Brachial Plexopathies: A Comprehensive Radiologic Method Integrating Ultrasound and MRI
Abstract
1. Introduction
2. Anatomical Components
3. Key Imaging Landmarks
4. Imaging Modalities and Techniques
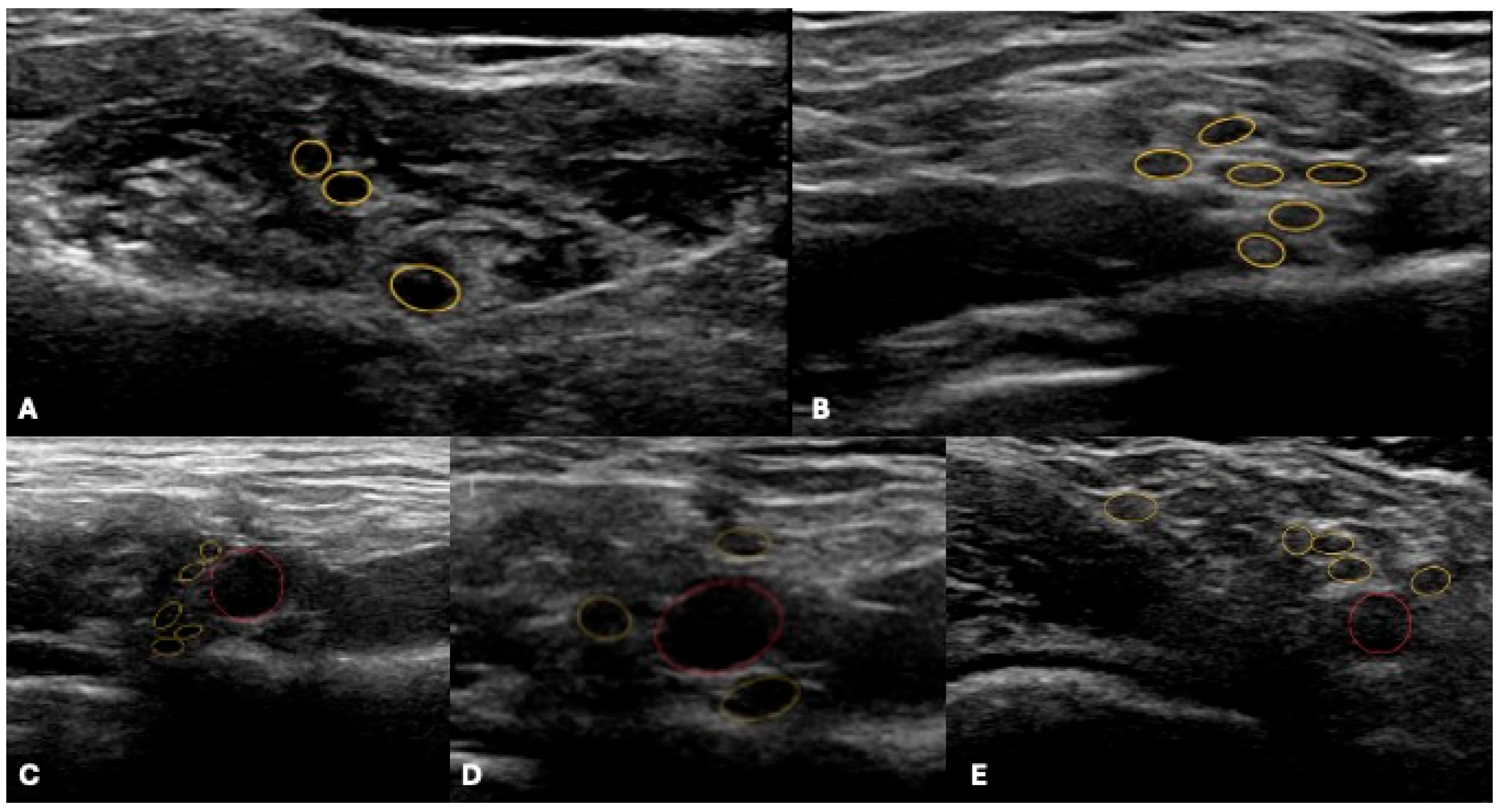
4.1. Ultrasound
Emerging US Tools
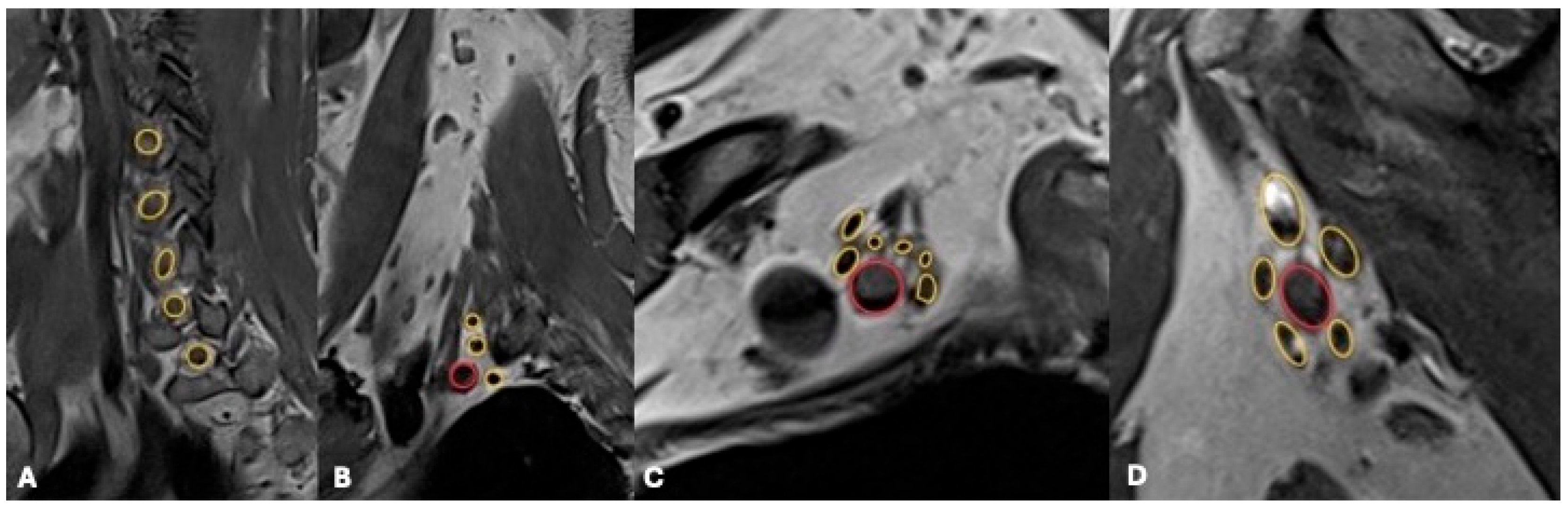
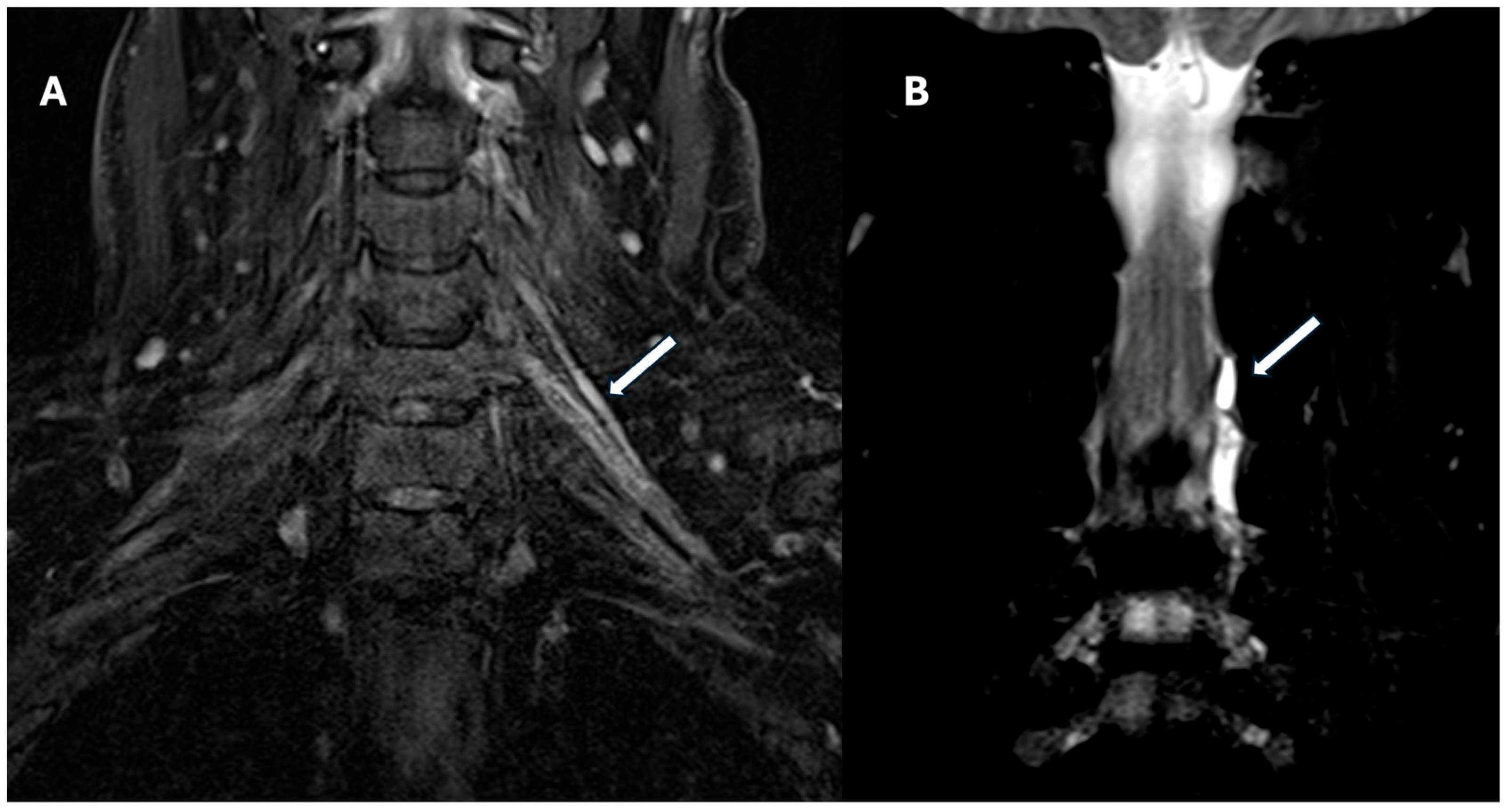
4.2. Magnetic Resonance Imaging (MRI)
Advanced MRI Techniques
5. Diagnostic Patterns and Differential Diagnosis
5.1. Traumatic Plexopathy
5.2. Neoplastic Plexopathy
5.3. Inflammatory and Iatrogenic Plexopathy
6. Integrated Diagnostic Workflow
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| US | Ultrasound |
| MRI | Magnetic Resonance Imaging |
| MRN | Magnetic Resonance Neurography |
| CEUS | Contrast-Enhanced Ultrasound |
| SWE | Shear Wave Elastography |
| DTI | Diffusion Tensor Imaging |
| PNST | Peripheral Nerve Sheath Tumor |
| MPNST | Malignant Peripheral Nerve Sheath Tumor |
| TOS | Thoracic Outlet Syndrome |
| CIDP | Chronic Inflammatory Demyelinating Polyradiculoneuropathy |
| MMN | Multifocal Motor Neuropathy |
| EMG | Electromyography |
| SNR | Signal-to-Noise Ratio |
| CNR | Contrast-to-Noise Ratio |
| FA | Fractional Anisotropy |
References
- Johnson, E.O.; Vekris, M.D.; Zoubos, A.B.; Soucacos, P.N. Neuroanatomy of the Brachial Plexus: The Missing Link in the Continuity between the Central and Peripheral Nervous Systems. Microsurgery 2006, 26, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease-Garcia, B.M.; Deshmukh, S.D.; Parsons, M.S. Anatomy, Imaging, and Pathologic Conditions of the Brachial Plexus. RadioGraphics 2020, 40, 1686–1714. [Google Scholar] [CrossRef]
