Action Observation Training for Upper Limb Stroke Rehabilitation: A Pilot Study on the Role of Attention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. AOT Protocol
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Group without attention deficit |
| AOT | Action observation training |
| BI | Barthel index |
| BBT | Box and block test |
| CI | Confidence interval |
| CRIq | Cognitive reserve index questionnaire |
| FMA-UE | Fugl-Meyer assessment for upper extremity |
| LCD | Liquid crystal display |
| MNS | Mirror neuron system |
| NIHSS | National Institute of Health Stroke Scale |
| No_AD | Group without attention deficit |
| RM-ANOVA | Repeated-measures analysis of variance |
| SD | Standard deviation |
| TAP | Test of attentional performance |
| W | Week |
References
- Brainin, M.; Tuomilehto, J.; Heiss, W.-D.; Bornstein, N.M.; Bath, P.M.W.; Teuschl, Y.; Richard, E.; Guekht, A.; Quinn, T.; Post Stroke Cognition Study Group. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur. J. Neurol. 2015, 22, 229-e16. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Schöttke, H.; Giabbiconi, C.-M. Post-stroke depression and post-stroke anxiety: Prevalence and predictors. Int. Psychogeriatr. 2015, 27, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.; Domingo, J.; Rodríguez-Garcia, E.; Castro, M.-D.; del Ser, T. Frequency of cognitive impairment without dementia in patients with stroke: A two-year follow-up study. Stroke 2007, 38, 105–110. [Google Scholar] [CrossRef]
- Min, K.; Min, J. Health-related quality of life is associated with stroke deficits in older adults. Age Ageing 2015, 44, 700–704. [Google Scholar] [CrossRef]
- Tsalta-Mladenov, M.; Andonova, S. Health-related quality of life after ischemic stroke: Impact of sociodemographic and clinical factors. Neurol. Res. 2021, 43, 553–561. [Google Scholar] [CrossRef]
- Cramer, S.C.; Richards, L.G.; Bernhardt, J.; Duncan, P. Cognitive Deficits After Stroke. Stroke 2023, 54, 5–9. [Google Scholar] [CrossRef]
- O’Donoghue, M.; Leahy, S.; Boland, P.; Galvin, R.; McManus, J.; Hayes, S. Rehabilitation of Cognitive Deficits Poststroke: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stroke 2022, 53, 1700–1710. [Google Scholar] [CrossRef]
- Hyndman, D.; Pickering, R.M.; Ashburn, A. The influence of attention deficits on functional recovery post stroke during the first 12 months after discharge from hospital. J. Neurol. Neurosurg. Psychiatry 2008, 79, 656–663. [Google Scholar] [CrossRef]
- Hyndman, D.; Ashburn, A. People with stroke living in the community: Attention deficits, balance, ADL ability and falls. Disabil. Rehabil. 2003, 25, 817–822. [Google Scholar] [CrossRef]
- Loetscher, T.; Potter, K.-J.; Wong, D.; das Nair, R. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst. Rev. 2019, 2019, CD002842. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Tang, P.-F.; Peng, Y.-C.; Chen, H.-Y. Meta-analysis of type and complexity of a secondary task during walking on the prediction of elderly falls. Geriatr. Gerontol. Int. 2013, 13, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lequerica, A.H.; Donnell, C.S.; Tate, D.G. Patient engagement in rehabilitation therapy: Physical and occupational therapist impressions. Disabil. Rehabil. 2009, 31, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Wang, D.; Rezaei, M.J. Stroke rehabilitation: From diagnosis to therapy. Front. Neurol. 2024, 15, 1402729. [Google Scholar] [CrossRef] [PubMed]
- Oyake, K.; Suzuki, M.; Otaka, Y.; Momose, K.; Tanaka, S. Motivational Strategies for Stroke Rehabilitation: A Delphi Study. Arch. Phys. Med. Rehabil. 2020, 101, 1929–1936. [Google Scholar] [CrossRef]
