Is Periodontal Inflammation Associated with Liver Cirrhosis? A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
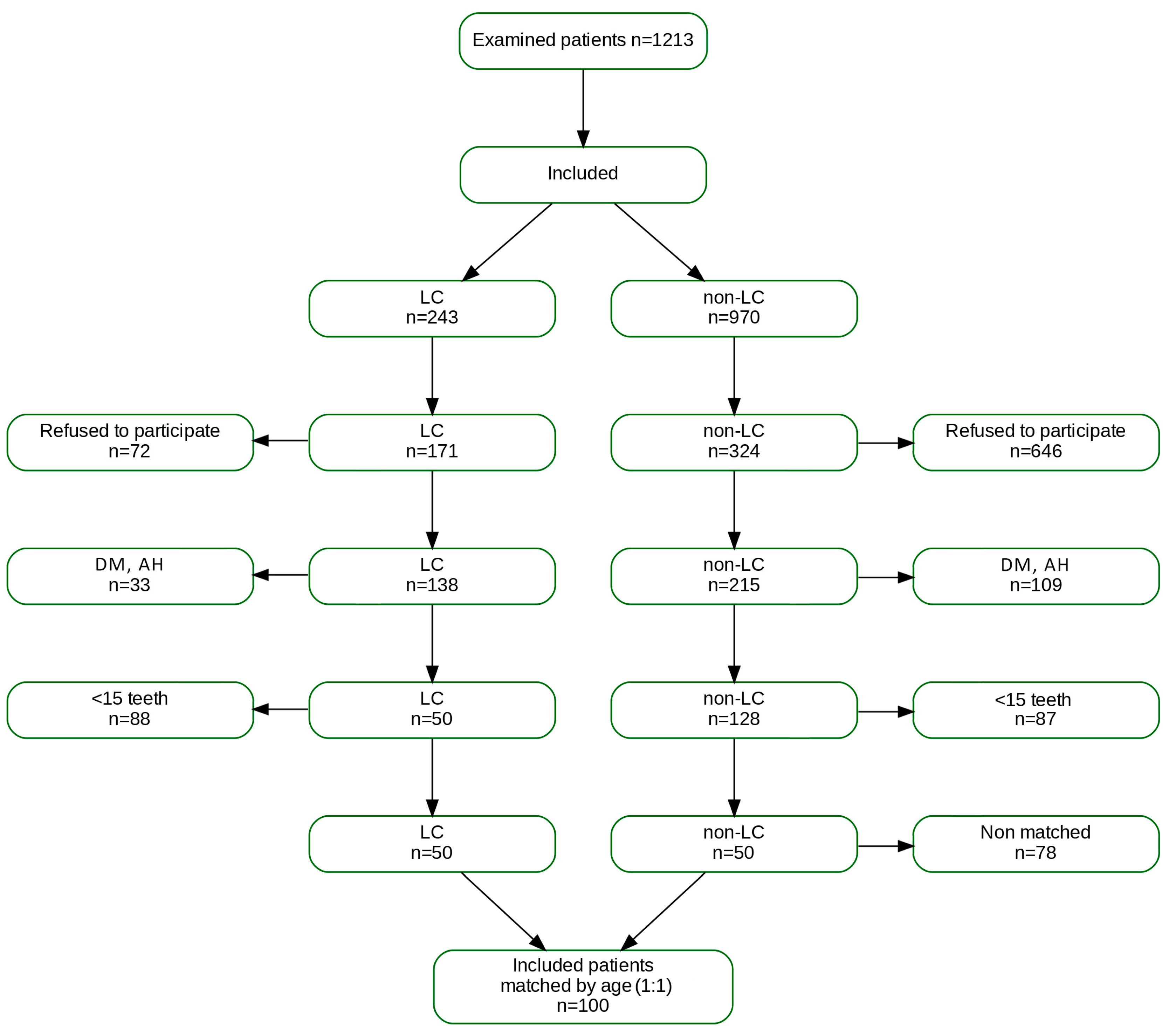
2.2. Participants
2.3. Clinical Medical Data and Assessment
2.3.1. Parameters from Medical History
2.3.2. Laboratory Parameters
2.4. Clinical Periodontal Assessment
PISA—Periodontal Inflamed Surface Area (PISA)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Liver Cirrhosis |
| CLD | Chronic Liver Disease |
| MELD | Model for End-Stage Liver Disease |
| INR | International Normalized Ratio |
| BMI | Body Mass Index |
| PI | Plaque Index |
| BoP | Bleeding on Probing |
| PPD | Probing Pocket Depth |
| GR | Gingival Recession |
| CAL | Clinical Attachment Level |
| PESA | Periodontal Epithelial Surface Area |
| PISA | Periodontal Inflamed Surface Area |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| OR | Odds Ratio |
| CI | Confidence Interval |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| GGT | Gamma-Glutamyl Transferase |
| EFP | European Federation of Periodontology |
| WONCA | World Organization of Family Doctors |
References
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Richard Hobbs, F.D.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef]
- Goyal, L.; Gupta, S.; Perambudhuru, Y. Association between periodontitis and cognitive impairment in adults. Evid. Based Dent. 2023, 24, 123–124. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Sahingur, S.E. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontology 2022, 89, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral. Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Gendron, R.; Grenier, D.; Maheu-Robert, L.F. The oral cavity as a reservoir of bacterial pathogens for focal infections. Can. J. Infect. Dis. 2000, 11, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Villoria, G.E.M.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal disease: A systemic condition. Periodontology 2024, 104, 12–28. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Chen, M.K. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J. Gastroenterol. 2022, 28, 5910–5930. [Google Scholar] [CrossRef]
- Schlichting, P.; Christensen, E.; Fauerholdt, L.; Poulsen, H.; Juhl, E.; Tygstrup, N. Main causes of death in cirrhosis. Scand. J. Gastroenterol. 1983, 18, 881–888. [Google Scholar] [CrossRef]
- Grønkjær, L.L. Periodontal disease and liver cirrhosis: A systematic review. SAGE Open Med. 2015, 3, 2050312115598129. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.; Ayares, G.; Taboun, Z.; Malhi, G.; Idalsoaga, F.; Mortuza, R.; Souyet, M.; Ramirez-Cadiz, C.; Díaz, L.A.; Arrese, M.; et al. Periodontal disease and cirrhosis: Current concepts and future prospects. eGastroenterology 2025, 3, e100140. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’AMico, G.; Dickson, R.E.; Kim, R.W. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Child, C.; Turcotte, J. Surgical consideration in portal hypertension. In The Liver: Biology and Pathobiology; Saunders: Philadelphia, PA, USA, 1964; pp. 50–56. [Google Scholar]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the esophagus for bleeding esophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef]
