2024, 55, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.E.; Harshfield, E.L.; Gensicke, H.; Wegener, S.; Michel, P.; Kägi, G.; Nedeltchev, K.; Kellert, L.; Rosenbaum, S.; Nolte, C.H.; et al. Antithrombotic Treatment for Cervical Artery Dissection: A Systematic Review and Individual Patient Data Meta-Analysis. JAMA Neurol. 2024, 81, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. Traumatic and spontaneous extracranial internal carotid artery dissections. J. Neurol. 1990, 237, 356–361. [Google Scholar] [CrossRef]
- Safouris, A.; Krogias, C.; Sharma, V.K.; Katsanos, A.H.; Faissner, S.; Roussopoulou, A.; Zompola, C.; Kneiphof, J.; Kargiotis, O.; Deftereos, S.; et al. Statin Pretreatment and Microembolic Signals in Large Artery Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1415–1422. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Sharma, V.K.; Krogias, C.; Mikulik, R.; Vadikolias, K.; Mijajlovic, M.; Safouris, A.; Zompola, C.; Faissner, S.; et al. Statin pretreatment is associated with better outcomes in large artery atherosclerotic stroke. Neurology 2016, 86, 1103–1111. [Google Scholar] [CrossRef]
- Jalal, H.; Pechlivanoglou, P.; Krijkamp, E.; Alarid-Escudero, F.; Enns, E.; Hunink, M.G.M. An Overview of R in Health Decision Sciences. Med. Decis. Making 2017, 37, 735–746. [Google Scholar] [CrossRef]
- Griffin, K.J.; Harmsen, W.S.; Mandrekar, J.; Brown, R.D.; Keser, Z. Epidemiology of Spontaneous Cervical Artery Dissection: Population-Based Study. Stroke 2024, 55, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Pannier, B.; Chabriat, H.; Nedeltchev, K.; Stapf, C.; Buffon, F.; Crassard, I.; Thomas, F.; Guize, L.; Baumgartner, R.W.; et al. Vascular risk factors and morphometric data in cervical artery dissection: A case-control study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 232–234. [Google Scholar] [CrossRef]
- Arnold, M.; De Marchis, G.M.; Stapf, C.; Baumgartner, R.W.; Nedeltchev, K.; Buffon, F.; Galimanis, A.; Sarikaya, H.; Mattle, H.P.; Bousser, M.G. Triple and quadruple spontaneous cervical artery dissection: Presenting characteristics and long-term outcome. J. Neurol. Neurosurg. Psychiatry 2009, 80, 171–174. [Google Scholar] [CrossRef]