- Bright, F.A.S.; Kayes, N.M.; Worrall, L.; McPherson, K.M. A conceptual review of engagement in healthcare and rehabilitation. Disabil. Rehabil. 2015, 37, 643–654. [Google Scholar] [CrossRef]
- Danzl, M.M.; Etter, N.M.; Andreatta, R.D.; Kitzman, P.H. Facilitating neurorehabilitation through principles of engagement. J. Allied Health 2012, 41, 35–41. [Google Scholar]
- Kringle, E.A.; Terhorst, L.; Butters, M.A.; Skidmore, E.R. Clinical Predictors of Engagement in Inpatient Rehabilitation Among Stroke Survivors With Cognitive Deficits: An Exploratory Study. J. Int. Neuropsychol. Soc. 2018, 24, 572–583. [Google Scholar] [CrossRef]
- Teo, J.L.; Zheng, Z.; Bird, S.R. Identifying the factors affecting ‘patient engagement’ in exercise rehabilitation. BMC Sports Sci. Med. Rehabil. 2022, 14, 18. [Google Scholar] [CrossRef]
- Brett, C.E.; Sykes, C.; Pires-Yfantouda, R. Interventions to increase engagement with rehabilitation in adults with acquired brain injury: A systematic review. Neuropsychol. Rehabil. 2017, 27, 959–982. [Google Scholar] [CrossRef]
- Lequerica, A.H.; Rapport, L.J.; Whitman, R.D.; Millis, S.R.; Vangel, S.J., Jr.; Hanks, R.A.; Axelrod, B.N. Psychometric properties of the rehabilitation therapy engagement scale when used among individuals with acquired brain injury. Rehabil. Psychol. 2006, 51, 331–337. [Google Scholar] [CrossRef]
- Evangelista, G.G.; Egger, P.; Brügger, J.; Beanato, E.; Koch, P.J.; Ceroni, M.; Fleury, L.; Cadic-Melchior, A.; Meyer, N.H.; de León Rodríguez, D.; et al. Differential Impact of Brain Network Efficiency on Poststroke Motor and Attentional Deficits. Stroke 2023, 54, 955–963. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, D.; Romeo, Z.; Maistrello, L.; Durgoni, E.; Della Pietà, C.; De Filippo De Grazia, M.; Meneghello, F.; Turolla, A.; Zorzi, M. Sensorimotor, Attentional, and Neuroanatomical Predictors of Upper Limb Motor Deficits and Rehabilitation Outcome after Stroke. Neural Plast. 2021, 2021, 8845685. [Google Scholar] [CrossRef] [PubMed]
- James, J.; McGlinchey, M.P. How active are stroke patients in physiotherapy sessions and is this associated with stroke severity? Disabil. Rehabil. 2022, 44, 4408–4414. [Google Scholar] [CrossRef]
- Stolwyk, R.J.; Mihaljcic, T.; Wong, D.K.; Chapman, J.E.; Rogers, J.M. Poststroke Cognitive Impairment Negatively Impacts Activity and Participation Outcomes: A Systematic Review and Meta-Analysis. Stroke 2021, 52, 748–760. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Antonioni, A.; Galluccio, M.; Baroni, A.; Fregna, G.; Pozzo, T.; Koch, G.; Manfredini, F.; Fadiga, L.; Malerba, P.; Straudi, S. Event-related desynchronization during action observation is an early predictor of recovery in subcortical stroke: An EEG study. Ann. Phys. Rehabil. Med. 2024, 67, 101817. [Google Scholar] [CrossRef]
- Antonioni, A.; Raho, E.M.; Straudi, S.; Granieri, E.; Koch, G.; Fadiga, L. The cerebellum and the Mirror Neuron System: A matter of inhibition? From neurophysiological evidence to neuromodulatory implications. A narrative review. Neurosci. Biobehav. Rev. 2024, 164, 105830. [Google Scholar] [CrossRef]
- Sarasso, E.; Gemma, M.; Agosta, F.; Filippi, M.; Gatti, R. Action observation training to improve motor function recovery: A systematic review. Arch. Physiother. 2015, 5, 14. [Google Scholar] [CrossRef]
- Zhang, J.J.Q.; Fong, K.N.K.; Welage, N.; Liu, K.P.Y. The Activation of the Mirror Neuron System during Action Observation and Action Execution with Mirror Visual Feedback in Stroke: A Systematic Review. Neural Plast. 2018, 2018, 2321045. [Google Scholar] [CrossRef]
