Sequential Treatment for Osteoporosis After Teriparatide: A Real-Life Long-Term Comparison Between Zoledronic Acid and Denosumab
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. BMD Changes
3.2. Incidence of FX
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BMD | Bone mineral density |
| BTMs | Bone Turnover Markers |
| CTX | C-terminal telopeptide |
| DEXA | Dual-Energy X-ray Absorptiometry |
| DMAB | Denosumab |
| EUROFORS | European Study of Forsteo |
| FPT | Fracture Prevention Trial |
| FX | Fracture(s) |
| LS | Lumbar Spine |
| P1NP | Procollagen Type 1 N-terminal Propeptide |
| SD | Standard Deviation |
| SDI | Spine Deformity Index |
| SQ | Semi-quantitative |
| TH | Total hip |
| TPTD | Teriparatide |
| US | United States |
| ZOL | Zoledronic acid |
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, dgaa048. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Lewiecki, E.M.; Eastell, R.; Ebeling, P.R.; Jan De Beur, S.; Langdahl, B.; Rhee, Y.; Fuleihan, G.E.-H.; Kiel, D.P.; Schousboe, J.T.; et al. Goal-Directed Osteoporosis Treatment: ASBMR/BHOF Task Force Position Statement 2024. J. Bone Miner. Res. 2024, 39, 1393–1405. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of Parathyroid Hormone (1–34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Marin, F.; Zerbini, C.A.F.; Russo, L.A.; Greenspan, S.L.; Zikan, V.; Bagur, A.; Malouf-Sierra, J.; Lakatos, P.; Fahrleitner-Pammer, A.; et al. Effects of Teriparatide and Risedronate on New Fractures in Post-Menopausal Women with Severe Osteoporosis (VERO): A Multicentre, Double-Blind, Double-Dummy, Randomised Controlled Trial. Lancet 2018, 391, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kurland, E.S.; Cosman, F.; McMahon, D.J.; Rosen, C.J.; Lindsay, R.; Bilezikian, J.P. Parathyroid Hormone as a Therapy for Idiopathic Osteoporosis in Men: Effects on Bone Mineral Density and Bone Markers. J. Clin. Endocrinol. Metab. 2000, 85, 3069–3076. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Scheele, W.H.; Paul, S.; Adami, S.; Syversen, U.; Diez-Perez, A.; Kaufman, J.M.; Clancy, A.D.; Gaich, G.A. The Effect of Teriparatide [Human Parathyroid Hormone (1–34)] Therapy on Bone Density in Men with Osteoporosis. J. Bone Miner. Res. 2003, 18, 9–17. [Google Scholar] [CrossRef]