- Yip, S.W.Y.; Griffith, J.F.; Tong, C.S.L.; Cheung, K.K.; Tsoi, C.; Hung, E.H.Y. Ultrasound Accuracy for Brachial Plexus Pathology. Clin. Radiol. 2024, 79, e916–e923. [Google Scholar] [CrossRef]
- Griffith, J.F.; Lalam, R.K. Top-Ten Tips for Imaging the Brachial Plexus with Ultrasound and MRI. Semin. Musculoskelet. Radiol. 2019, 23, 405–418. [Google Scholar] [CrossRef]
- Samara, O.; Al Khatib, F.; Braik, R.; Alsayouri, T.; Al-Qawasmeh, A.; Afaneh, O.; Al Ryalat, N.; Badran, D.; Shatarat, A. Cross-sectional Study of the Anatomic Variation of Brachial Plexus’ Nerve Roots Origin in Jordan: Prefixed and Postfixed. Jordan Med. J. 2025, 59. [Google Scholar] [CrossRef]
- Caldana, W.C.I.; Kodaira, S.K.; Cavalcanti, C.F.A.; Rodrigues, M.B.; Saito, O.C.; Buchpiguel, C.A. Value of Ultrasound in the Anatomical Evaluation of the Brachial Plexus: Correlation with Magnetic Resonance Imaging. Radiol. Bras. 2018, 51, 358–365. [Google Scholar] [CrossRef]
- Bridgwater, H.; Hector, L.R.; Xiang, P.; Sardesai, N.; Brassett, C.; Sardesai, A. Ultrasonographic Visualization of Anatomical Variations of the Supraclavicular Nerves. Clin. Anat. 2023, 37, 834–839. [Google Scholar] [CrossRef]
