Effect of Leukocyte- and Platelet-Rich Fibrin on Peri-Implant Mucosal Thickness in Edentulous Patients Treated with Mandibular Implant-Retained Overdentures: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Screening and Consenting
2.3. Outcome Measures
2.4. Surgical Protocol
2.5. Statistical Analysis
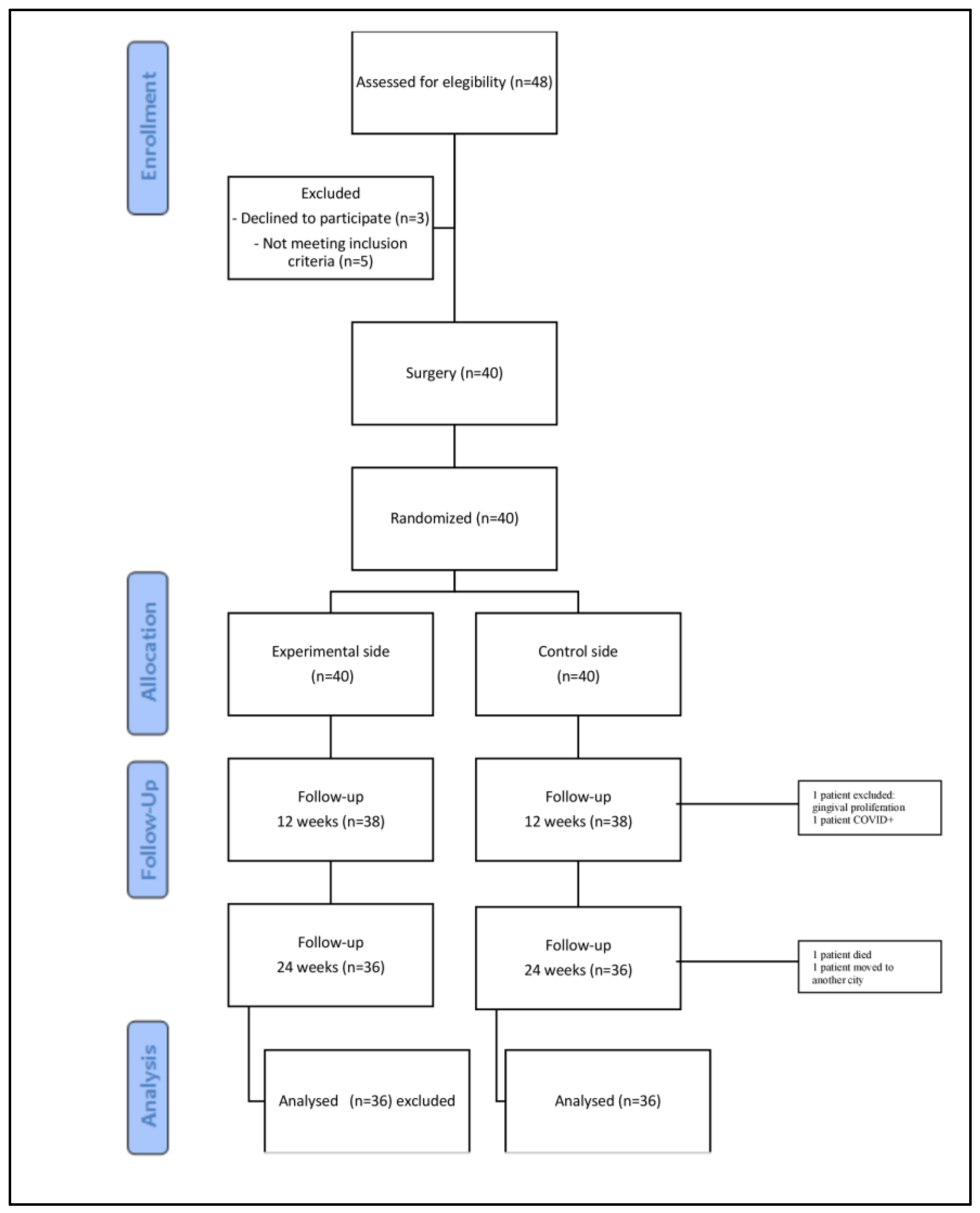
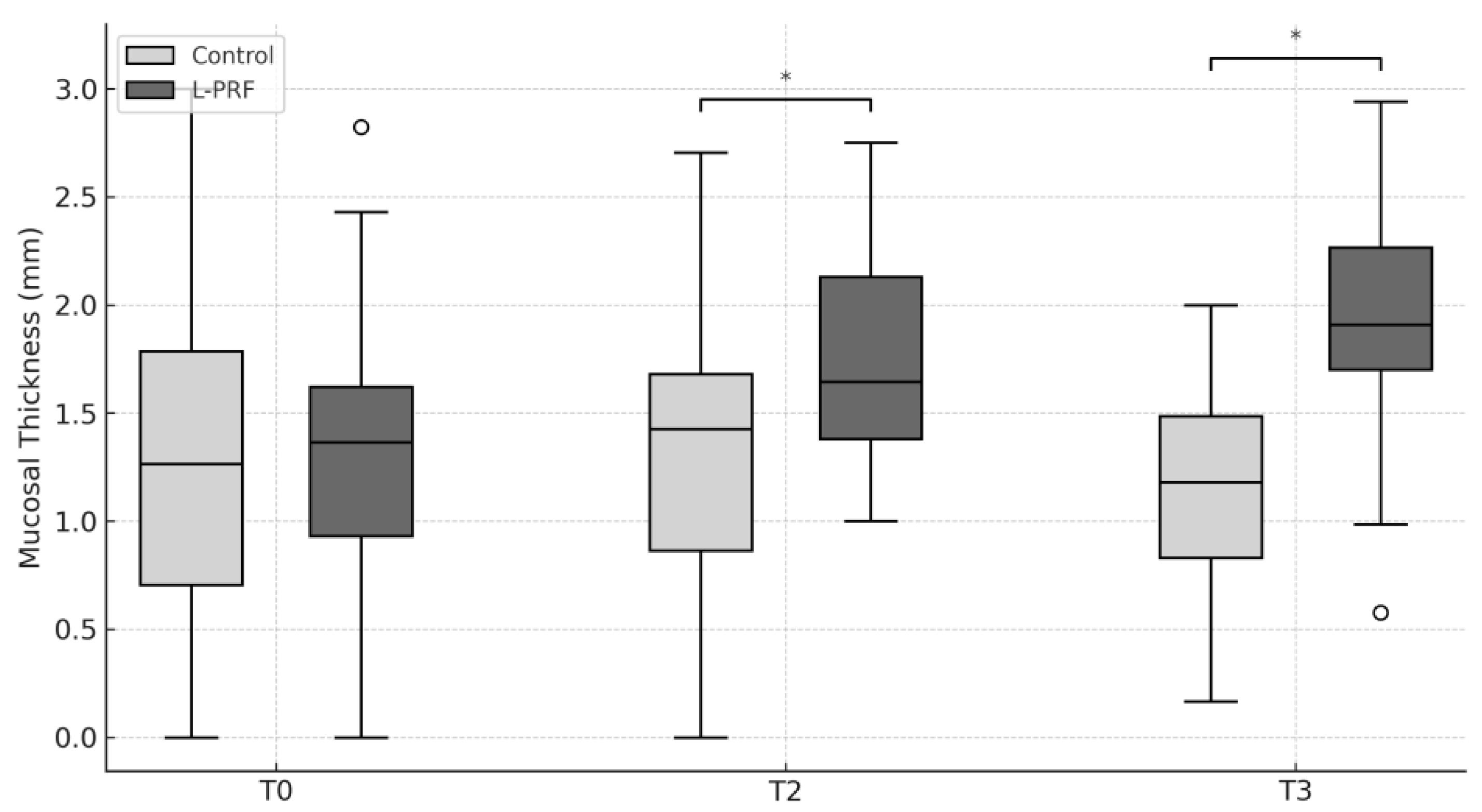
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enlow, D.H.; Bianco, H.J.; Eklund, S. The remodeling of the edentulous mandible. J. Prosthet. Dent. 1976, 36, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, B.K.; Heap, P.F. The effect of fluoride on iron, calcium and phosphorus distribution in the rat incisor. Caries Res. 1976, 10, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Siles, M.; Ballester-Ferrandis, J.F.; Salazar-Sanchez, N.; Gomez-Garcia, F.J.; Moraleja-Ruiz, R.; Camacho-Alonso, F. Long-term evaluation of quality of life and satisfaction between implant bar overdentures and conventional complete dentures: A 23 years retrospective study. Clin. Implant Dent. Relat. Res. 2018, 20, 208–214. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, C.; Shang, Z.; Niu, A.; Liang, X. Quality of Life of Implant-Supported Overdenture and Conventional Complete Denture in Restoring the Edentulous Mandible: A Systematic Review. Implant Dent. 2017, 26, 945–950. [Google Scholar] [CrossRef]
- Morais, J.A.; Heydecke, G.; Pawliuk, J.; Lund, J.P.; Feine, J.S. The effects of mandibular two-implant overdentures on nutrition in elderly edentulous individuals. J. Dent. Res. 2003, 82, 53–58. [Google Scholar] [CrossRef]
- Becker, S.T.; Beck-Broichsitter, B.E.; Rossmann, C.M.; Behrens, E.; Jochens, A.; Wiltfang, J. Long-term survival of Straumann dental implants with TPS surfaces: A retrospective study with a follow-up of 12 to 23 years. Clin. Implant Dent. Relat. Res. 2015, 18, 480–488. [Google Scholar] [CrossRef]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef]
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef]