- Costa, F.O.; Lages, E.J.P.; Lages, E.M.B.; Cota, L.O.M. Periodontitis in individuals with liver cirrhosis: A case–control study. J. Clin. Periodontol. 2019, 46, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, B.; Inoue, G.; Marui, V.C.; de França, B.N.; Romito, G.A.; Ortega, K.L.; Holzhausen, M.; Pannuti, C.M. Periodontal status of liver transplant candidates and healthy controls. J. Periodontol. 2018, 89, 1383–1389. [Google Scholar] [CrossRef]
- Rai, J.; Shah, V.; Shah, M. Periodontitis severity grading scale and C-reactive protein: A possible relation. Cureus 2023, 15, e41503. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal inflammation and systemic diseases: An overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Ladegaard Grønkjær, L.; Holmstrup, P.; Schou, S.; Jepsen, P.; Vilstrup, H. Severe periodontitis and higher cirrhosis mortality. United Eur. Gastroenterol. J. 2018, 6, 73–80. [Google Scholar] [CrossRef]
- Åberg, F.; Helenius-Hietala, J.; Meurman, J.; Isoniemi, H. Association between dental infections and the clinical course of chronic liver disease. Hepatol. Res. 2014, 44, 349–353. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 642, 433–485. [Google Scholar] [CrossRef]
- Tritto, G.; Bechlis, Z.; Stadlbauer, V.; Davies, N.; Francés, R.; Shah, N.; Mookerjee, R.P.; Such, J.; Jalan, R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J. Hepatol. 2011, 55, 574–581. [Google Scholar] [CrossRef]
- Mookerjee, R.P.; Stadlbauer, V.; Lidder, S.; Wright, G.A.K.; Hodges, S.J.; Davies, N.A.; Jalan, R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology 2007, 46, 831–840. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Kunz, D.; Yagmur, E.; Timmer-Stranghöner, A.; Vidacek, D.; Siewert, E.; Bach, J.; Geier, A.; Purucker, E.A.; Gressner, A.M.; et al. Patients with acute-on-chronic liver failure display ‘sepsis-like’ immune paralysis. J. Hepatol. 2005, 42, 195–201. [Google Scholar] [CrossRef] [PubMed]
- McGovern, B.H.; Golan, Y.; Lopez, M.; Pratt, D.; Lawton, A.; Moore, G.; Epstein, M.; Knox, T.A. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin. Infect. Dis. 2007, 44, 431–437. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, Y.; Gao, B. Natural killer cells in liver disease. Hepatology 2013, 57, 1654–1662. [Google Scholar] [CrossRef]
- Nagao, Y.; Tanigawa, T. Red complex periodontal pathogens are risk factors for liver cirrhosis. Biomed. Rep. 2019, 11, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Grønkjær, L.L.; Holmstrup, P.; Schou, S.; Kongstad, J.; Jepsen, P.; Vilstrup, H. Periodontitis in patients with cirrhosis: A cross-sectional study. BMC Oral Health 2018, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Amaral, C.S.F.; Vettore, M.V.; Leão, A. The relationship of alcohol dependence and alcohol consumption with periodontitis: A systematic review. J. Dent. 2009, 37, 643–651. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Zanatta, F.B.; Costa, S.A.; Pelissari, T.R.; Baumeister, S.E.; Demarco, F.F.; Nascimento, G. The alcohol harm paradox in periodontitis. J. Dent. Res. 2024, 103, 605–611. [Google Scholar] [CrossRef]
