Performance of Stratification Scores on the Risk of Stroke After a Transient Ischemic Attack: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Methods
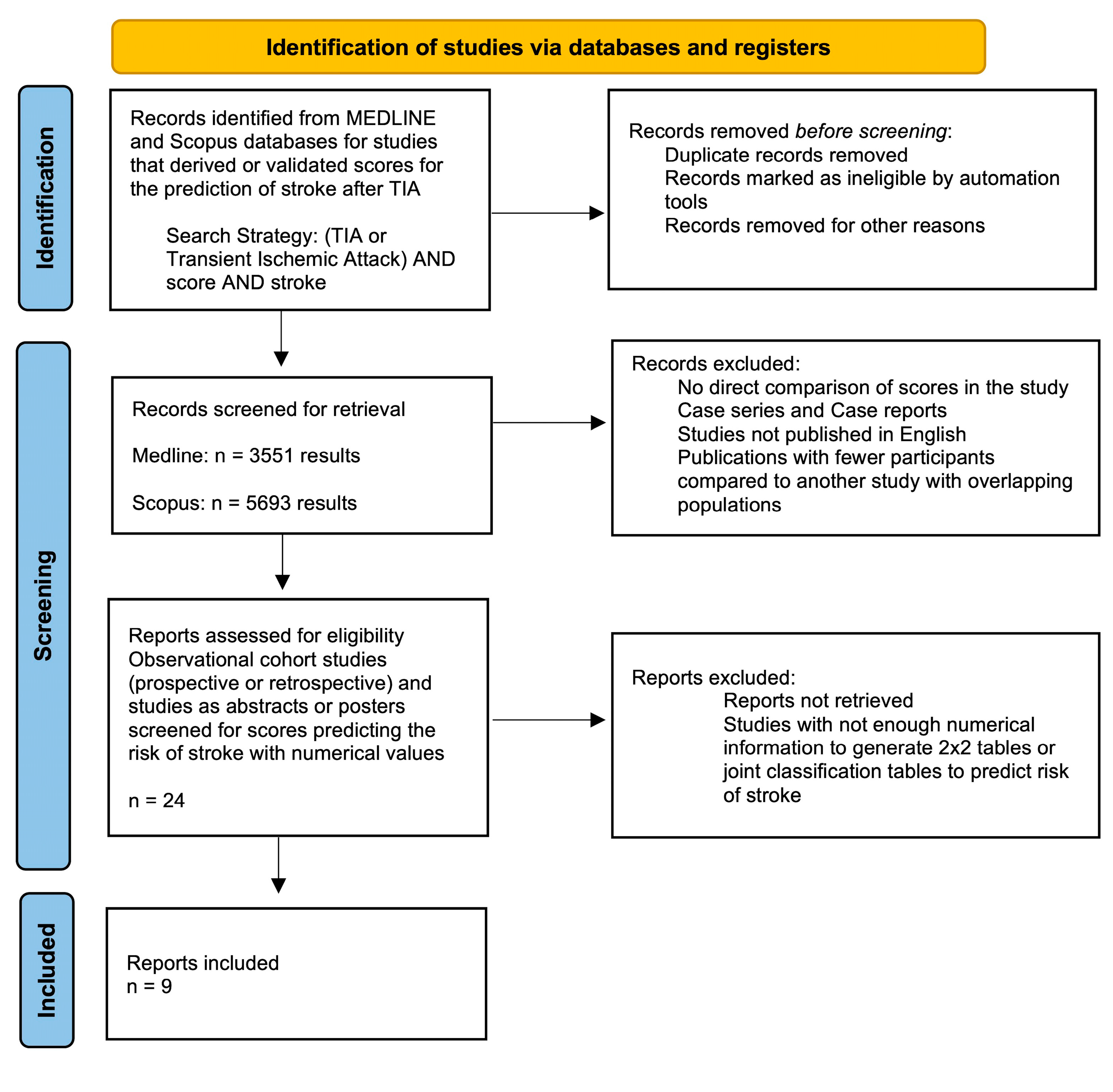
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Outcome Measure
2.4. Within-Study Bias Assessment
2.5. Data Extraction and Quality Assessment
2.6. Quantitative Synthesis
3. Results
3.1. Quality Assessment
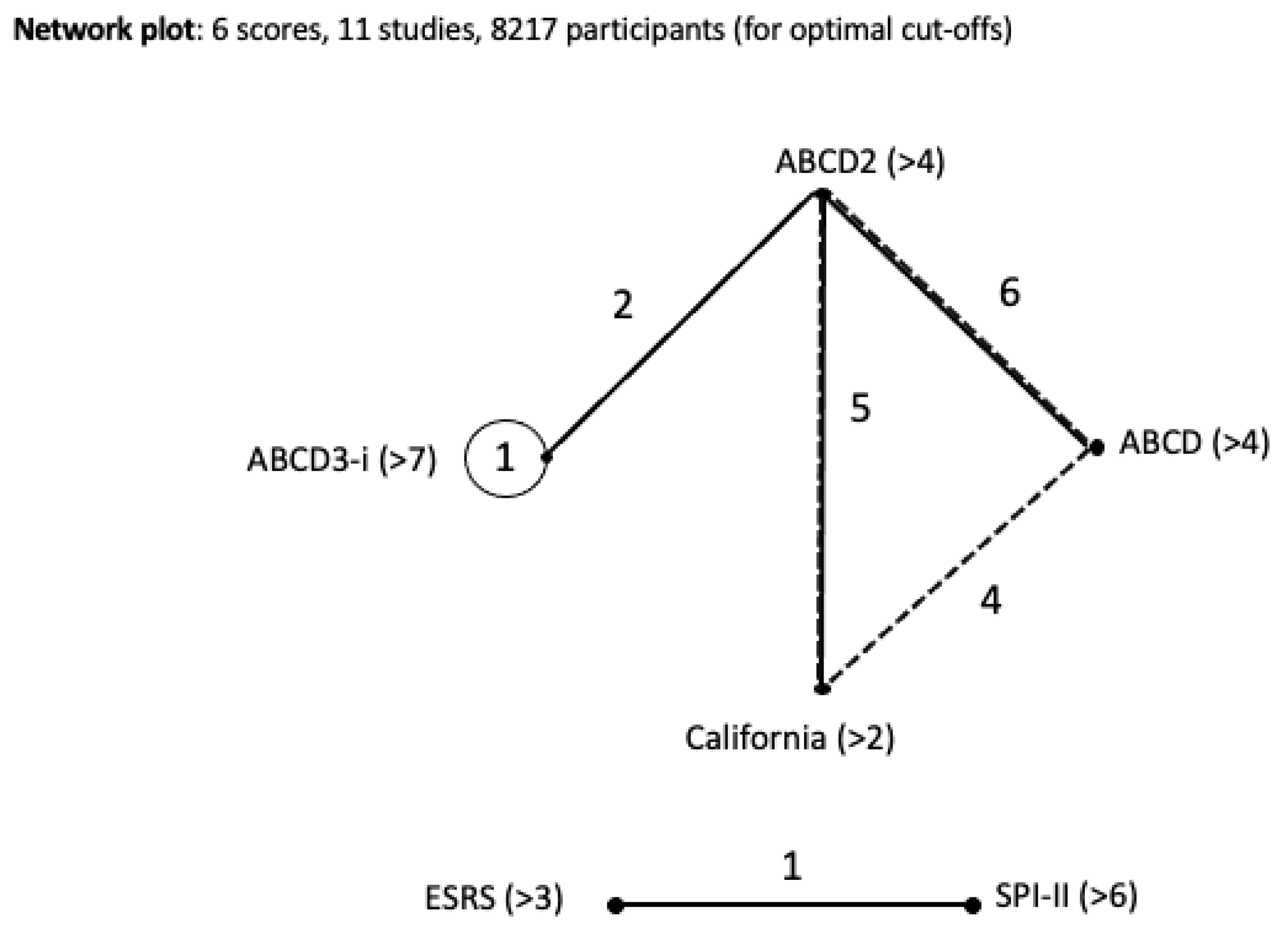
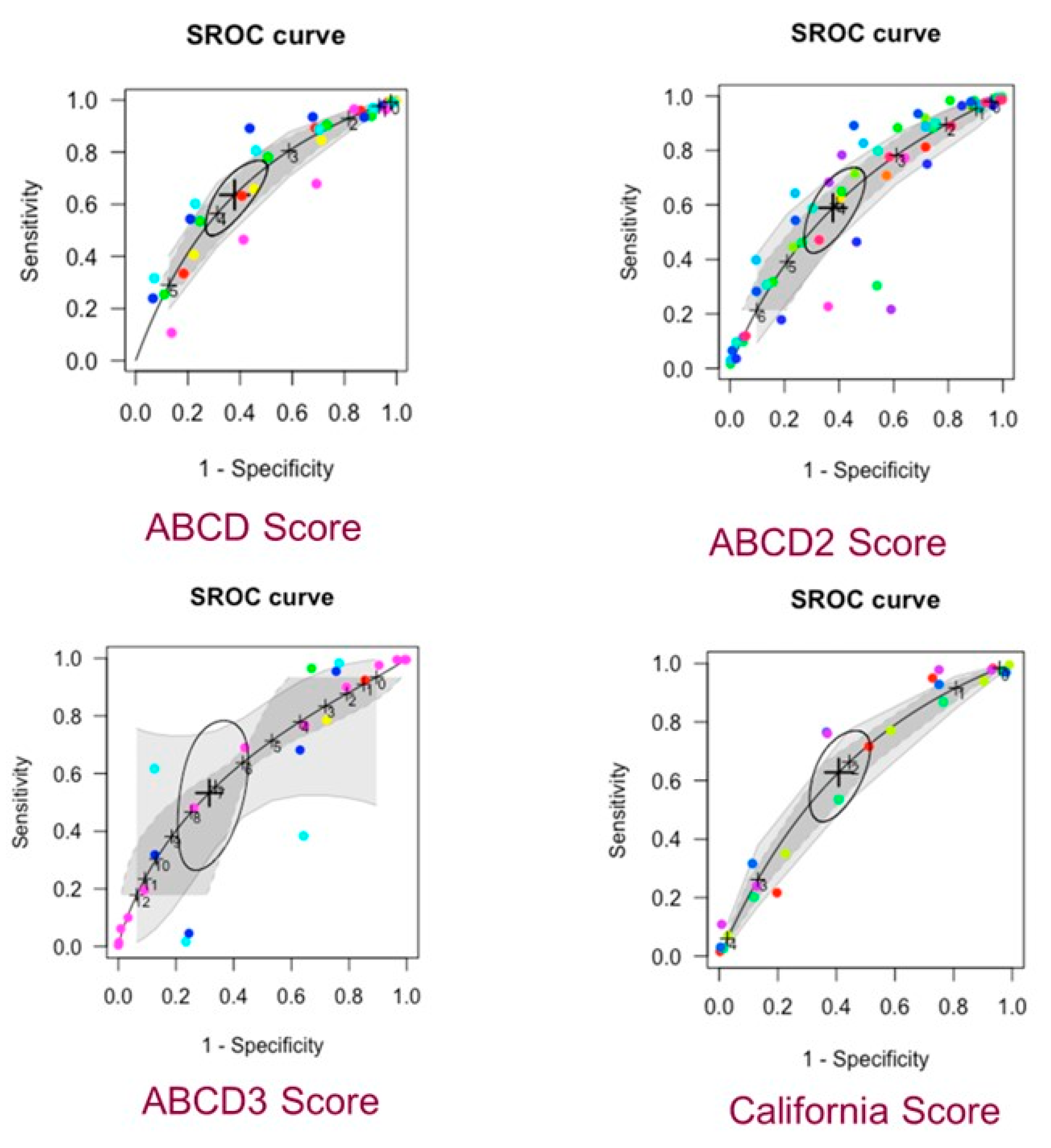
3.2. Network Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TIA | Transient Ischemic Attack |
| CVA | Cerebrovascular Accident |
| ED | Emergency Department |
| MRI | Magnetic Resonance Imaging |
| DWI | Diffusion-Weighted Imaging |
| AUC | Area Under the Curve |
| SROC | Summary Receiver Operating Characteristic |
| NMA | Network Meta-Analysis |
| CrI | Credible Interval |
| ABCD | Age, Blood pressure, Clinical features, Duration of symptoms |
| ABCD2 | Age, Blood pressure, Clinical features, Duration of symptoms, Diabetes |
| ABCD3 | ABCD2 plus Dual TIA events (≥2 in 7 days), and imaging |