- Nevins, M.; Kao, R.T.; McGuire, M.K.; McClain, P.K.; Hinrichs, J.E.; McAllister, B.S.; Reddy, M.S.; Nevins, M.L.; Genco, R.J.; Lynch, S.E.; et al. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J. Periodontol. 2013, 84, 456–464. [Google Scholar] [CrossRef]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Öncü, E.; Alaaddinoglu, E.E. The effect of platelet-rich fibrin on implant stability. Int. J. Oral Maxillofac. Implants 2015, 30, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Gaydarov, N.; Badoud, I.; Vazquez, L.; Bernard, J.P.; Ammann, P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: A prospective randomized controlled study. Implant Dent. 2013, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Giammarinaro, E.; Baldini, N.; Covani, U.; Menini, M.; Pesce, P.; Marconcini, S. Does platelet-rich fibrin enhance the outcomes of peri-implant soft tissues? A systematic review. BMC Oral Health 2025, 25, 615. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, B.B.; Penmetsa, G.S.; Raju, M.S.; Haripriya, N.; Gera, D.; Ramesh, K. Peri-implant mucosal enhancement using leukocyte platelet rich fibrin under Sohn’s poncho technique: A randomized controlled clinical trial. Clin. Adv. Periodontics 2024, 14, 134–141. [Google Scholar] [CrossRef]
- Razi, M.A.; Mahajan, A.; Zarrin, R.; Roy, S.; Singh, M.K.; Kumari, S. Impact of Platelet-Rich Fibrin (PRF) Versus Freeze-Dried Bone Allograft (FDBA) on Peri-Implant Soft and Hard Tissue in Alveolar Ridge Preservation. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S4), S3550–S3552. [Google Scholar] [CrossRef]
- Temmerman, A.; Cleeren, G.J.; Castro, A.B.; Teughels, W.; Quirynen, M. L-PRF for increasing the width of keratinized mucosa around implants: A split-mouth, randomized, controlled pilot clinical trial. J. Periodontal Res. 2018, 53, 793–800. [Google Scholar] [CrossRef]
- Al-Ekrish, A.A. Comparative study of the accuracy of CBCT implant site measurements using different software programs. Saudi Dent. J. 2021, 33, 355–361. [Google Scholar] [CrossRef]
- Swennen, G.R.; Mollemans, W.; De Clercq, C.; Abeloos, J.; Lamoral, P.; Lippens, F.; Neyt, N.; Casselman, J.; Schutyser, F. A cone-beam computed tomography triple scan procedure to obtain a three-dimensional augmented virtual skull model appropriate for orthognathic surgery planning. J. Craniofacial Surg. 2009, 20, 297–307. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stähli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implants Res. 2018, 29 (Suppl. S18), 6–19. [Google Scholar] [CrossRef]
