Correlation Between Fungal and Bacterial Populations in Periodontitis Through Targeted Sequencing: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in U.S. adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588. [Google Scholar] [CrossRef]
- Parker, M.L.; Thornton-Evans, G.O.; Wei, L.; Griffin, S.O. Prevalence of and changes in tooth loss among adults aged ≥50 years with selected chronic conditions—United States, 1999–2004 and 2011–2016. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 641–646. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Simpson, T.C.; Clarkson, J.E.; Worthington, H.V.; MacDonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2022, 4, CD004714. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Simonson, L.G.; McMahon, K.T.; Childers, D.W.; Morton, H.E. Bacterial synergy of Treponema denticola and Porphyromonas gingivalis. Oral Microbiol. Immunol. 1992, 7, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.; Mulla, M.; Hegde, S.; Koshy, A.V. In vitro assessment of probiotic Lactobacillus reuteri on peri-implantitis microflora. BMC Oral Health 2021, 21, 408. [Google Scholar] [CrossRef]
- Grant, D.A.; Grant, D.A.; Flynn, M.J.; Slots, J. Periodontal microbiota of mobile and non-mobile teeth. J. Periodontol. 1995, 66, 386–390. [Google Scholar] [CrossRef]
- Chaves, E.S.; Jeffcoat, M.K.; Ryerson, C.C.; Snyder, B. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J. Clin. Periodontol. 2000, 27, 897–903. [Google Scholar] [CrossRef]
- Van Winkelhoff, A.J.; Loos, B.G.; Van Der Reijden, W.A.; Van Der Velden, U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J. Clin. Periodontol. 2002, 29, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Wu, J.; Hayes, R.B.; Ahn, J. The oral fungal mycobiome: Characteristics and relation to periodontitis in a pilot study. BMC Microbiol. 2017, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and early childhood caries: A systematic review and meta-analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Grier, A.; Faustoferri, R.C.; Alzoubi, S.; Gill, A.L.; Feng, C.; Liu, Y.; Quivey, R.G.; Kopycka-Kedzierawski, D.T.; Koo, H.; et al. Association between oral Candida and bacteriome in children with severe ECC. J. Dent. Res. 2018, 97, 1468–1476. [Google Scholar] [CrossRef]