- Pezzini, A.; Del Zotto, E.; Archetti, S.; Negrini, R.; Bani, P.; Albertini, A.; Grassi, M.; Assanelli, D.; Gasparotti, R.; Vignolo, L.A.; et al. Plasma homocysteine concentration, C677T MTHFR genotype, and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombotic stroke. Stroke 2002, 33, 664–669. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, S.; Li, W. Ischemic stroke in young Asians caused by spontaneous cervical artery dissection may be due to slightly increased homocysteine. Front. Neurol. 2025, 16, 1527896. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cumurciuc, R.; Stapf, C.; Favrole, P.; Berthet, K.; Bousser, M.G. Pain as the only symptom of cervical artery dissection. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Mantatzis, M.; Vadikolias, K.; Heliopoulos, I.; Charalampopoulos, K.; Mitsoglou, A.; Georgiadis, G.S.; Giannopoulos, S.; Piperidou, C. Internal carotid artery dissection presenting as new-onset cluster headache. Neurol. Sci. 2013, 34, 1251–1252. [Google Scholar] [CrossRef]
- Kargiotis, O.; Safouris, A.; Varaki, K.; Magoufis, G.; Stamboulis, E.; Tsivgoulis, G. Yin-Yang vascular imaging sign in basilar artery dissection. J. Neurol. Sci. 2016, 366, 177–179. [Google Scholar] [CrossRef] [PubMed]
- von Babo, M.; De Marchis, G.M.; Sarikaya, H.; Stapf, C.; Buffon, F.; Fischer, U.; Heldner, M.R.; Gralla, J.; Jung, S.; Simonetti, B.G.; et al. Differences and similarities between spontaneous dissections of the internal carotid artery and the vertebral artery. Stroke 2013, 44, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Visser, J.; Arnold, M.; Sarikaya, H.; van den Berg, R.; Nederkoorn, P.J.; Leys, D.; Calvet, D.; Kloss, M.; Pezzini, A.; et al. Triple and quadruple cervical artery dissections: A systematic review of individual patient data. J. Neurol. 2019, 266, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Goeggel Simonetti, B.; Schilling, S.; Martin, J.J.; Kloss, M.; Sarikaya, H.; Hausser, I.; Engelter, S.; Metso, T.M.; Pezzini, A.; et al. Familial occurrence and heritable connective tissue disorders in cervical artery dissection. Neurology 2014, 83, 2023–2031. [Google Scholar] [CrossRef]
- Grond-Ginsbach, C.; Brandt, T.; Kloss, M.; Aksay, S.S.; Lyrer, P.; Traenka, C.; Erhart, P.; Martin, J.J.; Altintas, A.; Siva, A.; et al. Next generation sequencing analysis of patients with familial cervical artery dissection. Eur. Stroke J. 2017, 2, 137–143. [Google Scholar] [CrossRef]
- Debette, S.; Metso, T.; Pezzini, A.; Abboud, S.; Metso, A.; Leys, D.; Bersano, A.; Louillet, F.; Caso, V.; Lamy, C.; et al. Association of vascular risk factors with cervical artery dissection and ischemic stroke in young adults. Circulation 2011, 123, 1537–1544. [Google Scholar] [CrossRef]
- Le Grand, Q.; Ecker Ferreira, L.; Metso, T.M.; Schilling, S.; Tatlisumak, T.; Grond-Ginsbach, C.; Engelter, S.T.; Lyrer, P.; Majersik, J.J.; Worrall, B.B.; et al. Genetic Insights on the Relation of Vascular Risk Factors and Cervical Artery Dissection. J. Am. Coll. Cardiol. 2023, 82, 1411–1423. [Google Scholar] [CrossRef]