- Kaufman, J.-M.; Orwoll, E.; Goemaere, S.; San Martin, J.; Hossain, A.; Dalsky, G.P.; Lindsay, R.; Mitlak, B.H. Teriparatide Effects on Vertebral Fractures and Bone Mineral Density in Men with Osteoporosis: Treatment and Discontinuation of Therapy. Osteoporos. Int. 2005, 16, 510–516. [Google Scholar] [CrossRef]
- Saag, K.G.; Shane, E.; Boonen, S.; Marín, F.; Donley, D.W.; Taylor, K.A.; Dalsky, G.P.; Marcus, R. Teriparatide or Alendronate in Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2007, 357, 2028–2039. [Google Scholar] [CrossRef]
- Ebina, K.; Hirao, M.; Hashimoto, J.; Hagihara, K.; Kashii, M.; Kitaguchi, K.; Matsuoka, H.; Iwahashi, T.; Chijimatsu, R.; Yoshikawa, H. Assessment of the Effects of Switching Oral Bisphosphonates to Denosumab or Daily Teriparatide in Patients with Rheumatoid Arthritis. J. Bone Miner. Metab. 2018, 36, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; San Martin, J.; Muñoz-Torres, M.; Econs, M.J.; Xie, L.; Dalsky, G.P.; McClung, M.; Felsenberg, D.; Brown, J.P.; Brandi, M.L.; et al. Effect of Raloxifene after Recombinant Teriparatide [hPTH(1–34)] Treatment in Postmenopausal Women with Osteoporosis. Osteoporos. Int. 2008, 19, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Nickelsen, T.; Marin, F.; Barker, C.; Hadji, P.; Farrerons, J.; Audran, M.; Boonen, S.; Brixen, K.; Gomes, J.M.; et al. Sequential Treatment of Severe Postmenopausal Osteoporosis after Teriparatide: Final Results of the Randomized, Controlled European Study of Forsteo (EUROFORS). J. Bone Miner. Res. 2009, 24, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Scheele, W.H.; Neer, R.; Pohl, G.; Adami, S.; Mautalen, C.; Reginster, J.-Y.; Stepan, J.J.; Myers, S.L.; Mitlak, B.H. Sustained Vertebral Fracture Risk Reduction after Withdrawal of Teriparatide in Postmenopausal Women with Osteoporosis. Arch. Intern. Med. 2004, 164, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.; Sipos, A.; Hossain, A.; Syversen, U.; Ish-Shalom, S.; Marcinowska, E.; Halse, J.; Lindsay, R.; Dalsky, G.P.; Mitlak, B.H. Sustained Nonvertebral Fragility Fracture Risk Reduction after Discontinuation of Teriparatide Treatment. J. Bone Miner. Res. 2005, 20, 1507–1513. [Google Scholar] [CrossRef]
- Fahrleitner-Pammer, A.; Langdahl, B.L.; Marin, F.; Jakob, F.; Karras, D.; Barrett, A.; Ljunggren, Ö.; Walsh, J.B.; Rajzbaum, G.; Barker, C.; et al. Fracture Rate and Back Pain during and after Discontinuation of Teriparatide: 36-Month Data from the European Forsteo Observational Study (EFOS). Osteoporos. Int. 2011, 22, 2709–2719. [Google Scholar] [CrossRef]