- Krom, B.P.; Kidwai, S.; Ten Cate, J.M. Candida and other fungal species: Forgotten players of healthy oral microbiota. J. Dent. Res. 2014, 93, 445–451. [Google Scholar] [CrossRef]
- Vesty, A.; Biswas, K.; Taylor, M.W.; Gear, K.; Douglas, R.G. Evaluating the impact of DNA extraction method on the representation of human oral bacterial and fungal communities. PLoS ONE 2017, 12, e0169877. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Irinyi, L.; Meyer, W. DNA barcoding of human and animal pathogenic fungi: The ISHAM-ITS database. Microbiol. Aust. 2015, 36, 44–48. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. In Breakthroughs in Statistics: Methodology and Distribution; Springer: New York, NY, USA, 1992; pp. 196–202. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the oral microbiome: Beyond bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef]
- McLean, J.S. Advancements toward a systems level understanding of the human oral microbiome. Front. Cell. Infect. Microbiol. 2014, 4, 98. [Google Scholar] [CrossRef]
- Janus, M.M.; Crielaard, W.; Volgenant, C.M.C.; Van Der Veen, M.H.; Brandt, B.W.; Krom, B.P. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J. Oral Microbiol. 2017, 9, 1270613. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Ghedin, E. The human mycobiome in health and disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef]
- Rizzetto, L.; De Filippo, C.; Cavalieri, D. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Eur. J. Immunol. 2014, 44, 3166–3181. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, L.; Chen, P.; Han, M.; Song, L.; Tong, X.; Sun, X.; Yang, F.; Lin, Z.; Liu, X.; et al. Over 50,000 metagenomically assembled draft genomes for the human oral microbiome reveal new taxa. Genom. Proteom. Bioinform. 2022, 20, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Henriques, M.; Hayes, A.; Oliveira, R.; Azeredo, J.; Williams, D.W. Candida glabrata and Candida albicans co-infection of an in vitro oral epithelium. J. Oral Pathol. Med. 2011, 40, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Hong, B.-Y.; Dupuy, A.K.; Strausbaugh, L.D. Mining the oral mycobiome: Methods, components, and meaning. Virulence 2017, 8, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Dongari-Bagtzoglou, A. Critically appraising the significance of the oral mycobiome. J. Dent. Res. 2021, 100, 133–140. [Google Scholar] [CrossRef]