- Doukhi, D.; Debette, S.; Mawet, J. Headaches attributed to cranial and cervical artery dissections. J. Headache Pain 2025, 26, 28. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.J.; Brandt, T.; Buggle, F.; Orberk, E.; Mytilineos, J.; Werle, E.; Conradt; Krause, M.; Winter, R.; Hacke, W. Association of cervical artery dissection with recent infection. Arch. Neurol. 1999, 56, 851–856. [Google Scholar] [CrossRef]
- Tai, M.S.; Sia, S.F.; Kadir, K.A.A.; Idris, M.I.; Tan, K.S. Cough-Induced Extracranial Internal Carotid Artery Dissection. Case Rep. Neurol. 2020, 12, 149–155. [Google Scholar] [CrossRef]
- Naggara, O.; Touzé, E.; Marsico, R.; Leclerc, X.; Nguyen, T.; Mas, J.L.; Pruvo, J.P.; Meder, J.F.; Oppenheim, C. High-resolution MR imaging of periarterial edema associated with biological inflammation in spontaneous carotid dissection. Eur. Radiol. 2009, 19, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Alalfi, M.O.; Cau, R.; Argiolas, G.M.; Scicolone, R.; Mantini, C.; Nardi, V.; Benson, J.C.; Suri, J.S.; Keser, Z.; Lerman, A.; et al. Assessment of Attenuation in Pericarotid Fat among Patients with Carotid Plaque and Spontaneous Carotid Dissection. AJNR Am. J. Neuroradiol. 2025, 46, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, K.; Magoufis, G.; Kargiotis, O.; Safouris, A.; Bakola, E.; Chondrogianni, M.; Zis, P.; Stamboulis, E.; Tsivgoulis, G. Ultrasound Assessment of Extracranial Carotids and Vertebral Arteries in Acute Cerebral Ischemia. Medicina 2020, 56, 711. [Google Scholar] [CrossRef]
- Alexander, M.D.; Yuan, C.; Rutman, A.; Tirschwell, D.L.; Palagallo, G.; Gandhi, D.; Sekhar, L.N.; Mossa-Basha, M. High-resolution intracranial vessel wall imaging: Imaging beyond the lumen. J. Neurol. Neurosurg. Psychiatry 2016, 87, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Gao, P. High-resolution vessel wall magnetic resonance imaging of carotid and intracranial vessels. Acta Radiol. 2019, 60, 1329–1340. [Google Scholar] [CrossRef]
- Shao, S.; Wang, G. High-resolution magnetic resonance vessel wall imaging in extracranial cervical artery dissection. Front. Neurol. 2025, 16, 1536581. [Google Scholar] [CrossRef]
- Bapst, B.; Amegnizin, J.L.; Vignaud, A.; Kauv, P.; Maraval, A.; Kalsoum, E.; Tuilier, T.; Benaissa, A.; Brugières, P.; Leclerc, X.; et al. Post-contrast 3D T1-weighted TSE MR sequences (SPACE, CUBE, VISTA/BRAINVIEW, isoFSE, 3D MVOX): Technical aspects and clinical applications. J. Neuroradiol. 2020, 47, 358–368. [Google Scholar] [CrossRef]