- Benes, M.; Kachlik, D.; Belbl, M.; Kunc, V.; Havlikova, S.; Whitley, A.; Kunc, V. A meta-analysis on the anatomical variability of the brachial plexus: Part I—Roots, trunks, divisions and cords. Ann. Anat. Anat. Anz. 2021, 238, 151751. [Google Scholar] [CrossRef] [PubMed]
- Falyar, C.R.; Shaffer, K.M.; Perera, R.A. Localization of the Brachial Plexus: Sonography versus Anatomic Landmarks. J. Clin. Ultrasound 2016, 44, 411–415. [Google Scholar] [CrossRef]
- Mathew, A.; Panwar, J.; Shanmugasundaram, D.; Thomas, B.P. Will Preoperative Combined MRI and High-Resolution Ultrasonography Redefine Brachial Plexus Imaging? A Comparative Study of Preoperative MRI versus Combined MRI and High-Resolution Ultrasonography in Assessing Usable C5, C6 Root-Stumps for Intra-Plexal Nerve Grafting. Clin. Radiol. 2023, 78, e1023–e1031. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.K.; Pakpirom, J.; Songthamwat, B.; Areeruk, P. High Definition Ultrasound Imaging of the Individual Elements of the Brachial Plexus above the Clavicle. Reg. Anesth. Pain. Med. 2020, 45, 344–350. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Chang, K.-V.; Mezian, K.; Naňka, O.; Wu, W.-T.; Yang, Y.-C.; Meng, S.; Ricci, V.; Özçakar, L. Sonographic Pearls for Imaging the Brachial Plexus and Its Pathologies. Diagnostics 2020, 10, 324. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Seelig, M.J.; Baker, J.C.; Mackinnon, S.E.; Pestronk, A. Detection of Peripheral Nerve Pathology. Neurology 2013, 80, 1634–1640. [Google Scholar] [CrossRef]