- Egan, M.W.; Spratt, D.A.; Ng, Y.-L.; Lam, J.M.; Moles, D.R.; Gulabivala, K. Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int. Endod. J. 2002, 35, 321–329. [Google Scholar] [CrossRef]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Boekhout, T.; Dawson, T.L., Jr. Skin diseases associated with Malassezia species. J. Am. Acad. Dermatol. 2004, 51, 785–798. [Google Scholar] [CrossRef]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: Discovery of Malassezia as a prominent commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef]
- Olm, M.R.; Brown, C.T.; Brooks, B.; Firek, B.; Baker, R.; Burstein, D.; Soenjoyo, K.; Thomas, B.C.; Morowitz, M.; Banfield, J.F. Identical Bacterial Populations Colonize Premature Infant Gut, Skin, and Oral Microbiomes and Exhibit Different In Situ Growth Rates. Genome Res. 2017, 27, 601–612. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef]
- Canabarro, A.; Valle, C.; Farias, M.R.; Santos, F.B.; Lazera, M.; Wanke, B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J. Periodontal Res. 2013, 48, 428–432. [Google Scholar] [CrossRef]
- Slazhneva, E.; Tikhomirova, E.; Tsarev, V.; Orekhova, L.; Loboda, E.; Atrushkevich, V. Candida species detection in patients with chronic periodontitis: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2022, 8, 1354–1375. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.L.; Willems, H.M.E.; Jayatilake, J.A.M.S.; Bruno, V.M.; Peters, B.M.; Shirtliff, M.E. Candida–bacteria interactions: Their impact on human disease. In Virulence Mechanisms of Bacterial Pathogens; ASM Press: Washington, DC, USA, 2016; pp. 103–136. [Google Scholar]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Strausbaugh, L.D.; Dongari-Bagtzoglou, A. Fungal-bacterial interactions and their relevance to oral health: Linking the clinic and the bench. Front. Cell. Infect. Microbiol. 2014, 4, 101. [Google Scholar] [CrossRef]
- Chinnici, J.; Yerke, L.; Tsou, C.; Busarajan, S.; Mancuso, R.; Sadhak, N.D.; Kim, J.; Maddi, A. Candida albicans cell wall integrity transcription factors regulate polymicrobial biofilm formation with Streptococcus gordonii. PeerJ 2019, 7, e7870. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Dongari-Bagtzoglou, A. Streptococcus oralis and Candida albicans synergistically activate μ-calpain to degrade E-cadherin from oral epithelial junctions. J. Infect. Dis. 2016, 214, 925–934. [Google Scholar] [CrossRef]