- Lin, X.; Guo, W.; She, D.; Kang, Y.; Xing, Z.; Cao, D. Initial and follow-up high-resolution vessel wall MRI study of spontaneous cervicocranial artery dissection. Eur. Radiol. 2024, 34, 1704–1715. [Google Scholar] [CrossRef]
- Schievink, W.I. Spontaneous dissection of the carotid and vertebral arteries. N. Engl. J. Med. 2001, 344, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Morris, N.A.; Merkler, A.E.; Gialdini, G.; Kamel, H. Timing of Incident Stroke Risk After Cervical Artery Dissection Presenting Without Ischemia. Stroke 2017, 48, 551–555. [Google Scholar] [CrossRef]
- Yaghi, S.; Shu, L.; Mandel, D.; Leon Guerrero, C.R.; Henninger, N.; Muppa, J.; Affan, M.; Ul Haq Lodhi, O.; Heldner, M.R.; Antonenko, K.; et al. Antithrombotic Treatment for Stroke Prevention in Cervical Artery Dissection: The STOP-CAD Study. Stroke 2024, 55, 908–918. [Google Scholar] [CrossRef]
- Kloss, M.; Grond-Ginsbach, C.; Ringleb, P.; Hausser, I.; Hacke, W.; Brandt, T. Recurrence of cervical artery dissection: An underestimated risk. Neurology 2018, 90, e1372–e1378. [Google Scholar] [CrossRef]
- Salih, M.; Taussky, P.; Ogilvy, C.S. Association between cervicocerebral artery dissection and tortuosity—A review on quantitative and qualitative assessment. Acta Neurochir. 2024, 166, 285. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Suess, L.; Knoflach, M.; Peball, T.; Mangesius, S.; Steiger, R.; Pereverzyev, S.; Lerchner, H.; Blache, L.; Mayr, M.; Ratzinger, G.; et al. Cervical Artery Tortuosity Is Associated with Dissection Occurrence and Late Recurrence: A Nested Case-Control Study. Stroke 2025, 56, 413–419. [Google Scholar] [CrossRef] [PubMed]

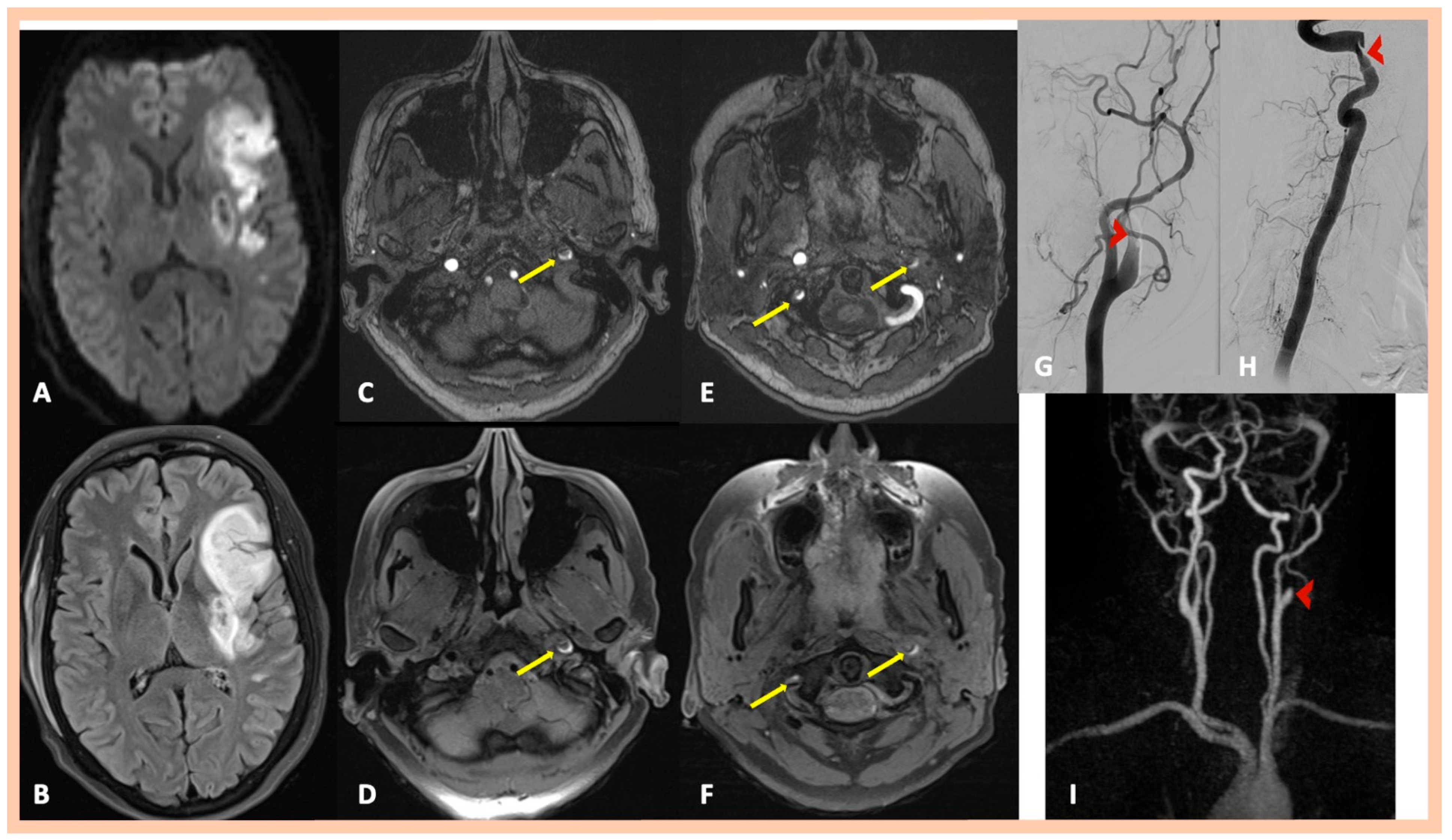
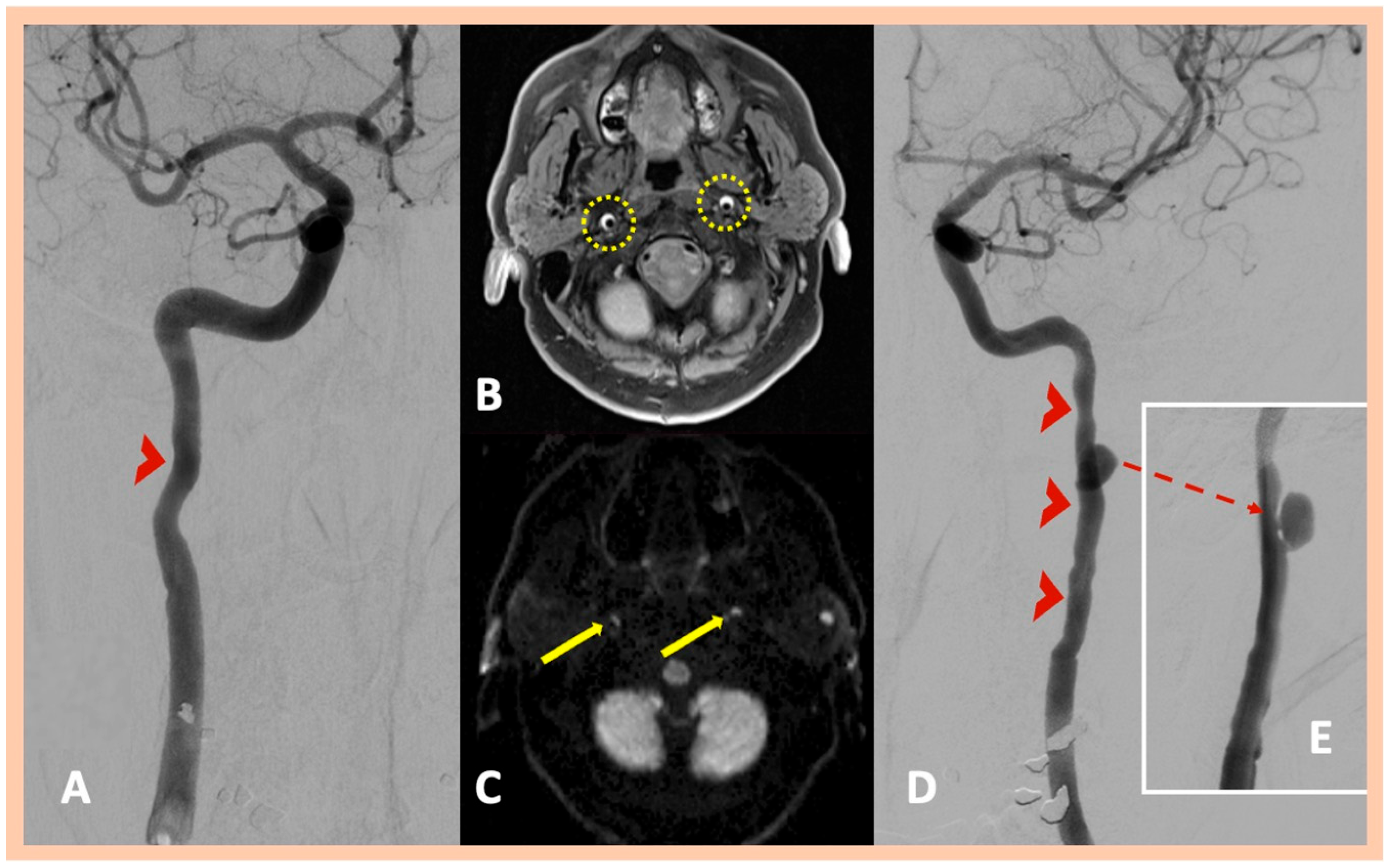
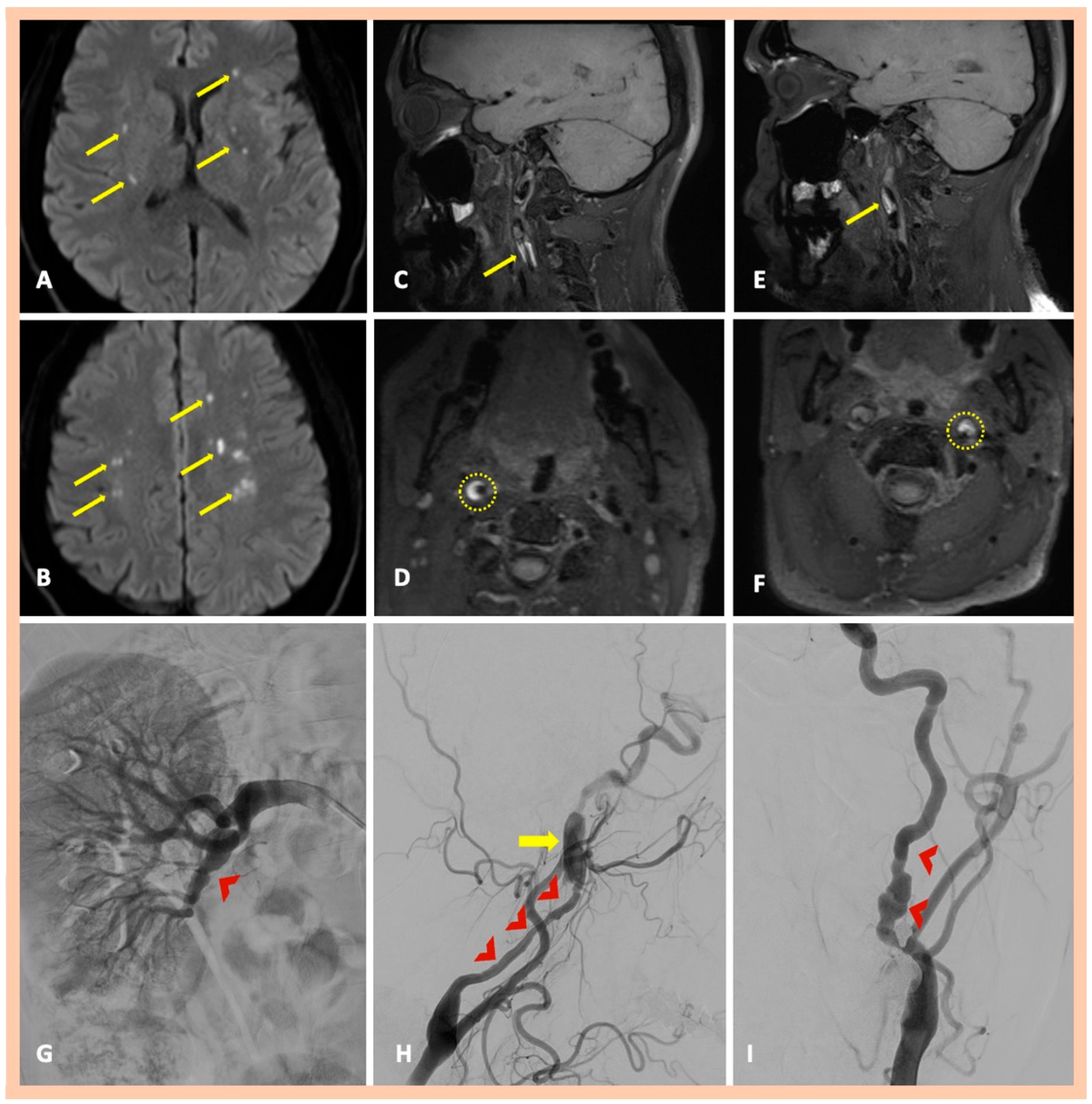
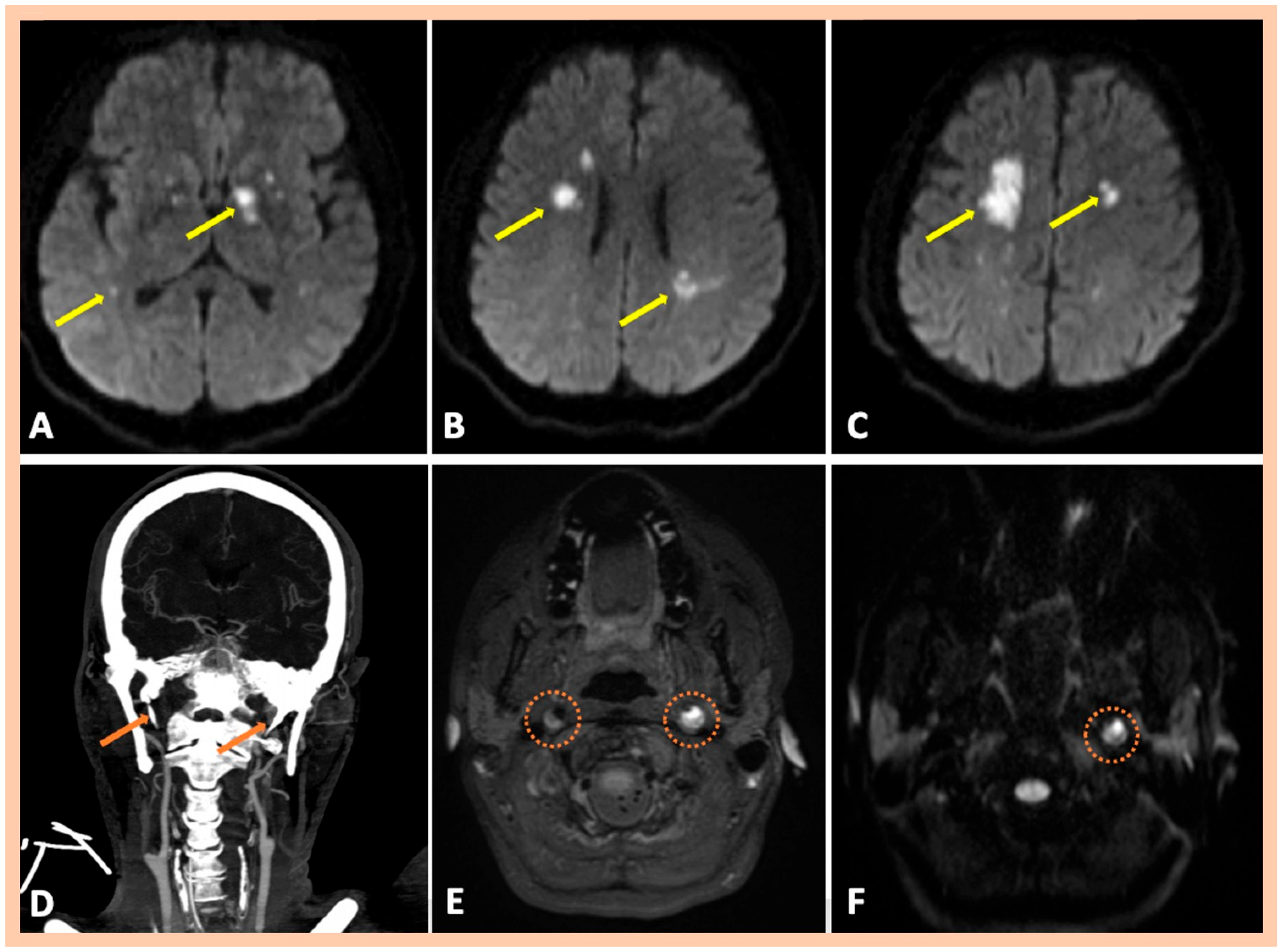
| Age | Sex | Medical History | History of Trauma | Baseline Clinical Presentation | Fibromuscular Dysplasia | Treatment | Outcome | Three-Month mRS | |
|---|---|---|---|---|---|---|---|---|---|
| Pat. #1 | 55 | F | Arterial hypertension | No | Sudden onset of right-sided Horner syndrome and left-sided hemiparesis with sensory deficits—NIHSS:4 | No | Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day | Substantial clinical recovery—NIHSS: 0 at discharge | 0 |
| Pat. #2 | 61 | F | Paroxysmal atrial fibrillation | No | Right-sided hemiparesis and hemihypesthesia, aphasia, dysarthria, right-sided hemianopsia and facial paresis. NIHSS: 9 | No | Intravenous thrombolysis with alteplase. Anticoagulation with low-molecularweight heparin, followed by Rivaroxaban (20 mg/day) | Significant clinical recovery—NIHSS: 4 at discharge | 2 |