| ABCD3-I | ABCD3 plus Imaging (carotid stenosis and DWI positivity) |
| ESRS | Essen Stroke Risk Score |
| SPI-II | Stroke Prognosis Instrument II |
| QUIPS | Quality in Prognostic Studies |
| CHARMS | Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies |
| TP | True Positive |
| FP | False Positive |
| TN | True Negative |
| FN | False Negative |
| PRISMA-NMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
References
- Wu, C.M.; McLaughlin, K.; Lorenzetti, D.L.; Hill, M.D.; Manns, B.J.; Ghali, W.A. Early risk of stroke after transient ischemic attack: A systematic review and meta-analysis. Arch. Intern. Med. 2007, 167, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, P.; Amarenco, P. TIA clinic: A major advance in management of transient ischemic attacks. Front. Neurol. Neurosci. 2014, 33, 30–40. [Google Scholar] [PubMed]
- Cucchiara, B.L.; Messe, S.R.; Taylor, R.A.; Pacelli, J.; Maus, D.; Shah, Q.; Kasner, S.E. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke 2006, 37, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Ildstad, F.; Ellekjær, H.; Wethal, T.; Lydersen, S.; Fjærtoft, H.; Indredavik, B. ABCD3-I and ABCD2 Scores in a TIA Population with Low Stroke Risk. Stroke Res. Treat. 2021, 2021, 8845898. [Google Scholar] [CrossRef]