- Bassolino, M.; Campanella, M.; Bove, M.; Pozzo, T.; Fadiga, L. Training the Motor Cortex by Observing the Actions of Others During Immobilization. Cereb. Cortex 2014, 24, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Nummenmaa, L.; Smirnov, D.; Lahnakoski, J.M.; Glerean, E.; Jääskeläinen, I.P.; Sams, M.; Hari, R. Mental action simulation synchronizes action-observation circuits across individuals. J. Neurosci. 2014, 34, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Kemmerer, D. What modulates the Mirror Neuron System during action observation?: Multiple factors involving the action, the actor, the observer, the relationship between actor and observer, and the context. Prog. Neurobiol. 2021, 205, 102128. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, G.; Gonzalez, C.C.; Nowicky, A.V.; Williams, A.M.; Bishop, D.T. Motor resonance during action observation is gaze-contingent: A TMS study. Neuropsychologia 2017, 103, 77–86. [Google Scholar] [CrossRef]
- Sánchez Silverio, V.; Abuín Porras, V.; Rodríguez Costa, I.; Cleland, J.A.; Villafañe, J.H. Effects of action observation training on the walking ability of patients post stroke: A systematic review. Disabil. Rehabil. 2022, 44, 7339–7348. [Google Scholar] [CrossRef]
- Caligiore, D.; Mustile, M.; Fineschi, A.; Romano, L.; Piras, F.; Assogna, F.; Pontieri, F.E.; Spalletta, G.; Baldassarre, G. Action Observation With Dual Task for Improving Cognitive Abilities in Parkinson’s Disease: A Pilot Study. Front. Syst. Neurosci. 2019, 13, 7. [Google Scholar] [CrossRef]
- Lim, H.; Jeong, C.H.; Kang, Y.J.; Ku, J. Attentional State-Dependent Peripheral Electrical Stimulation During Action Observation Enhances Cortical Activations in Stroke Patients. Cyberpsychol. Behav. Soc. Netw. 2023, 26, 408–416. [Google Scholar] [CrossRef]
- Borges, L.R.; Fernandes, A.B.; Oliveira Dos Passos, J.; Rego, I.A.O.; Campos, T.F. Action observation for upper limb rehabilitation after stroke. Cochrane Database Syst. Rev. 2022, 8, CD011887. [Google Scholar] [CrossRef]
- Zhang, B.; Kan, L.; Dong, A.; Zhang, J.; Bai, Z.; Xie, Y.; Liu, Q.; Peng, Y. The effects of action observation training on improving upper limb motor functions in people with stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221166. [Google Scholar] [CrossRef]
- Boni, S.; Galluccio, M.; Baroni, A.; Martinuzzi, C.; Milani, G.; Emanuele, M.; Straudi, S.; Fadiga, L.; Pozzo, T. Action Observation Therapy for Arm Recovery after Stroke: A Preliminary Investigation on a Novel Protocol with EEG Monitoring. J. Clin. Med. 2023, 12, 1327. [Google Scholar] [CrossRef]
- Sugg, K.; Müller, S.; Winstein, C.; Hathorn, D.; Dempsey, A. Does Action Observation Training With Immediate Physical Practice Improve Hemiparetic Upper-Limb Function in Chronic Stroke? Neurorehabil. Neural Repair 2015, 29, 807–817. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; PAFS Consensus Group. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [CrossRef]
- Zimmermann, P.; Fimm, B. A test battery for attentional performance. In Applied Neuropsychology of Attention; Psychology Press: Abingdon, UK, 2002; ISBN 978-0-203-30701-4. [Google Scholar]
- Schmidt, G.J.; Boechat, Y.E.M.; van Duinkerken, E.; Schmidt, J.J.; Moreira, T.B.; Nicaretta, D.H.; Schmidt, S.L. Detection of Cognitive Dysfunction in Elderly with a Low Educational Level Using a Reaction-Time Attention Task. J. Alzheimer’s Dis. 2020, 78, 1197–1205. [Google Scholar] [CrossRef]
- Schmidt, S.L.; Boechat, Y.E.M.; Schmidt, G.J.; Nicaretta, D.; van Duinkerken, E.; Schmidt, J.J. Clinical Utility of a Reaction-Time Attention Task in the Evaluation of Cognitive Impairment in Elderly with High Educational Disparity. J. Alzheimer’s Dis. 2021, 81, 691–697. [Google Scholar] [CrossRef]
- Schumacher, R.; Halai, A.D.; Lambon Ralph, M.A. Attention to attention in aphasia-elucidating impairment patterns, modality differences and neural correlates. Neuropsychologia 2022, 177, 108413. [Google Scholar] [CrossRef]