- Forsteo|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/forsteo (accessed on 8 June 2024).
- Ebina, K.; Hashimoto, J.; Kashii, M.; Hirao, M.; Kaneshiro, S.; Noguchi, T.; Tsukamoto, Y.; Yoshikawa, H. The Effects of Switching Daily Teriparatide to Oral Bisphosphonates or Denosumab in Patients with Primary Osteoporosis. J. Bone Miner. Metab. 2017, 35, 91–98. [Google Scholar] [CrossRef]
- Niimi, R.; Kono, T.; Nishihara, A.; Hasegawa, M.; Kono, T.; Sudo, A. Efficacy of Switching from Teriparatide to Bisphosphonate or Denosumab: A Prospective, Randomized, Open-Label Trial. JBMR Plus 2018, 2, 289–294. [Google Scholar] [CrossRef]
- Kocjan, T.; Rajic, A.S.; Janez, A.; Vidmar, G.; Orehek, N.; Marc, J.; Ostanek, B. Switching to Denosumab or Bisphosphonates After Completion of Teriparatide Treatment in Women with Severe Postmenopausal Osteoporosis. Endocr. Pract. 2021, 27, 941–947. [Google Scholar] [CrossRef]
- Liu, J.; Laster, A.; Xu, X.; Guo, H.; Oates, M.; Gandra, S.R. Patterns of Teriparatide and Sequential Antiresorptive Agent Treatment Among Elderly Female Medicare Beneficiaries. J. Bone Miner. Res. 2021, 36, 2309–2316. [Google Scholar] [CrossRef]
- Sarli, M.A.; Habib, C.; Zanchetta, M.B. Which Is the Best Antiresorptive Treatment after Finishing Teriparatide? Medicina 2021, 81, 749–753. [Google Scholar]
- Dito, G.; Lugaresi, M.; Degradi, C.; Guabello, G.; Longhi, M.; Corbetta, S. Efficacy of Switching from Teriparatide to Zoledronic Acid or Denosumab on Bone Mineral Density and Biochemical Markers of Bone Turnover in Older Patients with Severe Osteoporosis: A Real-Life Study. Endocrine 2023, 82, 181–189. [Google Scholar] [CrossRef]
- Reid, I.R.; Black, D.M.; Eastell, R.; Bucci-Rechtweg, C.; Su, G.; Hue, T.F.; Mesenbrink, P.; Lyles, K.W.; Boonen, S. HORIZON Pivotal Fracture Trial and HORIZON Recurrent Fracture Trial Steering Committees Reduction in the Risk of Clinical Fractures after a Single Dose of Zoledronic Acid 5 Milligrams. J. Clin. Endocrinol. Metab. 2013, 98, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Bolland, M.J.; Horne, A.; Mihov, B.; Gamble, G.; Reid, I.R. Bone Mineral Density and Bone Turnover 10 Years After a Single 5 mg Dose or Two 5-Yearly Lower Doses of Zoledronate in Osteopenic Older Women: An Open-Label Extension of a Randomized Controlled Trial. J. Bone Miner. Res. 2022, 37, 3–11. [Google Scholar] [CrossRef]