- Koo, H.; Bowen, W.H. Candida albicans and Streptococcus mutans: A potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014, 9, 1295–1297. [Google Scholar] [CrossRef]

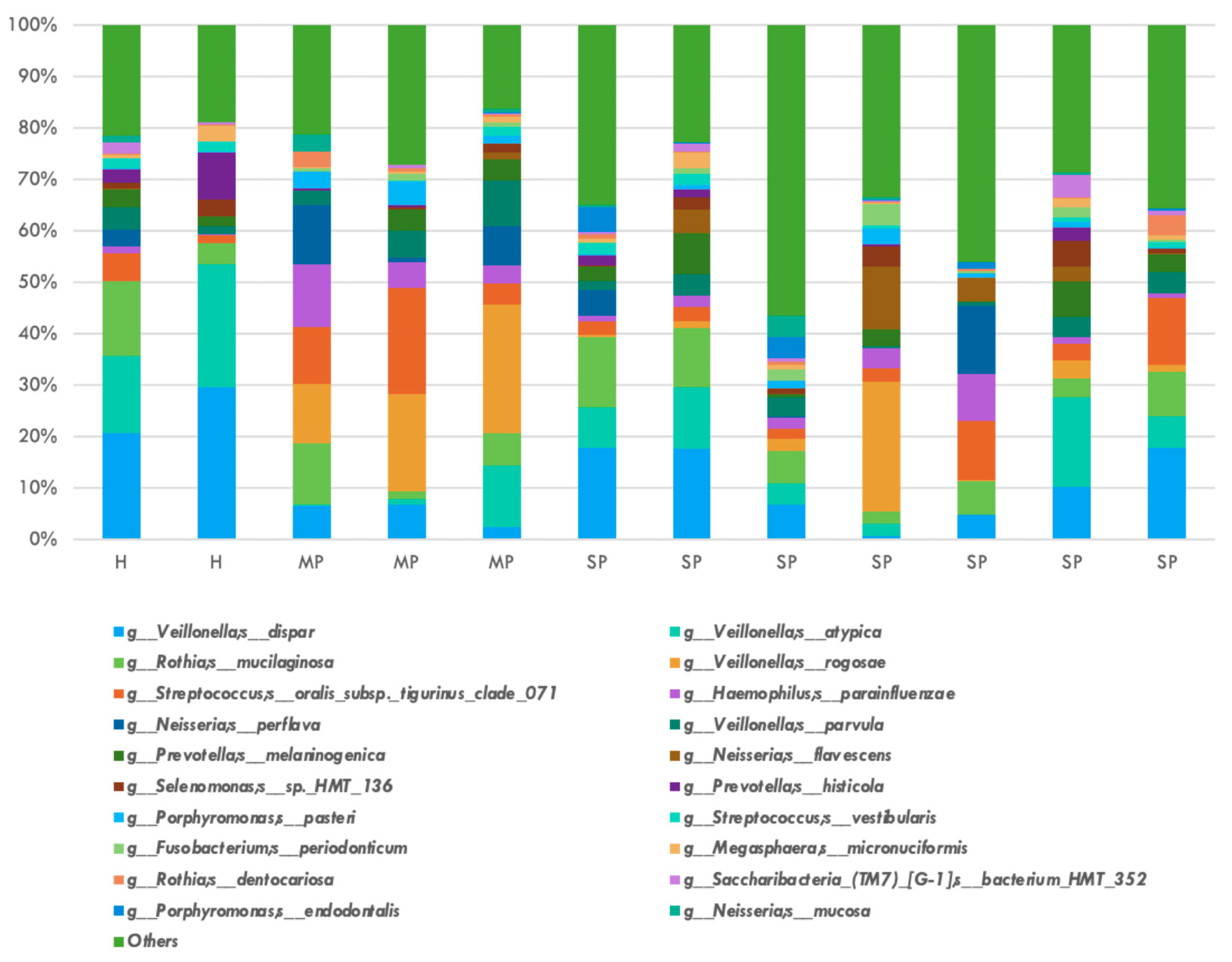
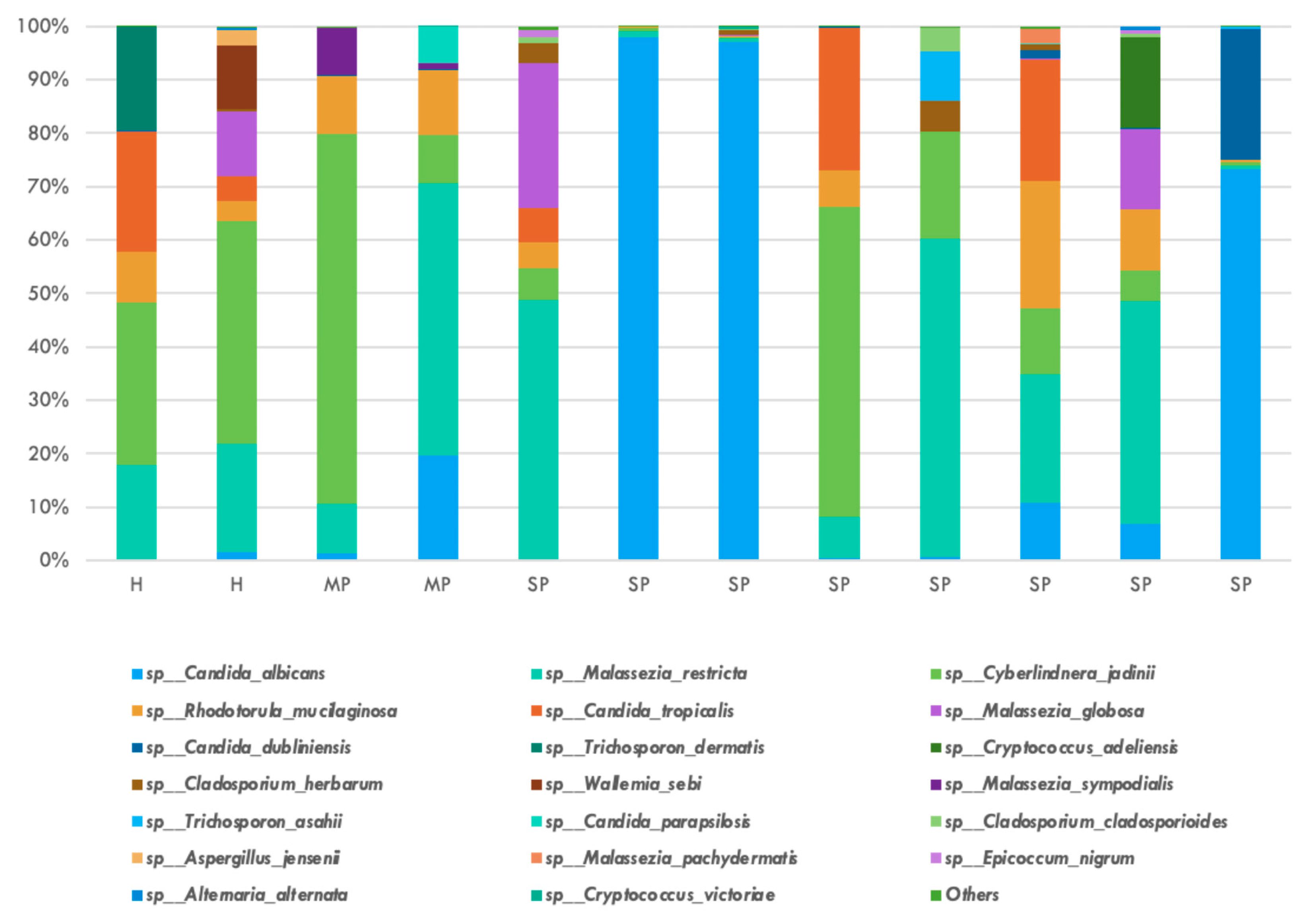
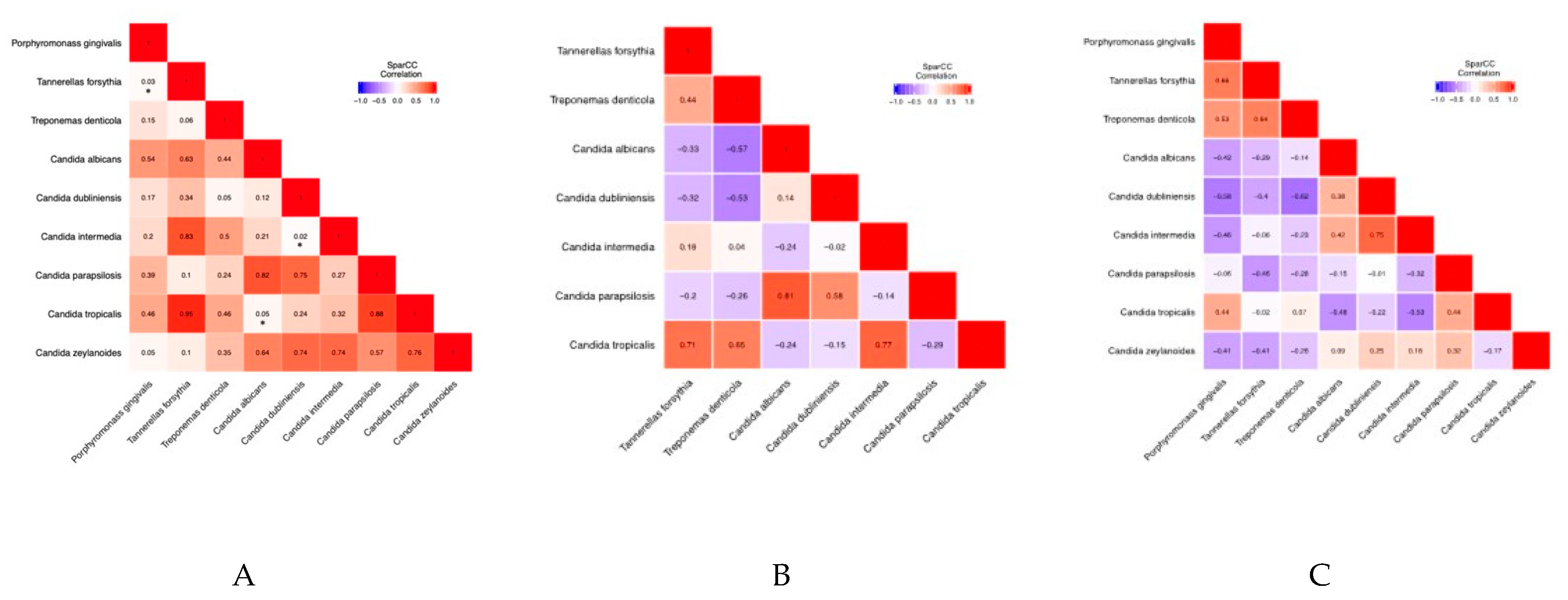
| Type of Sequencing | Mycobiome (Fungi) | Microbiome (Bacteria) |
|---|---|---|
| Region of Amplification | Internal transcriber sequence ITS1 region | 16s rRNA V3-V4 regions |
| Primer Sequences | ITS1F-CTTGGTCATTTAGAGGAAGTAA ITS1R-GCTGCGTTCTTCATCGATGC | F-AGAGTTTGATCCTGGCTCAG R-ACGGCTACCTTGTTACGACTT |
| PCR 1 Conditions | 95 °C for 3 min 30 s 95 °C 30 s 50 °C Repeat 25 cycles 30 s 72 °C 5 min 72 °C | 95 °C for 3 min 30 s 95 °C 45 sec 55 °C Repeat 25 cycles 30 s 72 °C 5 min 72 °C |
| PCR 2 Conditions | 3 min 95 °C 30 s 95 °C 30 s 55 °C Repeat 8 cycles 30 s 72 °C 5 min 72 °C | 3 min 95 °C 30 s 95 °C 30 s 55 °C Repeat 8 cycles 30 s 72 °C 5 min 72 °C |
| Correlation Coefficients | Porphyromonas gingivalis | Tannerella forsythia | Treponema denticola | Candida albicans | Candida dubliniensis | Candida intermedia | Candida parapsilosis | Candida tropicalis | Candida zeylanoides |
|---|---|---|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | 1 | 0.53731278 | 0.333041183 | −0.151767367 | −0.351388456 | −0.299375574 | −0.20625247 | 0.183436106 | −0.339953215 |
| Tannerella forsythia | 0.53731278 | 1 | 0.441430423 | −0.125639606 | −0.253119816 | −0.047184177 | −0.399397455 | −0.015253018 | −0.30703011 |
| Treponema denticola | 0.333041183 | 0.441430423 | 1 | −0.202235196 | −0.489641502 | −0.149063176 | −0.260111701 | 0.185394178 | −0.158882632 |
| Candida albicans | −0.151767367 | −0.125639606 | −0.202235196 | 1 | 0.425660058 | 0.298167467 | 0.067746325 | −0.498225752 | −0.102562846 |
| Candida dubliniensis | −0.351388456 | −0.253119816 | −0.489641502 | 0.425660058 | 1 | 0.602334681 | 0.07850208 | −0.31303916 | 0.072137738 |
| Candida intermedia | −0.299375574 | −0.047184177 | −0.149063176 | 0.298167467 | 0.602334681 | 1 | −0.238488494 | −0.235386894 | 0.066417965 |
| Candida parapsilosis | −0.20625247 | −0.399397455 | −0.260111701 | 0.067746325 | 0.07850208 | −0.238488494 | 1 | 0.039675969 | 0.10113917 |