- Mayer, L.; Ferrari, J.; Krebs, S.; Boehme, C.; Toell, T.; Matosevic, B.; Tinchon, A.; Brainin, M.; Gattringer, T.; Sommer, P.; et al. ABCD3-I score and the risk of early or 3-month stroke recurrence in tissue- and time-based definitions of TIA and minor stroke. J. Neurol. 2018, 265, 530–534. [Google Scholar] [CrossRef]
- Weimar, C.; Diener, H.C.; Alberts, M.J.; Steg, P.G.; Bhatt, D.L.; Wilson, P.W.F.; Mas, J.L.; Röther, J. The Essen Stroke Risk Score Predicts Recurrent Cardiovascular Events. Stroke 2009, 40, 350–354. [Google Scholar] [CrossRef]
- Kernan, W.N.; Viscoli, C.M.; Brass, L.M.; Makuch, R.W.; Sarrel, P.M.; Roberts, R.S.; Gent, M.; Rothwell, P.; Sacco, R.L.; Liu, R.-C.; et al. The Stroke Prognosis Instrument II (SPI-II). Stroke 2000, 31, 456–462. [Google Scholar] [CrossRef]
- Amin, H.P.; Madsen, T.E.; Bravata, D.M.; Wira, C.R.; Johnston, S.C.; Ashcraft, S.; Burrus, T.M.; Panagos, P.D.; Wintermark, M.; Esenwa, C.; et al. Diagnosis, Workup, Risk Reduction of Transient Ischemic Attack in the Emergency Department Setting: A Scientific Statement From the American Heart Association. Stroke 2023, 54, e109–e121. [Google Scholar] [CrossRef]
- Sorensen, A.G.; Ay, H. Transient Ischemic Attack: Definition, Diagnosis, and Risk Stratification. Neuroimaging Clin. N. Am. 2011, 21, 303–313. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V.; Flemyng, E. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5 (Updated August 2024); Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 10 January 2025).
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Groot, J.A.H.; de Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies: The CHARMS Checklist. PLOS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, S.; Schumacher, M.; Rücker, G. Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med. Res. Methodol. 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Steinhauser, S.; Kolampally, S.; Schwarzer, G. Package ‘Diagmeta’. 2022. Available online: https://cran.r-project.org/web/packages/diagmeta/diagmeta.pdf (accessed on 25 April 2025).