- Dunstan, D.A.; Scott, N.; Todd, A.K. Screening for anxiety and depression: Reassessing the utility of the Zung scales. BMC Psychiatry 2017, 17, 329. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S.; et al. Fugl-Meyer assessment of sensorimotor function after stroke: Standardized training procedure for clinical practice and clinical trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef]
- Hsiao, C.-P.; Zhao, C.; Do, E.Y.-L. The Digital Box and Block Test Automating traditional post-stroke rehabilitation assessment. In Proceedings of the 2013 IEEE International Conference on Pervasive Computing and Communications Workshops (PERCOM Workshops), San Diego, CA, USA, 18–22 March 2013; pp. 360–363. [Google Scholar]
- Galeoto, G.; Formica, M.C.; Mercuri, N.B.; Santilli, V.; Berardi, A.C.; Castiglia, S.F.; Mollica, R.; Servadio, A. Evaluation of the psychometric properties of the Barthel Index in an Italian ischemic stroke population in the acute phase: A cross-sectional study. Funct. Neurol. 2019, 34, 29–34. [Google Scholar]
- Galeoto, G.; Iori, F.; De Santis, R.; Santilli, V.; Mollica, R.; Marquez, M.A.; Sansoni, J.; Berardi, A. The outcome measures for loss of functionality in the activities of daily living of adults after stroke: A systematic review. Top. Stroke Rehabil. 2019, 26, 236–245. [Google Scholar] [CrossRef]
- Draheim, C.; Pak, R.; Draheim, A.A.; Engle, R.W. The role of attention control in complex real-world tasks. Psychon. Bull. Rev. 2022, 29, 1143–1197. [Google Scholar] [CrossRef]
- Buccino, G. Action observation treatment: A novel tool in neurorehabilitation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130185. [Google Scholar] [CrossRef]
- Errante, A.; Saviola, D.; Cantoni, M.; Iannuzzelli, K.; Ziccarelli, S.; Togni, F.; Simonini, M.; Malchiodi, C.; Bertoni, D.; Inzaghi, M.G.; et al. Effectiveness of action observation therapy based on virtual reality technology in the motor rehabilitation of paretic stroke patients: A randomized clinical trial. BMC Neurol. 2022, 22, 109. [Google Scholar] [CrossRef]
- Antonioni, A.; Cellini, N.; Baroni, A.; Fregna, G.; Lamberti, N.; Koch, G.; Manfredini, F.; Straudi, S. Characterizing practice-dependent motor learning after a stroke. Neurol. Sci. 2025, 46, 1245–1255. [Google Scholar] [CrossRef]
- Alawieh, A.; Zhao, J.; Feng, W. Factors affecting post-stroke motor recovery: Implications on neurotherapy after brain injury. Behav. Brain Res. 2018, 340, 94–101. [Google Scholar] [CrossRef]
- Chen, C.; Leys, D.; Esquenazi, A. The interaction between neuropsychological and motor deficits in patients after stroke. Neurology 2013, 80, S27–S34. [Google Scholar] [CrossRef]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.-B.; Askim, T.; Beyer, M.K.; Næss, H.; et al. Associations between post-stroke motor and cognitive function: A cross-sectional study. BMC Geriatr. 2021, 21, 103. [Google Scholar] [CrossRef]
- Shimonaga, K.; Hama, S.; Tsuji, T.; Yoshimura, K.; Nishino, S.; Yanagawa, A.; Soh, Z.; Matsushige, T.; Mizoue, T.; Onoda, K.; et al. The right hemisphere is important for driving-related cognitive function after stroke. Neurosurg. Rev. 2021, 44, 977–985. [Google Scholar] [CrossRef]
- Spaccavento, S.; Marinelli, C.V.; Nardulli, R.; Macchitella, L.; Bivona, U.; Piccardi, L.; Zoccolotti, P.; Angelelli, P. Attention Deficits in Stroke Patients: The Role of Lesion Characteristics, Time from Stroke, and Concomitant Neuropsychological Deficits. Behav. Neurol. 2019, 2019, 7835710. [Google Scholar] [CrossRef]