- Tomasi, C.; Tessarolo, F.; Caola, I.; Wennström, J.; Nollo, G.; Berglundh, T. Morphogenesis of peri-implant mucosa revisited: An experimental study in humans. Clin. Oral Implants Res. 2014, 25, 997–1003. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thomsen, P. The soft tissue barrier at implants and teeth. Clin. Oral Implants Res. 1991, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29 (Suppl. S15), 32–49. [Google Scholar] [CrossRef] [PubMed]
- Nedir, R.; Bischof, M.; Szmukler-Moncler, S.; Bernard, J.P.; Samson, J. Predicting osseointegration by means of implant primary stability. Clin. Oral Implants Res. 2004, 15, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Ali, A.R.; Saini, N.; Gautam, K.; Goyal, A.; Prakash, V. Comparative evaluation of implant stability with and without autologous platelet-rich fibrin prior to prosthetic loading—A split-mouth randomized clinical trial. J. Indian Soc. Periodontol. 2022, 26, 137–142. [Google Scholar]
- Guan, S.; Xiao, T.; Bai, J.; Ning, C.; Zhang, X.; Yang, L.; Li, X. Clinical application of platelet-rich fibrin to enhance dental implant stability: A systematic review and meta-analysis. Heliyon 2023, 9, e13196. [Google Scholar] [CrossRef]
- Żurek, J.; Niemczyk, W.; Dominiak, M.; Niemczyk, S.; Wiench, R.; Skaba, D. Gingival Augmentation Using Injectable Platelet-Rich Fibrin (i-PRF)-A Systematic Review of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 5591. [Google Scholar] [CrossRef]
- Boora, P.; Rathee, M.; Bhoria, M. Effect of platelet rich fibrin (PRF) on peri-implant soft tissue and crestal bone in one-stage implant placement: A randomized controlled trial. J. Clin. Diagn. Res. 2015, 9, ZC18–ZC21. [Google Scholar] [CrossRef]
- Zhan, L.; Yang, Y.; Zhao, X.; Wang, C.; Man, Y.; Qu, Y. Clinical outcomes of a peri-implant keratinized mucosa augmentation procedure using concentrated growth factor: A prospective case series. BMC Oral Health 2025, 25, 1064. [Google Scholar] [CrossRef]