- Bolland, M.J.; Nisa, Z.; Mellar, A.; Gasteiger, C.; Pinel, V.; Mihov, B.; Bastin, S.; Grey, A.; Reid, I.R.; Gamble, G.; et al. Fracture Prevention with Infrequent Zoledronate in Women 50 to 60 Years of Age. N. Engl. J. Med. 2025, 392, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Fobelo Lozano, M.J.; Sánchez-Fidalgo, S. Adherence and Preference of Intravenous Zoledronic Acid for Osteoporosis versus Other Bisphosphonates. Eur. J. Hosp. Pharm. 2019, 26, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.J.; Rodriguez, E.G.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guanabens, N.; et al. Fracture Risk and Management of Discontinuation of Denosumab Therapy: A Systematic Review and Position Statement by ECTS. J. Clin. Endocrinol. Metab. 2021, 106, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). Executive Summary of European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Aging Clin. Exp. Res. 2019, 31, 15–17. [Google Scholar] [CrossRef]
- Binkley, N.; Kiebzak, G.M.; Lewiecki, E.M.; Krueger, D.; Gangnon, R.E.; Miller, P.D.; Shepherd, J.A.; Drezner, M.K. Recalculation of the NHANES Database SD Improves T-Score Agreement and Reduces Osteoporosis Prevalence. J. Bone Miner. Res. 2005, 20, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Wu, C.Y.; van Kuijk, C.; Nevitt, M.C. Vertebral Fracture Assessment Using a Semiquantitative Technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef]
- Kerkeni, S.; Kolta, S.; Fechtenbaum, J.; Roux, C. Spinal Deformity Index (SDI) Is a Good Predictor of Incident Vertebral Fractures. Osteoporos. Int. 2009, 20, 1547–1552. [Google Scholar] [CrossRef]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwinski, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 Years of Denosumab Treatment in Postmenopausal Women with Osteoporosis: Results from the Phase 3 Randomised FREEDOM Trial and Open-Label Extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Giveon, S.; Zacay, G.; Vered, I.; Foldes, A.J.; Tripto-Shkolnik, L. Zoledronic Acid Sequential to Teriparatide May Promote Greater Inhibition of Bone Resorption than Zoledronic Acid Alone. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231213639. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; Bolognese, M.A.; Brown, J.P.; Reginster, J.-Y.; Langdahl, B.L.; Maddox, J.; Shi, Y.; Rojeski, M.; Meisner, P.D.; Grauer, A. A Single Dose of Zoledronate Preserves Bone Mineral Density for up to 2 Years after a Second Course of Romosozumab. Osteoporos. Int. 2020, 31, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Hiligsmann, M.; Cornelissen, D.; Vrijens, B.; Abrahamsen, B.; Al-Daghri, N.; Biver, E.; Brandi, M.L.; Bruyère, O.; Burlet, N.; Cooper, C.; et al. Determinants, Consequences and Potential Solutions to Poor Adherence to Anti-Osteoporosis Treatment: Results of an Expert Group Meeting Organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Osteoporosis Foundation (IOF). Osteoporos. Int. 2019, 30, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Krege, J.H.; Marin, F.; Jin, L.; Stepan, J.J. Teriparatide for Osteoporosis: Importance of the Full Course. Osteoporos. Int. 2016, 27, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Jacques, R.M.; Boonen, S.; Cosman, F.; Reid, I.R.; Bauer, D.C.; Black, D.M.; Eastell, R. Relationship of Changes in Total Hip Bone Mineral Density to Vertebral and Nonvertebral Fracture Risk in Women with Postmenopausal Osteoporosis Treated with Once-Yearly Zoledronic Acid 5 Mg: The HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2012, 27, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.-F.; Girgis, C.M. Bisphosphonate Drug Holidays: Evidence From Clinical Trials and Real-World Studies. JBMR Plus 2022, 6, e10629. [Google Scholar] [CrossRef]
- Borgen, T.T.; Lee-Ødegård, S.; Eriksen, B.F.; Eriksen, E.F. Intermittent Dosing of Zoledronic Acid Based on Bone Turnover Marker Assessment Reduces Vertebral and Non-Vertebral Fractures. JBMR Plus 2024, 8, ziae072. [Google Scholar] [CrossRef]
- Grassi, G.; Chiodini, I.; Palmieri, S.; Cairoli, E.; Arosio, M.; Eller-Vainicher, C. Bisphosphonates after Denosumab Withdrawal Reduce the Vertebral Fractures Incidence. Eur. J. Endocrinol. 2021, 185, 387–396. [Google Scholar] [CrossRef]
- Grassi, G.; Ghielmetti, A.; Zampogna, M.; Chiodini, I.; Arosio, M.; Mantovani, G.; Eller Vainicher, C. Zoledronate after Denosumab Discontinuation: Is Repeated Administrations More Effective than Single Infusion? J. Clin. Endocrinol. Metab. 2024, 109, e1817–e1826. [Google Scholar] [CrossRef]