- Stein, M.S.; Kilbride, C.; Reynolds, F.A. What are the functional outcomes of right hemisphere stroke patients with or without hemi-inattention complications? A critical narrative review and suggestions for further research. Disabil. Rehabil. 2016, 38, 315–328. [Google Scholar] [CrossRef][Green Version]
- Corbetta, M.; Shulman, G.L. Spatial Neglect and Attention Networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef]
- Husain, M.; Shapiro, K.; Martin, J.; Kennard, C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature 1997, 385, 154–156. [Google Scholar] [CrossRef]
- Malhotra, P.; Coulthard, E.J.; Husain, M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 2009, 132, 645–660. [Google Scholar] [CrossRef]
- Robertson, I.H.; Manly, T.; Beschin, N.; Daini, R.; Haeske-Dewick, H.; Hömberg, V.; Jehkonen, M.; Pizzamiglio, G.; Shiel, A.; Weber, E. Auditory sustained attention is a marker of unilateral spatial neglect. Neuropsychologia 1997, 35, 1527–1532. [Google Scholar] [CrossRef]
- Bronnick, K.; Ehrt, U.; Emre, M.; Deyn, P.P.D.; Wesnes, K.; Tekin, S.; Aarsland, D. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1136–1142. [Google Scholar] [CrossRef]
- Cheng, S.-T. Cognitive Reserve and the Prevention of Dementia: The Role of Physical and Cognitive Activities. Curr. Psychiatry Rep. 2016, 18, 85. [Google Scholar] [CrossRef]
- Fritsch, T.; McClendon, M.J.; Smyth, K.A.; Lerner, A.J.; Friedland, R.P.; Larsen, J.D. Cognitive Functioning in Healthy Aging: The Role of Reserve and Lifestyle Factors Early in Life. Gerontologist 2007, 47, 307–322. [Google Scholar] [CrossRef]
- Umarova, R.M.; Sperber, C.; Kaller, C.P.; Schmidt, C.S.M.; Urbach, H.; Klöppel, S.; Weiller, C.; Karnath, H.-O. Cognitive Reserve Impacts on Disability and Cognitive Deficits in Acute Stroke. J. Neurol. 2019, 266, 2495–2504. [Google Scholar] [CrossRef]
- Ojala-Oksala, J.; Jokinen, H.; Kopsi, V.; Lehtonen, K.; Luukkonen, L.; Paukkunen, A.; Seeck, L.; Melkas, S.; Pohjasvaara, T.; Karhunen, P.; et al. Educational History Is an Independent Predictor of Cognitive Deficits and Long-Term Survival in Postacute Patients with Mild to Moderate Ischemic Stroke. Stroke 2012, 43, 2931–2935. [Google Scholar] [CrossRef]
- Spampinato, D.; Celnik, P. Multiple Motor Learning Processes in Humans: Defining Their Neurophysiological Bases. Neuroscientist 2021, 27, 246–267. [Google Scholar] [CrossRef]
- Peters, S.; Handy, T.C.; Lakhani, B.; Boyd, L.A.; Garland, S.J. Motor and Visuospatial Attention and Motor Planning After Stroke: Considerations for the Rehabilitation of Standing Balance and Gait. Phys. Ther. 2015, 95, 1423–1432. [Google Scholar] [CrossRef]
- Proto, D.; Pella, R.D.; Hill, B.D.; Gouvier, W.D. Assessment and rehabilitation of acquired visuospatial and proprioceptive deficits associated with visuospatial neglect. NeuroRehabilitation 2009, 24, 145–157. [Google Scholar] [CrossRef]
- Saxena, S.K.; Ng, T.-P.; Koh, G.; Yong, D.; Fong, N.P. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta Neurol. Scand. 2007, 115, 339–346. [Google Scholar] [CrossRef]
- Mao, H.; Li, Y.; Tang, L.; Chen, Y.; Ni, J.; Liu, L.; Shan, C. Effects of mirror neuron system-based training on rehabilitation of stroke patients. Brain Behav. 2020, 10, e01729. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabil. Neural. Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Matthews, G.; Warm, J.S.; Reinerman, L.E.; Langheim, L.K.; Saxby, D.J. Task Engagement, Attention, and Executive Control. In Handbook of Individual Differences in Cognition: Attention, Memory, and Executive Control; Gruszka, A., Matthews, G., Szymura, B., Eds.; Springer: New York, NY, USA, 2010; pp. 205–230. ISBN 978-1-4419-1210-7. [Google Scholar]