| Candida tropicalis | 0.183436106 | −0.015253018 | 0.185394178 | −0.498225752 | −0.31303916 | −0.235386894 | 0.039675969 | 1 | 0.074242306 |
| Candida zeylanoides | −0.339953215 | −0.30703011 | −0.158882632 | −0.102562846 | 0.072137738 | 0.066417965 | 0.10113917 | 0.074242306 | 1 |
| p-Values | Porphyromonas gingivalis | Tannerella forsythia | Treponema denticola | Candida albicans | Candida dubliniensis | Candida intermedia | Candida parapsilosis | Candida tropicalis | Candida zeylanoides |
|---|---|---|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | 1 | 0.026 | 0.148 | 0.536 | 0.173 | 0.2 | 0.388 | 0.458 | 0.05 |
| Tannerella forsythia | 0.026 | 1 | 0.055 | 0.633 | 0.342 | 0.832 | 0.098 | 0.946 | 0.103 |
| Treponema denticola | 0.148 | 0.055 | 1 | 0.436 | 0.054 | 0.503 | 0.243 | 0.456 | 0.35 |
| Candida albicans | 0.536 | 0.633 | 0.436 | 1 | 0.119 | 0.206 | 0.821 | 0.049 | 0.636 |
| Candida dubliniensis | 0.173 | 0.342 | 0.054 | 0.119 | 1 | 0.019 | 0.748 | 0.244 | 0.737 |
| Candida intermedia | 0.2 | 0.832 | 0.503 | 0.206 | 0.019 | 1 | 0.266 | 0.323 | 0.735 |
| Candida parapsilosis | 0.388 | 0.098 | 0.243 | 0.821 | 0.748 | 0.266 | 1 | 0.875 | 0.573 |
| Candida tropicalis | 0.458 | 0.946 | 0.456 | 0.049 | 0.244 | 0.323 | 0.875 | 1 | 0.759 |
| Candida zeylanoides | 0.05 | 0.103 | 0.35 | 0.636 | 0.737 | 0.735 | 0.573 | 0.759 | 1 |
| Correlation Coefficients | Tannerella forsythia | Treponema denticola | Candida albicans | Candida dubliniensis | Candida intermedia | Candida parapsilosis | Candida tropicalis |
|---|---|---|---|---|---|---|---|
| Tannerella forsythia | 1 | 0.43774298 | −0.333843702 | −0.316563422 | 0.1799742 | −0.201116436 | 0.706151712 |
| Treponema denticola | 0.43774298 | 1 | −0.570547057 | −0.533947765 | 0.036642522 | −0.255673848 | 0.651375479 |
| Candida albicans | −0.333843702 | −0.570547057 | 1 | 0.142774181 | −0.241524058 | 0.811733249 | −0.236286454 |
| Candida dubliniensis | −0.316563422 | −0.533947765 | 0.142774181 | 1 | −0.020422605 | 0.578807206 | −0.151123919 |
| Candida intermedia | 0.1799742 | 0.036642522 | −0.241524058 | −0.020422605 | 1 | −0.143151822 | 0.769874961 |
| Candida parapsilosis | −0.201116436 | −0.255673848 | 0.811733249 | 0.578807206 | −0.143151822 | 1 | −0.285399967 |
| Candida tropicalis | 0.706151712 | 0.651375479 | −0.236286454 | −0.151123919 | 0.769874961 | −0.285399967 | 1 |