| Pat. #3 | 42 | F | Unremarkable | Yes | Within 24 h following head and neck trauma, Horner syndrome and left-sided maxillofacial pain | No | Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day | Complete clinical recovery | 0 |
| Pat. #4 | 48 | F | Anorexia nervosa, smoking | No trauma, symptoms onset after intense physical exercise | Left-sided cervicocranial pain, frequently mimicking cluster headaches. Nausea and vomiting | Yes | Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day. | Complete clinical recovery | 0 |
| Pat. #5 | 60 | M | Arterial hypertension, hyperlipidemia | No | Horner syndrome right, headache, visual disturbances, dizziness, and gait imbalance. Symptoms onset 2 months ago. | NA | Clopidogrel 75 mg/day. | Complete clinical recovery | 0 |
| Pat. #6 | 42 | M | Unremarkable | No | Left frontal headache, vision disturbances, left-sided sensory deficits | Yes (confirmed in DSA) | Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day | Substantial clinical recovery—NIHSS: 0 at discharge | 0 |
| Pat. #7 | 41 | M | Arterial hypertension, hyperlipidemia, smoking | No | Sudden onset of right-sided hemiparesis with dysarthria/aphasia | Yes | Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day | Substantial clinical recovery—NIHSS: 0 at discharge | 0 |
| Pat. #8 | 57 | M | Arterial hypertension, papillary thyloid carcinoma 40 years ago, hypothyroidism | No | Sudden onset of right-sided hemiparesis and hemihypesthesia, left-sided amaurosis and dysarthria with mixed aphasia. NIHSS: 19 on admission | NA | Intravenous thrombolysis with tenecteplase. Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day | Substantial clinical recovery—NIHSS: 3 at discharge | 0 |
| Pat. #9 | 40 | F | Hyperlipidemia, smoking | No | Transient ischemic attack with right-hand weakness, followed by aphasia and right-sided hemiparesis and hemihypesthesia | No | Anticoagulation with low-molecular-weight heparin, followed by dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) after follow-up | Substantial clinical recovery—NIHSS: 0 at discharge | 0 |
| Pat. #10 | 51 | M | Hyperlipidemia, sleep apnoea, arterial hypertension, previous smoking | No | Right0sided cervicocranial pain, visual disturbances, mixed aphasia, gait disturbances NIHSS:3 | No | Anticoagulation with low-molecular-weight heparin | Recurrent ischemic stroke, frontal left Antiplatelet therapy (Clopidogrel 75 mg/day) was added to the already administered anticoagulation NIHSS:7 | 1 |