- Bosio, S.B.; Graffigna, G.; Vegni, E.; Bosio, A.C. Claudio The Challenges of Conceptualizing Patient Engagement in Health Care: A Lexicographic Literature Review. J. Particip. Med. 2014, 6, 259–267. [Google Scholar]
- Fisher, E.S.; McClellan, M.B.; Safran, D.G. Building the path to accountable care. N. Engl. J. Med. 2011, 365, 2445–2447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, B.; Liu, Z.; Mei, Y.; Li, X.; Ma, L.; Zhang, Z. Patient engagement in rehabilitation: An evolutionary concept analysis. Clin Rehabil 2025, 39, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Lequerica, A.H.; Kortte, K. Therapeutic Engagement: A Proposed Model of Engagement in Medical Rehabilitation. Am. J. Phys. Med. Rehabil. 2010, 89, 415. [Google Scholar] [CrossRef]
- Moucha, R.; Kilgard, M.P. Cortical plasticity and rehabilitation. Prog. Brain Res. 2006, 157, 111–122. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Marshall, I.J.; Wolfe, C.D.; Wang, Y.; O’Connell, M.D. Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Med. 2023, 20, e1004200. [Google Scholar] [CrossRef]
- Barker-Collo, S.L. Depression and anxiety 3 months post stroke: Prevalence and correlates. Arch. Clin. Neuropsychol. 2007, 22, 519–531. [Google Scholar] [CrossRef]
- Backhouse, E.V.; McHutchison, C.A.; Cvoro, V.; Shenkin, S.D.; Wardlaw, J.M. Cognitive ability, education and socioeconomic status in childhood and risk of post-stroke depression in later life: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0200525. [Google Scholar] [CrossRef]
- Verdelho, A.; Madureira, S.; Moleiro, C.; Ferro, J.M.; O’Brien, J.T.; Poggesi, A.; Pantoni, L.; Fazekas, F.; Scheltens, P.; Waldemar, G.; et al. Depressive symptoms predict cognitive decline and dementia in older people independently of cerebral white matter changes: The LADIS study. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1250–1254. [Google Scholar] [CrossRef]
- Rasquin, S.; Lodder, J.; Verhey, F. The association between psychiatric and cognitive symptoms after stroke: A prospective study. Cerebrovasc. Dis. 2005, 19, 309–316. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Dancause, N.; Lannin, N.A.; Ward, N.S.; Nudo, R.J.; Farrin, A.; Churilov, L.; Boyd, L.A.; Jones, T.A.; et al. A Stroke Recovery Trial Development Framework: Consensus-Based Core Recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Neurorehabil. Neural. Repair 2019, 33, 959–969. [Google Scholar] [CrossRef]
- Calabrò, R.S.; De Cola, M.C.; Leo, A.; Reitano, S.; Balletta, T.; Trombetta, G.; Naro, A.; Russo, M.; Bertè, F.; De Luca, R.; et al. Robotic neurorehabilitation in patients with chronic stroke: Psychological well-being beyond motor improvement. Int. J. Rehabil. Res. 2015, 38, 219. [Google Scholar] [CrossRef] [PubMed]
- Champod, A.S.; Eskes, G.A.; Barrett, A.M. Neuropsychological Rehabilitation. In Neurovascular Neuropsychology; Lazar, R.M., Pavol, M.A., Browndyke, J.N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 415–463. ISBN 978-3-030-49586-2. [Google Scholar]
- Betti, S.; Castiello, U.; Guerra, S.; Sartori, L. Overt orienting of spatial attention and corticospinal excitability during action observation are unrelated. PLoS ONE 2017, 12, e0173114. [Google Scholar] [CrossRef] [PubMed]
- Hétu, S.; Mercier, C.; Eugène, F.; Michon, P.-E.; Jackson, P.L. Modulation of brain activity during action observation: Influence of perspective, transitivity and meaningfulness. PLoS ONE 2011, 6, e24728. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Gignac, G.E.; Weinborn, M.; Green, S.; Pestell, C. A Meta-Analysis of Neuropsychological Predictors of Outcome Following Stroke and Other Non-Traumatic Acquired Brain Injuries in Adults. Neuropsychol. Rev. 2020, 30, 194–223. [Google Scholar] [CrossRef]