- Prakash, P.; Rath, S.K.; Mukherjee, M. Clinical efficacy of periosteal pedicle graft with subepithelial connective tissue graft in gingival recession coverage. J. Indian Soc. Periodontol. 2019, 23, 442–447. [Google Scholar] [CrossRef]
- Aldommari, E.A.; Omair, A.; Qasem, T. Titanium-prepared platelet-rich fibrin enhances alveolar ridge preservation: A randomized controlled clinical and radiographic study. Sci. Rep. 2025, 15, 2551. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Worsaae, N.; Gotfredsen, K. Tissue changes at implant sites in the anterior maxilla with and without connective tissue grafting: A five-year prospective study. Clin. Oral Implants Res. 2020, 31, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Zeltner, M.; Hammerle, C.H.F.; Jung, R.E.; Thoma, D.S. Non-interventional 1-year follow-up study of peri-implant soft tissues following previous soft tissue augmentation and crown insertion in single-tooth gaps. J. Clin. Periodontol. 2018, 45, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Majzoub, J.; Siqueira, R.; Mendonça, G.; Wang, H.L. Volumetric changes at implant sites: A systematic appraisal of traditional methods and optical scanning-based digital technologies. J. Clin. Periodontol. 2021, 48, 315–334. [Google Scholar] [CrossRef]
- Ramos, E.U.; Bizelli, V.F.; Pereira Baggio, A.M.; Ferriolli, S.C.; Silva Prado, G.A.; Farnezi Bassi, A.P. Do the New Protocols of Platelet-Rich Fibrin Centrifugation Allow Better Control of Postoperative Complications and Healing After Surgery of Impacted Lower Third Molar? A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2022, 80, 1238–1253. [Google Scholar] [CrossRef]
- Dohan, M.; Bielecki, T.; Jimbo, R.; Barbé, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (LPRF). Curr. Pharmacol. Biotechnol. 2012, 13, 1145–1152. [Google Scholar]
- Di Summa, F.; Kargarpour, Z.; Nasirzade, J.; Stähli, A.; Mitulović, G.; Panić-Janković, T.; Koller, V.; Kaltenbach, C.; Müller, H.; Panahipour, L.; et al. TGFβ activity released from platelet-rich fibrin adsorbs to titanium surface and collagen membranes. Sci. Rep. 2020, 10, 10203. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stähli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef]