- Nyaga, V.N.; Aerts, M.; Arbyn, M. ANOVA model for network meta-analysis of diagnostic test accuracy data. Stat. Methods Med. Res. 2018, 27, 1766–1784. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Vasiliadis, H.S.; Higgins, J.P.T.; Salanti, G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013, 42, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ades, A.E. Assessing Evidence Inconsistency in Mixed Treatment Comparisons. J. Am. Stat. Assoc. 2006, 101, 447–459. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Tsokani, S.; Rücker, G.; Mavridis, D.; Takwoingi, Y. Challenges in Comparative Meta-Analysis of the Accuracy of Multiple Diagnostic Tests. Methods Mol. Biol. 2022, 2345, 299–316. [Google Scholar]
- Dai, Q.; Sun, W.; Xiong, Y.; Hankey, G.J.; Xiao, L.; Zhu, W.; Ma, M.; Liu, W.; Liu, D.; Cai, Q.; et al. From clinical to tissue-based dual TIA: Validation and refinement of ABCD3-I score. Neurology 2015, 84, 1426–1432. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Weck, A.; Audebert, H.; Benik, S.; Foerch, C.; Buhl, D.; Schuetz, P.; Jung, S.; Seiler, M.; Morgenthaler, N.G.; et al. Copeptin for the prediction of recurrent cerebrovascular events after transient ischemic attack: Results from the CoRisk study. Stroke 2014, 45, 2918–2923. [Google Scholar] [CrossRef]
- Kiyohara, T.; Kamouchi, M.; Kumai, Y.; Ninomiya, T.; Hata, J.; Yoshimura, S.; Ago, T.; Okada, Y.; Kitazono, T.; Ishitsuka, T.; et al. ABCD3 and ABCD3-I Scores Are Superior to ABCD2 Score in the Prediction of Short- and Long-Term Risks of Stroke After Transient Ischemic Attack. Stroke 2014, 45, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Knoflach, M.; Lang, W.; Seyfang, L.; Fertl, E.; Oberndorfer, S.; Daniel, G.; Seifert-Held, T.; Brainin, M.; Krebs, S.; Matosevic, B.; et al. Predictive value of ABCD2 and ABCD3-I scores in TIA and minor stroke in the stroke unit setting. Neurology 2016, 87, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Fang, H.; Zhao, L.; Gao, Y.; Tan, S.; Lu, J.; Sun, S.; Chandra, A.; Wang, R.; Xu, Y. Validation of the ABCD3-I score to predict stroke risk after transient ischemic attack. Stroke 2013, 44, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Engelter, S.T.; Amort, M.; Jax, F.; Weisskopf, F.; Katan, M.; Burow, A.; Bonati, L.H.; Hatz, F.; Wetzel, S.G.; Fluri, F.; et al. Optimizing the risk estimation after a transient ischaemic attack—the ABCDE⊕ score. Eur. J. Neurol. 2012, 19, 55–61. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Liu, J. Evaluation of the ESRS and SPI-II scales for short-term prognosis of minor stroke and transient ischemic attack. Neurol. Res. 2013, 35, 568–572. [Google Scholar] [CrossRef]
- Johnston, S.C.; Rothwell, P.M.; Nguyen-Huynh, M.N.; Giles, M.F.; Elkins, J.S.; Bernstein, A.L.; Sidney, S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet Lond. Engl. 2007, 369, 283–292. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Tsokani, S.; Agarwal, R.; Pagkalidou, E.; Rücker, G.; Mavridis, D.; Takwoingi, Y. Diagnostic test accuracy network meta-analysis methods: A scoping review and empirical assessment. J. Clin. Epidemiol. 2022, 146, 86–96. [Google Scholar] [CrossRef]
- Power, M.; Fell, G.; Wright, M. Principles for high-quality, high-value testing. Evid. Based Med. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Fothergill, A.; Christianson, T.J.; Brown, R.D.; Rabinstein, A.A. Validation and Refinement of the ABCD2 Score: A population-based analysis. Stroke J. Cereb. Circ. 2009, 40, 2669–2673. [Google Scholar] [CrossRef]