- Hommel, B.; Chapman, C.S.; Cisek, P.; Neyedli, H.F.; Song, J.-H.; Welsh, T.N. No one knows what attention is. Atten. Percept. Psychophys. 2019, 81, 2288–2303. [Google Scholar] [CrossRef]
- Mengotti, P.; Käsbauer, A.S.; Fink, G.R.; Vossel, S. Lateralization, functional specialization, and dysfunction of attentional networks. Cortex 2020, 132, 206–222. [Google Scholar] [CrossRef]
- Barker-Collo, S.; Feigin, V.; Lawes, C.; Senior, H.; Parag, V. Natural history of attention deficits and their influence on functional recovery from acute stages to 6 months after stroke. Neuroepidemiology 2010, 35, 255–262. [Google Scholar] [CrossRef]
- Crotti, M.; Koschutnig, K.; Wriessnegger, S.C. Handedness impacts the neural correlates of kinesthetic motor imagery and execution: A FMRI study. J. Neurosci. Res. 2022, 100, 798–826. [Google Scholar] [CrossRef]
- Kirby, K.M.; Pillai, S.R.; Carmichael, O.T.; Van Gemmert, A.W.A. Brain functional differences in visuo-motor task adaptation between dominant and non-dominant hand training. Exp. Brain Res. 2019, 237, 3109–3121. [Google Scholar] [CrossRef]
- Embrechts, E.; Loureiro-Chaves, R.; Nijboer, T.C.W.; Lafosse, C.; Truijen, S.; Saeys, W. The Association of Personal Neglect with Motor, Activities of Daily Living, and Participation Outcomes after Stroke: A Systematic Review. Arch. Clin. Neuropsychol. 2024, 39, 249–264. [Google Scholar] [CrossRef]
- Hama, S.; Yoshimura, K.; Yanagawa, A.; Shimonaga, K.; Furui, A.; Soh, Z.; Nishino, S.; Hirano, H.; Yamawaki, S.; Tsuji, T. Relationships between motor and cognitive functions and subsequent post-stroke mood disorders revealed by machine learning analysis. Sci. Rep. 2020, 10, 19571. [Google Scholar] [CrossRef]
- Contador, I.; Alzola, P.; Stern, Y.; de la Torre-Luque, A.; Bermejo-Pareja, F.; Fernández-Calvo, B. Is cognitive reserve associated with the prevention of cognitive decline after stroke? A Systematic review and meta-analysis. Ageing Res. Rev. 2023, 84, 101814. [Google Scholar] [CrossRef] [PubMed]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage 2007, 36, T164–T173. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, K. Effects of purposeful action observation on kinematic patterns of upper extremity in individuals with hemiplegia. J. Phys. Ther. Sci. 2015, 27, 1809–1811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antonioni, A.; Galluccio, M.; Toselli, R.; Baroni, A.; Fregna, G.; Schincaglia, N.; Milani, G.; Cosma, M.; Ferraresi, G.; Morelli, M.; et al. A Multimodal Analysis to Explore Upper Limb Motor Recovery at 4 Weeks After Stroke: Insights From EEG and Kinematics Measures. Clin. EEG Neurosci. 2023, 55, 465–476. [Google Scholar] [CrossRef]
- Baroni, A.; Antonioni, A.; Fregna, G.; Lamberti, N.; Manfredini, F.; Koch, G.; D’Ausilio, A.; Straudi, S. The Effectiveness of Paired Associative Stimulation on Motor Recovery after Stroke: A Scoping Review. Neurol. Int. 2024, 16, 567–589. [Google Scholar] [CrossRef]
- Milani, G.; Antonioni, A.; Baroni, A.; Malerba, P.; Straudi, S. Relation Between EEG Measures and Upper Limb Motor Recovery in Stroke Patients: A Scoping Review. Brain Topogr. 2022, 35, 651–666. [Google Scholar] [CrossRef]
- Ordikhani-Seyedlar, M.; Lebedev, M.A.; Sorensen, H.B.D.; Puthusserypady, S. Neurofeedback Therapy for Enhancing Visual Attention: State-of-the-Art and Challenges. Front. Neurosci. 2016, 10, 352. [Google Scholar] [CrossRef]
- Kwakkel, G.; Stinear, C.; Essers, B.; Munoz-Novoa, M.; Branscheidt, M.; Cabanas-Valdés, R.; Lakičević, S.; Lampropoulou, S.; Luft, A.R.; Marque, P.; et al. Motor rehabilitation after stroke: European Stroke Organisation (ESO) consensus-based definition and guiding framework. Eur. Stroke J. 2023, 8, 880–894. [Google Scholar] [CrossRef]