- Wang, X.; Fok, M.R.; Pelekos, G.; Jin, L.; Tonetti, M.S. In Vitro and Ex Vivo Kinetic Release Profile of Growth Factors and Cytokines from Leucocyte- and Platelet-Rich Fibrin (L-PRF) Preparations. Cells 2022, 11, 2089. [Google Scholar] [CrossRef]
- Coucke, B.; Dilissen, E.; Cremer, J.; Schrijvers, R.; Theys, T.; Van Gerven, L. Leukocyte-and Platelet-Rich Fibrin for enhanced tissue repair: An in vitro study characterizing cellular composition, growth factor kinetics and transcriptomic insights. Mol. Biol. Rep. 2024, 51, 954. [Google Scholar] [CrossRef]

| L-PRF | Control | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T2 | T3 | T0 | T2 | T3 | T0 | T2 | T3 | |
| PD (mm) | 1.6 (±0.5) | 1.4 (±0.5) | 1.8 (±0.6) | 1.4 (±0.5) | 0.60 | 0.46 | |||
| KTW (mm) | 2.7 (±1.6) | 2.5 (±1.8) | 2.4 (±0.9) | 2.5 (±1.8) | 2.4 (±0.9) | 2.7 (±0.9) | 0.53 | 0.68 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, X.; Neira, P.; Strauss, F.J.; Mery, M.I.; Gruber, R.; Cavalla, F. Effect of Leukocyte- and Platelet-Rich Fibrin on Peri-Implant Mucosal Thickness in Edentulous Patients Treated with Mandibular Implant-Retained Overdentures: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 6917. https://doi.org/10.3390/jcm14196917
Moreno X, Neira P, Strauss FJ, Mery MI, Gruber R, Cavalla F. Effect of Leukocyte- and Platelet-Rich Fibrin on Peri-Implant Mucosal Thickness in Edentulous Patients Treated with Mandibular Implant-Retained Overdentures: A Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(19):6917. https://doi.org/10.3390/jcm14196917
Chicago/Turabian StyleMoreno, Ximena, Patricio Neira, Franz J. Strauss, María Ignacia Mery, Reinhard Gruber, and Franco Cavalla. 2025. "Effect of Leukocyte- and Platelet-Rich Fibrin on Peri-Implant Mucosal Thickness in Edentulous Patients Treated with Mandibular Implant-Retained Overdentures: A Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 19: 6917. https://doi.org/10.3390/jcm14196917
APA StyleMoreno, X., Neira, P., Strauss, F. J., Mery, M. I., Gruber, R., & Cavalla, F. (2025). Effect of Leukocyte- and Platelet-Rich Fibrin on Peri-Implant Mucosal Thickness in Edentulous Patients Treated with Mandibular Implant-Retained Overdentures: A Randomized Controlled Trial. Journal of Clinical Medicine, 14(19), 6917. https://doi.org/10.3390/jcm14196917