- Schüz, B.; Brick, C.; Wilding, S.; Conner, M. Socioeconomic status moderates the effects of health cognitions on health behaviors within participants: Two multibehavior studies. Ann. Behav. Med. 2020, 54, 36–48. [Google Scholar] [CrossRef]
- Khocht, A.; Schleifer, S.J.; Janal, M.N.; Keller, S. Dental care and oral disease in alcohol-dependent persons. J. Subst. Abuse Treat. 2009, 37, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Lile, I.E.; Hajaj, T.; Veja, I.; Hosszu, T.; Vaida, L.L.; Todor, L.; Stana, O.; Popovici, R.-A.; Marian, D. Comparative Evaluation of Natural Mouthrinses and Chlorhexidine in Dental Plaque Management: A Pilot Randomized Clinical Trial. Healthcare 2025, 13, 1181. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S.; Consultant, E.W.P.A.M. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef] [PubMed]
| Variable | All (N = 100) | Non-Cirrhosis (N = 50) | Cirrhosis (N = 50) | p |
|---|---|---|---|---|
| Age (mean, SD) | 56.79 (11.16) | 55.58 (10.797) | 58.00 (11.49) | 0.280 |
| Gender (male), n (%) | 58 (58) | 24 (48) | 34 (68) | 0.043 |
| Gender (female), n (%) | 42 (42) | 26 (52) | 16 (32) | |
| Body mass (median, IQR) | 80 (67.75–89) | 81.5 (69.25–89.75) | 80 (66.25–86.25) | 0.426 |
| Height (mean, SD) | 1.74 (0.10) | 1.75 (0.10) | 1.72 (0.09) | 0.191 |
| BMI (median, IQR) | 25.83 (22.99–30.11) | 26.31 (23.62–30.45) | 25.83 (22.91–28.12) | 0.657 |
| Level of education 8 years n (%) | 11 (11) | 0 (0) | 11(22) | 0.002 |
| Level of education 12 years n (%) | 63 (63) | 34 (68) | 29 (58) | |
| Level of education 16 years n (%) | 26 (26) | 16 (32) | 10 (20) | |
| No income n (%) | 10 (10) | 3 (6) | 7 (14) | 0.020 |
| Income < 700€ n (%) | 43 (43) | 16 (32) | 27 (54) | |
| Income 700–1300€ n (%) | 38 (38) | 24 (48) | 14 (28) | |
| Income >1300€ n (%) | 9 (9) | 7 (14) | 2 (4) | |
| OH habits < 1/day | 6 (6) | 2 (4) | 4 (8) | 0.002 |
| OH habits 1/day | 33 (33) | 9 (18) | 24 (48) | |
| OH habits 2/day | 61 (61) | 39 (78) | 22 (44) | |
| No smoking n (%) | 60 (60) | 34 (68) | 26 (52) | 0.211 |
| Smoking: < 20/day n (%) | 33 (33) | 14 (28) | 19 (38) | |
| Smoking: >20/day n (%) | 7 (7) | 2 (4) | 5 (10) | |
| No alcohol n (%) | 51 (51) | 41 (82) | 10 (20) | <0.001 |
| Alcohol: < 6 drinks/week n (%) | 16 (16) | 9 (0) | 7 (14) | |
| Alcohol: >6 drinks/week n (%) | 33 (33) | 0 (0) | 33 (66) | |
| No cirrhosis n (%) | 50 (50) | 50 (100) | 0 (0) | <0.001 |
| Cirrhosis with alcohol n (%) | 41 (41) | 0 (0) | 41 (82) | |
| Cirrhosis with no alcohol n (%) | 9 (9) | 0 (0) | 9 (18) |
| Variable | All (N = 100) | Non-Cirrhosis (N = 50) | Cirrhosis (N = 50) | p |
|---|---|---|---|---|
| Number of teeth median (IQR) | 24 (19–27) | 24 (19–26) | 24 (19–28) | 0.433 |
| Plaque index (PI), median (IQR) | 82.66 (70.95–93.39) | 76.27 (69.5–84.82) | 87.73 (75.42–95.6) | 0.003 |
| Bleeding on probing (BoP), median (IQR) | 57.61 (46.28–71.71) | 48.77 (43.88–57.48) | 68.75 (59.96–82.01) | <0.001 |