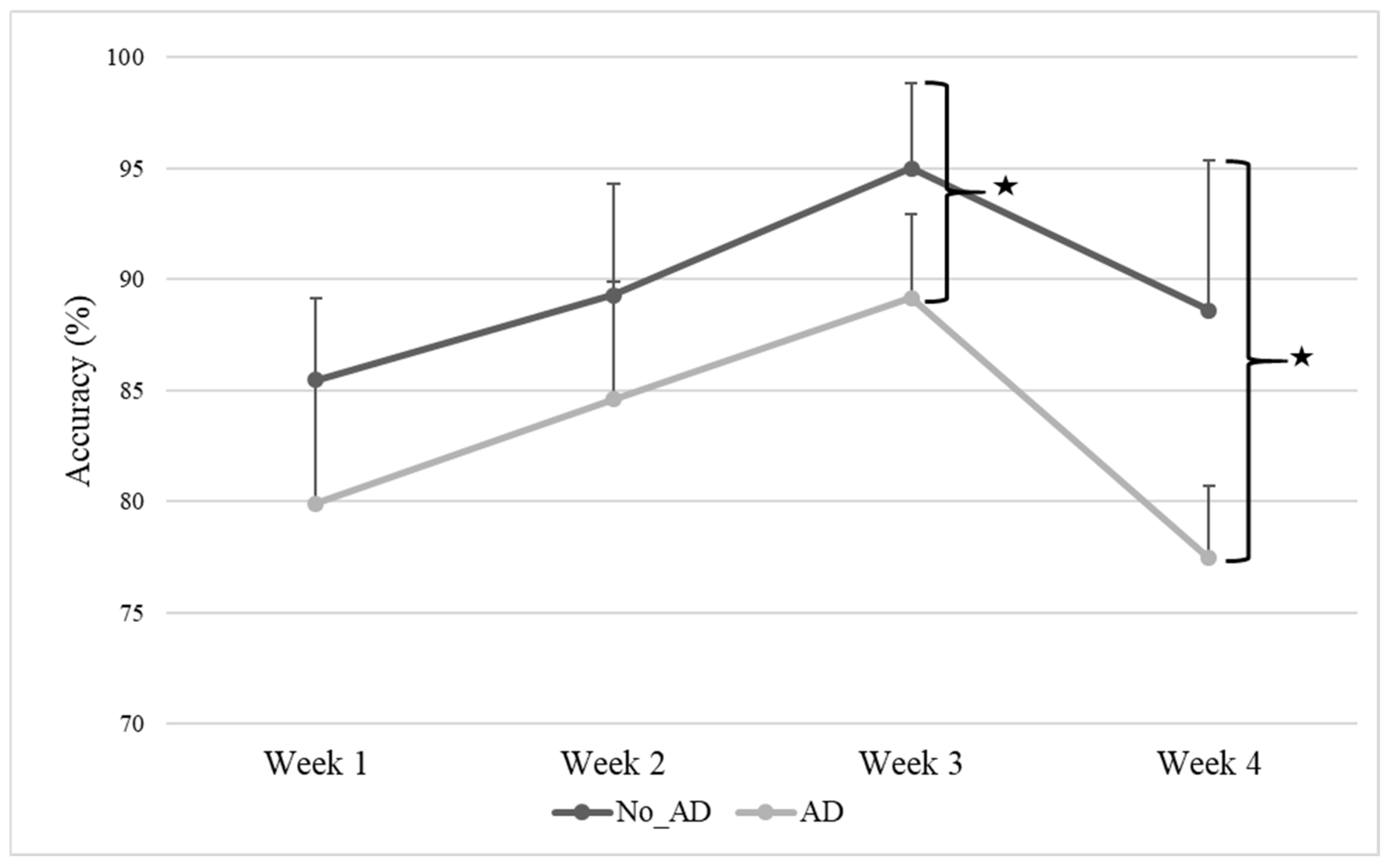

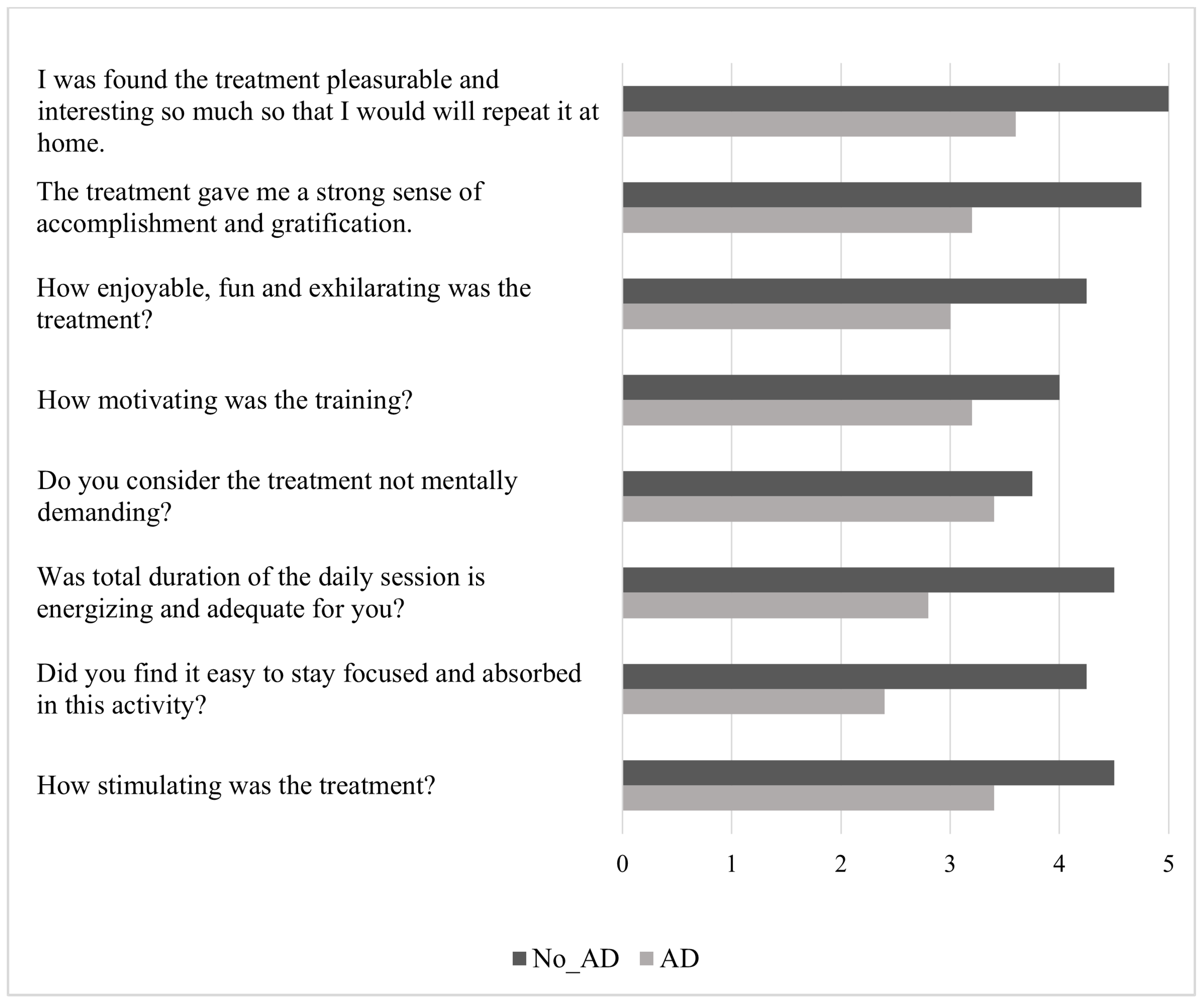
| Measures | Total Sample (N = 10) | No_AD Group (N = 4) | AD Group (N = 6) | p Value |
|---|---|---|---|---|
| Stroke severity (mild,%) | 5 (50%) | 3 (75%) | 2 (33.33%) | 0.242 |
| Sex (male,%) | 7 (70%) | 3 (75%) | 4 (66.66%) | 0.807 |
| Age (mean, SD) | 57.9 (9.22) | 56.5 (4.20) | 58.8 (11.8) | 0.719 |
| Stroke type (hemorrhagic, %) | 6 (60%) | 3 (75%) | 3 (50%) | 0.486 |
| Dominant hand (left, %) | 3 (30%) | 3 (75%) | 0 (0%) | 0.005 * |
| NIHSS entry (mean, SD) | 12.6 (3.24) | 10.3 (2.89) | 13.7 (3.01) | 0.157 |
| Measures | Total Sample (N = 10) | No_AD Group (N = 4) | AD Group (N = 6) | p Value |
|---|---|---|---|---|
| TAP: Go/Nogo_Reaction Time (mean, SD) [95%CI] | 591 (130) | 444.07 (40.57) [343.29, 544.85] | 678.95 (52.37) [613.93, 743.97] | <0.001 * |
| TAP: Dual task_Reaction Time (mean, SD) [95%CI] | 815 (185) | 667.64 (20.74) [638.34, 696.95] | 925.63 (173.98) [712.78, 1138.49] | 0.051 |
| Anxious and depressive signs/symptoms (n, %) | 4 (40%) | 0 (0%) | 4 (66.66%) | 0.035 * |
| Neglect (n, %) | 4 (40%) | 0 (0%) | 4 (66.66%) | 0.035 * |
| CRIq (mean, SD) [95%CI] | 105 (17.5) | 120 (14.9) [102.85, 137.15] | 92.2 (4.09) [87.99, 96.41] | 0.005 * |
| CRI-Education (mean, SD) [95%CI] | 96.2 (14.9) | 107.5 (8.13) [88.75, 126.25] | 87.2 (1.71) [83.25, 91.15] | 0.029 * |
| CRI-Working Activity (mean, SD) [95%CI] | 102 (12.1) | 110.75 (6.57) [95.59, 125.91] | 94.8 (1.98) [90.22, 99.38] | 0.037 * |
| CRI-Leisure Time (mean, SD) [95%CI] | 112 (16.7) | 126.25 (12.8) [111.44, 141.06] | 100.4 (7.83) [92.33, 108.47] | 0.007 * |
| Measures | Total Sample (N = 10) | No_AD Group (N = 4) | AD Group (N = 6) | p Value |
|---|---|---|---|---|
| ∆ FMA-UPPER LIMB (mean, SD) [95%CI] | 6.52 (4.680) | 10.8 (5.68) [4.33, 17.17] | 3.33 (1.21) [2.21, 4.45] | 0.013 * |
| ∆ FMA-WRIST (mean, SD) [95%CI] | 2.75 (3.178) | 4.25 (3.10) [0.75, 7.75] | 1.33 (3.33) [−1.74, 4.41] | 0.201 |
| ∆ FMA-HAND (mean, SD) [95%CI] | 3.81 (4.398) | 5.75 (6.29) [−1.37, 12.87] | 1.83 (3.06) [−0.99, 4.66] | 0.219 |
| ∆ FMA total (mean, SD) [95%CI] | 13.53 (11.772) | 21.0 (16.4) [2.41, 39.59] | 7.33 (6.25) [1.56, 13.11] | 0.096 |
| ∆ BBT—PARETIC LIMB (mean, SD) [95%CI] | 11.34 (12.500) | 13.8 (12.0) [0.17, 27.33] | 8.00 (15.0) [−5.06, 22.06] | 0.540 |
| ∆ BI (mean, SD) [95%CI] | 25.49 (20.99) | 38.8 (26.9) [8.34, 69.16] | 15.8 (16.6) [0.54, 31.12] | 0.130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, G.; Baroni, A.; Galluccio, M.; Fregna, G.; Antonioni, A.; Straudi, S.; Pozzo, T.; Fadiga, L. Action Observation Training for Upper Limb Stroke Rehabilitation: A Pilot Study on the Role of Attention. J. Clin. Med. 2025, 14, 6618. https://doi.org/10.3390/jcm14186618
Milani G, Baroni A, Galluccio M, Fregna G, Antonioni A, Straudi S, Pozzo T, Fadiga L. Action Observation Training for Upper Limb Stroke Rehabilitation: A Pilot Study on the Role of Attention. Journal of Clinical Medicine. 2025; 14(18):6618. https://doi.org/10.3390/jcm14186618
Chicago/Turabian StyleMilani, Giada, Andrea Baroni, Martina Galluccio, Giulia Fregna, Annibale Antonioni, Sofia Straudi, Thierry Pozzo, and Luciano Fadiga. 2025. "Action Observation Training for Upper Limb Stroke Rehabilitation: A Pilot Study on the Role of Attention" Journal of Clinical Medicine 14, no. 18: 6618. https://doi.org/10.3390/jcm14186618
APA StyleMilani, G., Baroni, A., Galluccio, M., Fregna, G., Antonioni, A., Straudi, S., Pozzo, T., & Fadiga, L. (2025). Action Observation Training for Upper Limb Stroke Rehabilitation: A Pilot Study on the Role of Attention. Journal of Clinical Medicine, 14(18), 6618. https://doi.org/10.3390/jcm14186618