- Kobayakawa, T.; Kanayama, Y.; Hirano, Y.; Yukishima, T.; Nakamura, Y. Therapy with Transitions from One Bone-Forming Agent to Another: A Retrospective Cohort Study on Teriparatide and Romosozumab. JBMR Plus 2024, 8, ziae131. [Google Scholar] [CrossRef]

| Variables at T0 | All (N = 77) | Group A (N = 56) | Group B (N = 21) | P |
|---|---|---|---|---|
| Age (years) | 71.1 (15.9) | 67.9 (16.6) | 74.0 (11.7) | 0.185 |
| Female gender | 64 (83.1) | 46 (82.1) | 18 (85.7) | 0.709 |
| Secondary OP | 37 (48.0) | 29 (51.8) | 8 (38.1) | 0.317 |
| ARs prior to TPTD | 65 (84.4) | 49 (87.5) | 16 (76.2) | 0.291 |
| Years of ARs prior to TPTD | 4.0 (6.5) | 4.5 (5.5) | 3.0 (8.5) | 0.648 |
| TPTD treatment (months) | 24 (5.1) | 23.9 (6.1) | 24.1 (0.35) | 0.140 |
| Vertebral FX (≥1) | 77 (100) | 56 (100) | 21 (100) | 1.000 |
| SDI | 7 (7) | 7 (9) | 9 (6) | 0.153 |
| Hip FX (≥1) | 8 (10.4) | 5 (8.9) | 3 (14.3) | 0.676 |
| FRAX (10 years major fracture risk, %) | 28.5 (21.3) | 27 (19.3) | 30.5 (27) | 0.143 |
| FRAX (10 years hip fracture risk, %) | 12.5 (16.1) | 11 (14.9) | 14 (23.3) | 0.162 |
| T-score TH | −2.26 ± 0.98 | −2.21 ± 0.99 | −2.42 ± 0.95 | 0.251 |
| T-score LS | −2.48 ± 1.25 | −2.51 ± 1.20 | −2.41 ± 1.38 | 0.320 |
| Serum calcium (mg/dL) | 9.54 ± 0.42 | 9.50 ± 0.39 | 9.65 ± 0.46 | 0.217 |
| Serum creatinine (mg/dL) | 0.81 (0.29) | 0.81 (0.32) | 0.81 (0.35) | 0.495 |
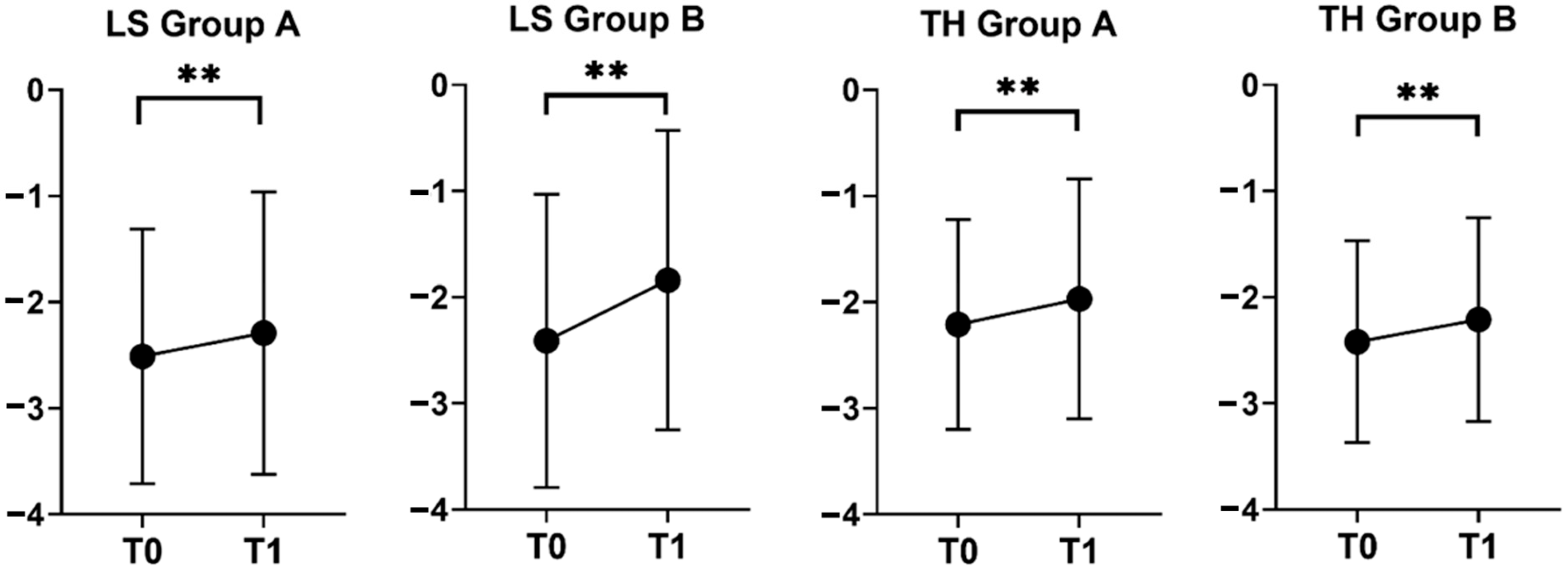
| Variable | Group A T0 (N = 56) | Group A T1 (N = 56) | P | Group B T0 (N = 21) | Group B T1 (N = 21) | P |
|---|---|---|---|---|---|---|
| T-score TH | −2.21 ± 0.99 | −1.97 ± 1.13 | 0.002 | −2.42 ± 0.95 | −2.21 ± 0.96 | 0.001 |
| T-score LS | −2.51 ± 1.20 | −2.29 ± 1.33 | 0.006 | −2.41 ± 1.38 | −1.84 ± 1.41 | <0.001 |
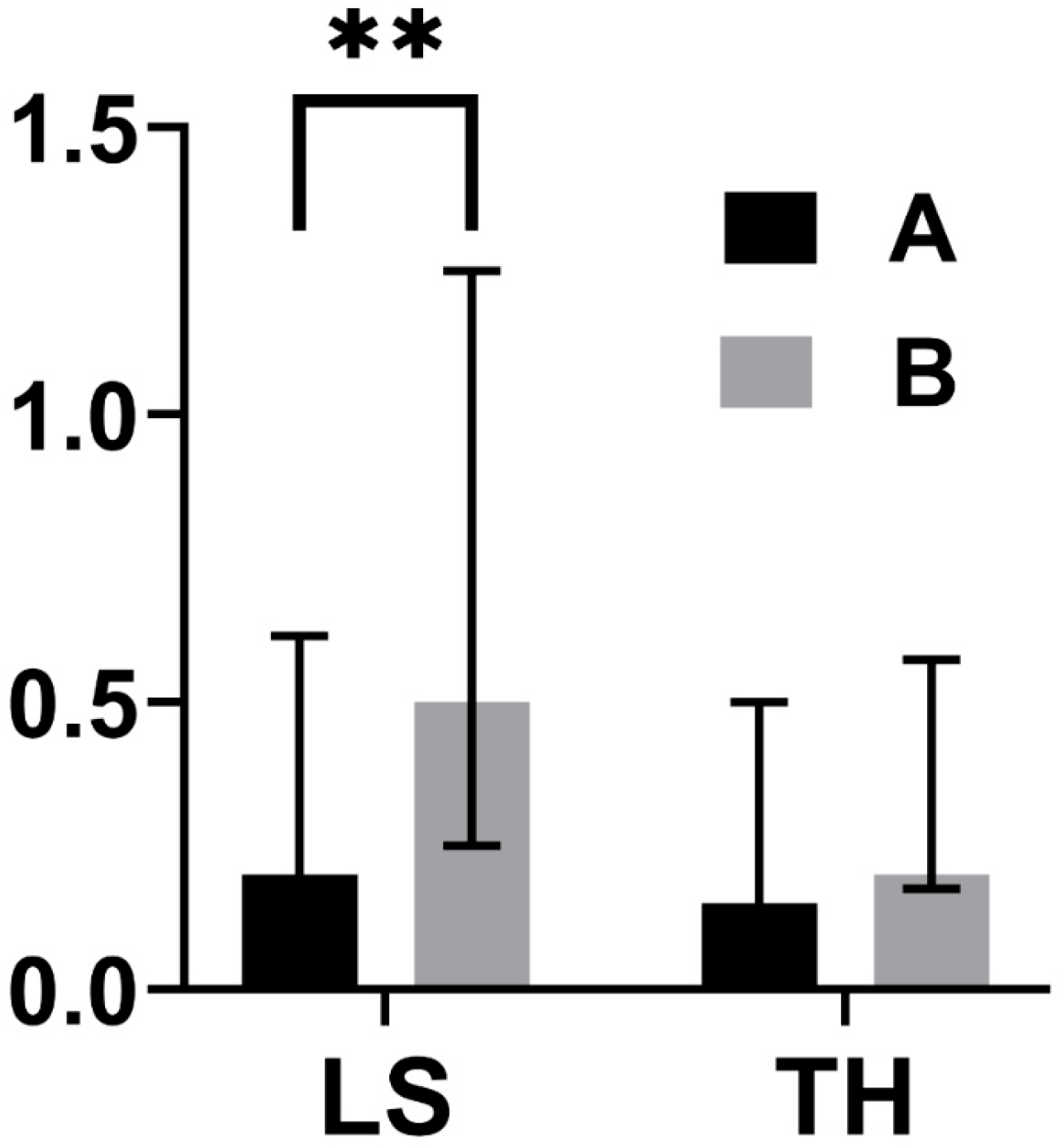
| Variable | Group A (N = 56) | Group B (N = 21) | P |
|---|---|---|---|
| ΔT TH (T1 − T0) | 0.15 (0.40) | 0.20 (0.35) | 0.740 |
| ΔT LS (T1 − T0) | 0.20 (0.43) | 0.50 (0.50) | 0.004 |
| Variable | Group A1 T0 (N = 15) | Group A1 T1 (N = 15) | Group A1 T2 (N = 15) | P Within A1 | Group A2 T0 (N = 17) | Group A2 T1 (N =17) | Group A2 T2 (N = 17) | P Within A2 | Group B T0 (N = 14) | Group B T1 (N = 14) | Group B T2 (N = 14) | P Within B | P Between Groups |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-score TH | −1.78 ± 0.87 | −1.49 ± 0.86 | −1.44 ± 0.98 | 0.004 * | −1.78 ± 0.79 | −1.58 ± 0.85 | −1.57 ± 0.93 | 0.008 § | −2.57 ± 0.72 | −2.32 ± 0.77 | −2.19 ± 0.68 | <0.001 # | T0: 0.030 ^ T1: 0.034 $ T2: 0.082 |