| Average probing depth (PD, mm), median (IQR) | 3.12 (2.79–3.64) | 2.980 (2.78–3.12) | 3.635 (3.027–4.24) | <0.001 |
| Average total CAL (mm), median (IQR) | 4.46 (3.88–5.29) | 4.05 (3.54–4.43) | 5.01 (4.54–6.09) | <0.001 |
| PESA, median (IQR) | 1555.39 (1246.4–1908.74) | 1348.915 (1159.71–1648.76) | 1827.8 (1470.46–2229.54) | <0.001 |
| PISA, median (IQR) | 972.16 (681.87–1310.81) | 710.235 (496.66–932.17) | 1308.270 (1078.89–1691.51) | <0.001 |
| Periodontitis: no n (%) | 6 (6) | 6 (12) | 0 (0) | 0.012 |
| Periodontitis: yes n (%) | 94 (94) | 44 (88) | 50 (100) | |
| Periodontitis stage I, II n (%) | 50 (50) | 46 (92) | 4 (8) | <0.001 |
| Periodontitis stage III, IV n (%) | 50 (50) | 4 (8) | 46 (92) |
| Variable, Median (IQR) | All (N = 100) | Non-Cirrhosis (N = 50) | Cirrhosis (N = 50) | p |
|---|---|---|---|---|
| MELD | NA | NA | 16 (9–21) | NA |
| Alkaline phosphatase | 83.5 (61.75–128) | 65 (56.5–82) | 115.5 (86.25–144.5) | <0.001 |
| AST | 26 (19–41.75) | 19.5 (16.25–25) | 42.5 (29–73.5) | <0.001 |
| ALT | 23 (15.75–35.25) | 19.5 (15–25.75) | 24 (18.25–39.25) | 0.012 |
| GGT | 33 (20.75–74.25) | 21.5 (16–33) | 56.5 (36.25–188) | <0.001 |
| Bilirubin total | 13 (8–32.5) | 9 (7–12) | 32.5 (14.75–82.75) | <0.001 |
| Albumin (g/L) | 38 (29–42) | 41 (37.25–43) | 32 (25.33–38.75) | <0.001 |
| Prothrombin time (%) | 99 (63.5–116.25) | 116 (105.25–121) | 63 (46.25–82.5) | <0.001 |
| Variable | 95%CI | 95%CI | |||||
|---|---|---|---|---|---|---|---|
| β | Low | hi | OR | Low | hi | p | |
| Gender (F) | 1.5 | −0.8 | 3.9 | 4.6 | 0.4 | 48.4 | 0.209 |
| Age | 1.0 | −0.1 | 2.1 | 2.7 | 0.9 | 8.3 | 0.080 |
| BMI | −0.9 | −1.8 | 0.0 | 0.4 | 0.2 | 1.0 | 0.051 |
| Socio-econom. status | −0.3 | −1.6 | 0.9 | 0.7 | 0.2 | 2.4 | 0.579 |
| Smoking | −1.2 | −3.1 | 0.8 | 0.3 | 0.0 | 2.2 | 0.244 |
| Alcohol consumption | 5.6 | 4.0 | 7.3 | 275.0 | 52.8 | 1432.9 | <0.001 |
| PISA | 3.3 | 2.1 | 4.6 | 28.3 | 8.3 | 96.8 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinčić, G.; Roguljić, M.; Rinčić, N.; Jukić, L.V.; Gaćina, P.; Božić, D.; Badovinac, A. Is Periodontal Inflammation Associated with Liver Cirrhosis? A Cross-Sectional Study. J. Clin. Med. 2025, 14, 6616. https://doi.org/10.3390/jcm14186616
Rinčić G, Roguljić M, Rinčić N, Jukić LV, Gaćina P, Božić D, Badovinac A. Is Periodontal Inflammation Associated with Liver Cirrhosis? A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(18):6616. https://doi.org/10.3390/jcm14186616
Chicago/Turabian StyleRinčić, Goran, Marija Roguljić, Nives Rinčić, Lucija Virović Jukić, Petar Gaćina, Darko Božić, and Ana Badovinac. 2025. "Is Periodontal Inflammation Associated with Liver Cirrhosis? A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 18: 6616. https://doi.org/10.3390/jcm14186616
APA StyleRinčić, G., Roguljić, M., Rinčić, N., Jukić, L. V., Gaćina, P., Božić, D., & Badovinac, A. (2025). Is Periodontal Inflammation Associated with Liver Cirrhosis? A Cross-Sectional Study. Journal of Clinical Medicine, 14(18), 6616. https://doi.org/10.3390/jcm14186616