| Pat. #11 | 33 | F | Childbirth with cesarean section one month earlier | No | Left-sided headache, transient ischemic attack with right-sided hemiparesis and hemihypesthesia | Yes | Dual antiplatelet therapy (Clopidogrel 75 mg/day plus Acetylsalicylic acid 100 mg/day) for 21 days, followed by Clopidogrel 75 mg/day | Complete clinical recovery | 0 |
| MRI Findings | MRA Findings | 3D T1 SPACE Findings | DSA Findings | Affected Cervical Arteries | Number of Dissected Vessels | Affected Renal Arteries | |
|---|---|---|---|---|---|---|---|
| Pat. #1 | Acute right MCA infarction | Right internal carotid “flame-like” occlusion (≈1 cm above the bifurcation) | Intramural hematoma along the distal cervical and proximal petrous artery segments and mural hematoma in the intraforaminal segment of the right (V2) vertebral artery | Confirmed MRA findings | Symptomatic right ICA and asymptomatic right V2 dissections | 2 | No |
| Pat. #2 | Acute left MCA infarction | Left internal carotid “flame-like” occlusion (≈1 cm above the bifurcation) | Intramural hematoma along the distal cervical and proximal petrous artery segments of the left ICA. and Mural hematoma in the intraforaminal segment of the right (V2) vertebral artery | Confirmed MRA findings | Symptomatic left ICA dissection and asymptomatic right V2 dissection | 2 | No |
| Pat. #3 | No ischemic lesions | Stenosis along the distal cervical and proximal petrous segments of the left ICA with dissecting pseudoaneurysm along the proximal segment of the left ICA dissection | Intramural hematoma along the distal cervical and petrous segments of the ICA bilaterally | Confirmed MRA findings | Asymptomatic right ICA and symptomatic left ICA dissections | 2 | No |
| Pat. #4 | No ischemic lesions | Stenosis along the distal cervical segment of the right ICA and the distal cervical and proximal petrous segments of the left ICA Reduction in the diameter at the peripheral half of the foraminal (V2) segment of the right vertebral artery | Intramural hematoma along the cervical segments of the ICA bilaterally | Confirmed MRA findings | Right ICA and left ICA dissections and highly suspected right V2 segment dissection | 2 | Yes—abnormal findings along the right renal artery |
| Pat. #5 | No ischemic lesions | Right ICA occlusion. Occlusion along the V4 segment of the left vertebral artery. Sequential stenosis along the V4 segment of the right vertebral artery | intramural hematoma along the cervical and proximal petrous artery segments of the right ICA and Mural hematoma in the V4 segments of the right and left vertebral arteries | DSA was recommended but declined by the patient | Right ICA dissection. Dissections along the right and left V4 segments of the vertebral arteries | 3 | No |