| T-score LS | −2.21 ± 1.17 | −1.94 ± 1.22 | −2.13 ± 1.54 | 0.041 + | −1.99 ± 1.17 | −1.87 ± 1.40 | −1.73 ± 1.43 | 0.203 | −2.67 ± 1.40 | −2.17 ± 1.38 | −2.02 ± 1.25 | <0.001 ° | T0: 0.400 T1: 0.992 T2: 0.583 |
| Median ΔT-Score | Group A1 T0T1 (N = 15) | Group A1 T1T2 (N = 15) | Group A1 T0T2 (N = 15) | P Within A1 1 | Group A2 T0T1 (N = 17) | Group A2 T1T2 (N = 17) | Group A2 T0T2 (N = 17) | P Within A2 1 | Group B T0T1 (N = 14) | Group B T1T2 (N = 14) | Group B T0T2 (N = 14) | P Within B 1 | P Between Groups |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TH | 0.3 (0.2) | 0.1 (0.5) | 0.3 (0.5) | 0.120 | 0.1 (0.4) | 0 (0.3) | 0.3 (0.5) | 0.139 | 0.2(0.4) | 0.1 (0.5) | 0.3 (0.3) | 0.360 | T0T1: 0.182 T1T2: 0.275 T0T2: 0.167 |
| LS | 0.3 (0.3) | −0.2 (0.9) | 0.2 (1.3) | 0.109 | 0.2 (0.8) | 0.1 (0.4) | 0.3 (0.8) | 0.820 | 0.5 (0.5) | 0.1 (0.8) | 0.6 (0.4) | 0.410 | T0T1: 0.607 T1T2: 0.487 T0T2: 0.547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghielmetti, A.; Grassi, G.; Zampogna, M.; Mantovani, G.; Chiodini, I.; Eller-Vainicher, C. Sequential Treatment for Osteoporosis After Teriparatide: A Real-Life Long-Term Comparison Between Zoledronic Acid and Denosumab. J. Clin. Med. 2025, 14, 6360. https://doi.org/10.3390/jcm14186360
Ghielmetti A, Grassi G, Zampogna M, Mantovani G, Chiodini I, Eller-Vainicher C. Sequential Treatment for Osteoporosis After Teriparatide: A Real-Life Long-Term Comparison Between Zoledronic Acid and Denosumab. Journal of Clinical Medicine. 2025; 14(18):6360. https://doi.org/10.3390/jcm14186360
Chicago/Turabian StyleGhielmetti, Alberto, Giorgia Grassi, Marta Zampogna, Giovanna Mantovani, Iacopo Chiodini, and Cristina Eller-Vainicher. 2025. "Sequential Treatment for Osteoporosis After Teriparatide: A Real-Life Long-Term Comparison Between Zoledronic Acid and Denosumab" Journal of Clinical Medicine 14, no. 18: 6360. https://doi.org/10.3390/jcm14186360
APA StyleGhielmetti, A., Grassi, G., Zampogna, M., Mantovani, G., Chiodini, I., & Eller-Vainicher, C. (2025). Sequential Treatment for Osteoporosis After Teriparatide: A Real-Life Long-Term Comparison Between Zoledronic Acid and Denosumab. Journal of Clinical Medicine, 14(18), 6360. https://doi.org/10.3390/jcm14186360