- Baute, V.; Strakowski, J.A.; Reynolds, J.W.; Karvelas, K.R.; Ehlers, P.; Brenzy, K.J.; Li, Z.J.; Cartwright, M.S. Neuromuscular Ultrasound of the Brachial Plexus: A Standardized Approach. Muscle Nerve 2018, 58, 618–624. [Google Scholar] [CrossRef]
- Baal, J.D.; Yoon, D.; Patel, R.P.; Chin, C.T.; Shah, V.N. Advanced Imaging of the Peripheral Nerves, From the AJR “How We Do It” Special Series. Am. J. Roentgenol. 2024, 223, e2430826. [Google Scholar] [CrossRef] [PubMed]
- Drakonaki, E.; Konschake, M.; Chlouverakis, G.; Tsiaoussis, J. Ultrasound morphometry of the cervical vagus nerve for daily clinical practice: Reference values for cross sectional area and fascicle count. Ann. Anat. 2023, 250, 152137. [Google Scholar] [CrossRef] [PubMed]
- Picasso, R.; Zaottini, F.; Pistoia, F.; Perez, M.M.; Klauser, A.; Rossi, F.; Schenone, A.; Tagliafico, A.S.; Martinoli, C. High-Resolution Ultrasound of Small Clinically Relevant Nerves Running across the Posterior Triangle of the Neck. Semin. Musculoskelet. Radiol. 2020, 24, 101–112. [Google Scholar] [CrossRef]
- Griffith, J.F. Ultrasound of the Brachial Plexus. Semin. Musculoskelet. Radiol. 2018, 22, 323–333. [Google Scholar] [CrossRef]
- Dominika Linda, D.; Harish, S.; Brian Stewart, F.G.; Finlay, K.; Parasu, N.; Paul Rebello, R. Education Exhibits Multimodality Imaging of Peripheral Neuropathies of the Upper Limb and Bra-chial Plexus 1 Cme Feature Learning Objectives for Test 6. RadioGraphics 2010, 30, 1373–1400. [Google Scholar] [CrossRef]