- Sheehan, O.C.; Kyne, L.; Kelly, L.A.; Hannon, N.; Marnane, M.; Merwick, A.; McCormack, P.M.; Duggan, J.; Moore, A.; Moroney, J.; et al. Population-Based Study of ABCD2 Score, Carotid Stenosis, and Atrial Fibrillation for Early Stroke Prediction After Transient Ischemic Attack. Stroke 2010, 41, 844–850. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zhang, D.; Zhang, Y.; Deng, X.; Zhao, J. Comparison of Stroke Prediction Accuracy of ABCD2 and ABCD3-I in Patients with Transient Ischemic Attack: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2017, 26, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Thomas, B.; Sudlow, C.L.M. Epidemiology of stroke and its subtypes in Chinese vs white populations. Neurology 2013, 81, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Trimble, B.; Morgenstern, L.B. Stroke in minorities. Neurol. Clin. 2008, 26, 1177–1190, xi. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.; Sivilotti, M.L.A.; Émond, M.; Stiell, I.G.; Stotts, G.; Lee, J.; Worster, A.; Morris, J.; Cheung, K.W.; Jin, A.Y.; et al. Prospective validation of Canadian TIA Score and comparison with ABCD2 and ABCD2i for subsequent stroke risk after transient ischaemic attack: Multicentre prospective cohort study. BMJ 2021, 372, n49. [Google Scholar] [CrossRef] [PubMed]
- Calvet, D.; Touzé, E.; Oppenheim, C.; Turc, G.; Meder, J.F.; Mas, J.L. DWI Lesions and TIA Etiology Improve the Prediction of Stroke After TIA. Stroke 2009, 40, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Jia, W.; Han, M.; Chen, Y.; Li, J.; Wu, B.; Yin, S.; Zhang, X.; Chen, J.; et al. Prediction of recurrence of ischemic stroke within 1 year of discharge based on machine learning MRI radiomics. Front. Neurosci. 2023, 17, 1110579. [Google Scholar] [CrossRef]
- Colangelo, G.; Ribo, M.; Montiel, E.; Dominguez, D.; Olivé-Gadea, M.; Muchada, M.; Garcia-Tornel, Á.; Requena, M.; Pagola, J.; Juega, J.; et al. PRERISK: A Personalized, Artificial Intelligence–Based and Statistically–Based Stroke Recurrence Predictor for Recurrent Stroke. Stroke 2024, 55, 1200–1209. [Google Scholar] [CrossRef]
| Study | QUIPS Issues to Consider | ||||||
|---|---|---|---|---|---|---|---|
| Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | Overall | |
| Dai et al., 2015 [21] | Low | Low | Moderate | Low | High | Low | Low-moderate |
| De Marchis et al., 2014 [22] | Moderate | Low | Low | Low | High | Low | Low-moderate |
| Kiyohara et al., 2014 [23] | Low | Low | Moderate | Moderate | Low | Low | Low-moderate |
| Knoflach et al., 2016 [24] | Low | Moderate | Low | Low | Moderate | Low | Low-moderate |
| Song et al., 2013 [25] | Low | Moderate | Low | Low | Low | Low | Low |
| Ildstad et al., 2021 [4] | Low | High | Moderate | Low | Moderate | Low | Low-moderate |
| Engelter et al., 2011 [26] | Low | Low | Low | Low | Moderate | Low | Low |
| Liu et al., 2013 [27] | Moderate | High | Moderate | Low | High | Low | Moderate |
| Johnston et al., 2007 [28] | Low | High | Low | Low | Moderate | Low | Low-moderate |
| Cohort Study | Location | Sample Source | Scores Compared | Type of Study | Participants | Timeline | Mean Age | % Female |
|---|---|---|---|---|---|---|---|---|
| Dai et al., 2015 [21] | Nanjing, China | Nanjing Stroke Registry Program | ABCD2 ABCD3-I | Registry | 658 | July 2009–December 2013 | 62 | 26.7 |
| De Marchis et al., 2014 [22] | Switzerland & Germany | University Hospitals ED | ABCD2 ABCD3-I Copeptin value | Cohort | 302 | March 2009–April 2011 | 69 | 37.1 |
| Kiyohara et al., 2014 [23] | Fukuoka, Japan | Fukuoka Stroke Registry | ABCD2 ABCD3 ABCD3-I | Registry | 693 | June 2007–August 2023 | 69 | 37.8 |
| Knoflach et al., 2016 [24] | Austria | Austrian Stroke Unit Registry | ABCD2 ABCD3-I | Registry | 2457 | December 2010–January 2014 | 71.9 | 34.3 |
| Song et al., 2013 [25] | Zhengzhou, China | Zhengzho University-affiliated hospital database | ABCD ABCD-I | Cohort | 239 | October 2010–December 2011 | 57.4 | 40.2 |
| Ildstad et al., 2021 [4] | Norway | 8 Central Norway region hospitals | ABCD2 ABCD3-I | Cohort | 305 | October 2012–July 2014 | 68 | 40 |