| Pat. #6 | Chronic ischemic lesion in the right inferior frontal gyrus | Focal stenoses along the distal cervical segments of the right and left ICA | Intimal flap along the distal cervical segment of the right ICA Intramural hematoma along the distal cervical segment of the left ICA | DSA confirmed the findings | Right and left ICA dissections | 2 | Yes—wall abnormalities along the right renal artery, indicative of fibromuscular dysplasia |
| Pat. #7 | Yes—multiple ischemic lesions in different arterial territories | Stenoses along the cervical segments of the right and left ICA. Characteristic “string-of-beads” appearance along the cervical segment of the left ICA | Intramural hematoma along the cervical segment of the right ICA. Intramural hematoma along the cervical segment of the left ICA, combined with the typical beaded appearance | DSA confirmed the findings and also revealed a focal stenosis of the right renal artery | Asymptomatic right and symptomatic left ICA dissections | 2 | Yes—focal stenosis of right renal artery |
| Pat. #8 | Yes—acute ischemic stroke in the territory of the MCA artery | Significant stenosis along the cervical segment of the left ICA | Focal intramural hematoma along the cervical segment of the right ICA Intramural hematoma along the cervical and proximal petrous segments of the left ICA | DSA was recommended but declined by the patient | Asymptomatic right and symptomatic left ICA dissections | 2 | No |
| Pat. #9 | Acute left MCA infarction | Right internal carotid near occlusion along the cervical segment of the artery Left internal carotid “flame-like” occlusion (≈0.5 cm above the bifurcation) | Intramural hematoma along the cervical and proximal petrous artery segments of the right and left ICAs | Confirmed MRA findings | Asymptomatic right and symptomatic left ICA dissections | 2 | No |
| Pat. #10 | Bilateral multiple ischemic lesions in different arterial territories | Stenosis along the distal cervical and proximal petrous segments of the right ICA. Stenosis along the distal cervical and proximal petrous segments of the right ICA. | Chronic intramural hematoma along the distal cervical segment of the right ICA. Intramural hematoma along the cervical and proximal petrous artery segments of the left ICA | Confirmed MRA findings CT revealed elongated styloid processes bilaterally, indicative of Eagle syndrome | Symptomatic right and left ICA dissections | 2 | No |