| p-Values | Tannerella forsythia | Treponema denticola | Candida albicans | Candida dubliniensis | Candida intermedia | Candida parapsilosis | Candida tropicalis |
|---|---|---|---|---|---|---|---|
| Tannerella forsythia | 1 | 0.333 | 0.442 | 0.528 | 0.712 | 0.541 | 0.07 |
| Treponema denticola | 0.333 | 1 | 0.239 | 0.316 | 0.932 | 0.401 | 0.081 |
| Candida albicans | 0.442 | 0.239 | 1 | 0.781 | 0.566 | 0.075 | 0.537 |
| Candida dubliniensis | 0.528 | 0.316 | 0.781 | 1 | 0.964 | 0.132 | 0.736 |
| Candida intermedia | 0.712 | 0.932 | 0.566 | 0.964 | 1 | 0.666 | 0.067 |
| Candida parapsilosis | 0.541 | 0.401 | 0.075 | 0.132 | 0.666 | 1 | 0.296 |
| Candida tropicalis | 0.07 | 0.081 | 0.537 | 0.736 | 0.067 | 0.296 | 1 |
| Correlation Coefficients | Porphyromonas gingivalis | Tannerella forsythia | Treponema denticola | Candida albicans | Candida dubliniensis | Candida intermedia | Candida parapsilosis | Candida tropicalis | Candida zeylanoides |
|---|---|---|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | 1 | 0.663026251 | 0.533469205 | −0.416165635 | −0.577078078 | −0.455449694 | −0.064539964 | 0.435096681 | −0.411388874 |
| Tannerella forsythia | 0.663026251 | 1 | 0.642781964 | −0.291249055 | −0.399786984 | −0.057060816 | −0.464981704 | −0.015279857 | −0.411604834 |
| Treponema denticola | 0.533469205 | 0.642781964 | 1 | −0.139752468 | −0.615080092 | −0.226454353 | −0.277915645 | 0.065709903 | −0.262961763 |
| Candida albicans | −0.416165635 | −0.291249055 | −0.139752468 | 1 | 0.376787143 | 0.415429972 | −0.149909207 | −0.480294922 | 0.085956511 |
| Candida dubliniensis | −0.577078078 | −0.399786984 | −0.615080092 | 0.376787143 | 1 | 0.753572716 | −0.011325989 | −0.223835122 | 0.251957054 |
| Candida intermedia | −0.455449694 | −0.057060816 | −0.226454353 | 0.415429972 | 0.753572716 | 1 | −0.32434038 | −0.534608342 | 0.175364742 |
| Candida parapsilosis | −0.064539964 | −0.464981704 | −0.277915645 | −0.149909207 | −0.011325989 | −0.32434038 | 1 | 0.441652891 | 0.317988583 |
| Candida tropicalis | 0.435096681 | −0.015279857 | 0.065709903 | −0.480294922 | −0.223835122 | −0.534608342 | 0.441652891 | 1 | −0.169806535 |
| Candida zeylanoides | −0.411388874 | −0.411604834 | −0.262961763 | 0.085956511 | 0.251957054 | 0.175364742 | 0.317988583 | −0.169806535 | 1 |