| Engelter et al., 2011 [26] | Switzerland | Basel Stroke Unit program registry | ABCD ABCDE+ | Registry | 248 | November 2006–November 2008 | 70 | 40 |
| Liu et al., 2013 [27] | Shikiazhuang, China | The Third Hospital of Hebei Medical University and the CHANCE database | ESRS SPI-II | Cohort | 167 | March 2009–October 2011 | 61.1 | 28.7 |
| Johnston et al., 2007 [28] | San Francisco, California (USA) | 16 California Emergency Departments | California and ABCD vs. ABCD2 | Cohort | 1707 | May 1997–February 1998 | Not reported | 53 |
| Johnston et al., 2007 [28] | Oxfordshire, UK | 10 Family practices | California and ABCD vs. ABCD2 | Cohort | 203 | January 1981–December 1986 | Not reported | 46 |
| Johnston et al., 2007 [28] | San Francisco, California (USA) | 16 California Emergency Departments | California and ABCD vs. ABCD2 | Cohort | 1069 | March 1998–February 1999 | Not reported | 52 |
| Johnston et al., 2007 [28] | San Francisco, California (USA) | 16 Primary Care clinics | California and ABCD vs. ABCD2 | Cohort | 962 | March 1998–February 1999 | Not reported | 53 |
| Johnston et al., 2007 [28] | Oxfordshire, UK | 9 family practices | California and ABCD vs. ABCD2 | Cohort | 545 | April 2002–March 2005 | Not reported | 55 |
| Johnston et al., 2007 [28] | Oxfordshire, UK | Hospital-based TIA clinic | California and ABCD vs. ABCD2 | Cohort | 315 | April 2002–March 2005 | Not reported | 54 |
| Score | Cut-Off Value | Sensitivity (95% Cr) | Specificity (95% Cr) |
|---|---|---|---|
| ABCD | ≥4 | 0.64 (0.51, 0.74) | 0.62 (0.52, 0.71) |
| ABCD2 | ≥4 | 0.59 (0.46, 0.71) | 0.62 (0.53, 0.71) |
| ABCD3-I | ≥7 | 0.53 (0.31, 0.74) | 0.68 (0.58, 0.77) |
| California Score | ≥2 | 0.63 (0.49, 0.74) | 0.59 (0.51, 0.67) |
| ESRS | ≥3 | 0.62 (0.41, 0.79) | 0.72 (0.64, 0.79) |
| SPI-II | ≥6 | 0.48 (0.28, 0.68) | 0.64 (0.56, 0.72) |
| Score | Diagnostic Odds Ratio | Between-Study Heterogeneity SD Sensitivity | Between-Study Heterogeneity SD Specificity |
|---|---|---|---|
| ABCD | 3.08 | 0.16 (0.00, 0.86) | 0.56 (0.14, 0.97) |
| ABCD2 | 2.30 | 0.70 (0.21, 0.98) | 0.60 (0.17, 0.97) |
| ABCD3-I | 4.44 | 0.42 (0.03, 0.96) | 0.49 (0.10, 0.96) |
| California | 2.87 | 0.40 (0.05, 0.96) | 0.43 (0.10, 0.95) |
| ESRS | 3.47 | 0.47 (0.01, 0.99) | 0.50 (0.03, 0.97) |
| SPI-II | 1.69 | 0.53 (0.04, 0.97) | 0.52 (0.03, 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deris, D.; Mastroianni, S.; Kan, J.; Veroniki, A.A.; Sharma, M.; Joundi, R.A.; Shoamanesh, A.; Srivastava, A.; Katsanos, A.H. Performance of Stratification Scores on the Risk of Stroke After a Transient Ischemic Attack: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2025, 14, 6268. https://doi.org/10.3390/jcm14176268
Deris D, Mastroianni S, Kan J, Veroniki AA, Sharma M, Joundi RA, Shoamanesh A, Srivastava A, Katsanos AH. Performance of Stratification Scores on the Risk of Stroke After a Transient Ischemic Attack: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2025; 14(17):6268. https://doi.org/10.3390/jcm14176268
Chicago/Turabian StyleDeris, Dimitrios, Sabrina Mastroianni, Jonathan Kan, Areti Angeliki Veroniki, Mukul Sharma, Raed A. Joundi, Ashkan Shoamanesh, Abhilekh Srivastava, and Aristeidis H. Katsanos. 2025. "Performance of Stratification Scores on the Risk of Stroke After a Transient Ischemic Attack: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 14, no. 17: 6268. https://doi.org/10.3390/jcm14176268
APA StyleDeris, D., Mastroianni, S., Kan, J., Veroniki, A. A., Sharma, M., Joundi, R. A., Shoamanesh, A., Srivastava, A., & Katsanos, A. H. (2025). Performance of Stratification Scores on the Risk of Stroke After a Transient Ischemic Attack: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 14(17), 6268. https://doi.org/10.3390/jcm14176268