| Pat. #11 | Acute multiple left MCA infarctions | Stenosis along the distal cervical and proximal petrous segments of the right ICA with dissecting pseudoaneurysm along the proximal segment of the right ICA dissection Left internal carotid “flame-like” occlusion, above the bifurcation | Intramural hematoma along the cervical segment of the right ICA Intramural hematoma along the cervical and proximal petrous artery segments of the left ICA | Confirmed MRA findings Mild wall abnormalities and segmental dilatations of the lumen were also observed along the V2 vertebral artery segments bilaterally | Asymptomatic right ICA dissection and symptomatic left ICA dissection | 2 | Mild wall abnormalities of the trunk of the right renal artery, without stenosis, indicative of fibromuscular dysplasia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foska, A.; Theodorou, A.; Chondrogianni, M.; Velonakis, G.; Lachanis, S.; Bakola, E.; Papagiannopoulou, G.; Akrivaki, A.; Fanouraki, S.; Moschovos, C.; et al. Spontaneous Multiple Cervical Artery Dissections Detected with High-Resolution MRI: A Prospective, Case-Series Study. J. Clin. Med. 2025, 14, 6635. https://doi.org/10.3390/jcm14186635
Foska A, Theodorou A, Chondrogianni M, Velonakis G, Lachanis S, Bakola E, Papagiannopoulou G, Akrivaki A, Fanouraki S, Moschovos C, et al. Spontaneous Multiple Cervical Artery Dissections Detected with High-Resolution MRI: A Prospective, Case-Series Study. Journal of Clinical Medicine. 2025; 14(18):6635. https://doi.org/10.3390/jcm14186635
Chicago/Turabian StyleFoska, Aikaterini, Aikaterini Theodorou, Maria Chondrogianni, Georgios Velonakis, Stefanos Lachanis, Eleni Bakola, Georgia Papagiannopoulou, Alexandra Akrivaki, Stella Fanouraki, Christos Moschovos, and et al. 2025. "Spontaneous Multiple Cervical Artery Dissections Detected with High-Resolution MRI: A Prospective, Case-Series Study" Journal of Clinical Medicine 14, no. 18: 6635. https://doi.org/10.3390/jcm14186635
APA StyleFoska, A., Theodorou, A., Chondrogianni, M., Velonakis, G., Lachanis, S., Bakola, E., Papagiannopoulou, G., Akrivaki, A., Fanouraki, S., Moschovos, C., Tsalouchidou, P.-E., Papageorgiou, E., Andrikopoulou, A., Psychogios, K., Kargiotis, O., Safouris, A., Koutsouraki, E., Magoufis, G., Mitsikostas, D.-D., ... Tsivgoulis, G. (2025). Spontaneous Multiple Cervical Artery Dissections Detected with High-Resolution MRI: A Prospective, Case-Series Study. Journal of Clinical Medicine, 14(18), 6635. https://doi.org/10.3390/jcm14186635