| p-Values | Porphyromonas gingivalis | Tannerellas forsythia | Treponemas denticola | Candida albicans | Candida dubliniensis | Candida intermedia | Candida parapsilosis | Candida tropicalis | Candida zeylanoides |
|---|---|---|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | 1 | 0.038 | 0.112 | 0.246 | 0.101 | 0.184 | 0.837 | 0.186 | 0.132 |
| Tannerellas forsythia | 0.038 | 1 | 0.041 | 0.44 | 0.327 | 0.866 | 0.15 | 0.963 | 0.141 |
| Treponemas denticola | 0.112 | 0.041 | 1 | 0.739 | 0.073 | 0.523 | 0.376 | 0.863 | 0.356 |
| Candida albicans | 0.246 | 0.44 | 0.739 | 1 | 0.369 | 0.253 | 0.698 | 0.153 | 0.801 |
| Candida dubliniensis | 0.101 | 0.327 | 0.073 | 0.369 | 1 | 0.029 | 0.977 | 0.626 | 0.479 |
| Candida intermedia | 0.184 | 0.866 | 0.523 | 0.253 | 0.029 | 1 | 0.295 | 0.084 | 0.518 |
| Candida parapsilosis | 0.837 | 0.15 | 0.376 | 0.698 | 0.977 | 0.295 | 1 | 0.164 | 0.303 |
| Candida tropicalis | 0.186 | 0.963 | 0.863 | 0.153 | 0.626 | 0.084 | 0.164 | 1 | 0.616 |
| Candida zeylanoides | 0.132 | 0.141 | 0.356 | 0.801 | 0.479 | 0.518 | 0.303 | 0.616 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayers, J.; Chinnici, J.; Sabharwal, A.; Emecen-Huja, P.; Chien, H.-H.; Joshi, S.; Maddi, A. Correlation Between Fungal and Bacterial Populations in Periodontitis Through Targeted Sequencing: A Pilot Study. J. Clin. Med. 2025, 14, 6418. https://doi.org/10.3390/jcm14186418
Ayers J, Chinnici J, Sabharwal A, Emecen-Huja P, Chien H-H, Joshi S, Maddi A. Correlation Between Fungal and Bacterial Populations in Periodontitis Through Targeted Sequencing: A Pilot Study. Journal of Clinical Medicine. 2025; 14(18):6418. https://doi.org/10.3390/jcm14186418
Chicago/Turabian StyleAyers, Jacob, Jennifer Chinnici, Amarpreet Sabharwal, Pinar Emecen-Huja, Hua-Hong Chien, Shilpi Joshi, and Abhiram Maddi. 2025. "Correlation Between Fungal and Bacterial Populations in Periodontitis Through Targeted Sequencing: A Pilot Study" Journal of Clinical Medicine 14, no. 18: 6418. https://doi.org/10.3390/jcm14186418
APA StyleAyers, J., Chinnici, J., Sabharwal, A., Emecen-Huja, P., Chien, H.-H., Joshi, S., & Maddi, A. (2025). Correlation Between Fungal and Bacterial Populations in Periodontitis Through Targeted Sequencing: A Pilot Study. Journal of Clinical Medicine, 14(18), 6418. https://doi.org/10.3390/jcm14186418