- Hayashi, T.; Matsumoto, N.; Hatake, S.; Takeshi, Y.; Suzuki, K.; Nishiyama, Y.; Nagayama, H.; Kimura, K. Nerve Sonography to Detect Intraneural Microvascularity in Patients with Peripheral Neuropathy. Clin. Neurophysiol. 2024, 166, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Pedro, M.T.; Antoniadis, G.; Scheuerle, A.; Pham, M.; Wirtz, C.R.; Koenig, R.W. Intraoperative High-Resolution Ultrasound and Contrast-Enhanced Ultrasound of Peripheral Nerve Tumors and Tumorlike Lesions. Neurosurg. Focus 2015, 39, E5. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, Y.; Wu, X.; Hou, X.; Qu, X. Myxofibrosarcoma Involving Brachial Plexus Diagnoses by Contrast-Enhanced Ultrasound: A Case Report. Medicine 2023, 102, E36626. [Google Scholar] [CrossRef]
- Nouh, M.R.; Abdel-Naby, H.M.; El Sakka, T.; El-Shafei, M. Peripheral nerve ultrasound: A survival guide for the practicing radiologist with updates. Ultrasound J. 2025, 17, 21. [Google Scholar] [CrossRef]
- Aslan, A.; Aktan, A.; Aslan, M.; Gülseren, Y.; Kabaalioğlu, A. Shear Wave and Strain Elastographic Features of the Brachial Plexus in Healthy Adults: Reliability of the Findings—A Pilot Study. J. Ultrasound Med. 2018, 37, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Neto, T.; Johannsson, J.; Andrade, R.J. Using ultrasound shear wave elastography to characterize peripheral nerve mechanics: A systematic review on the normative reference values in healthy individuals. Ultrasonography 2024, 43, 169–178. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Hayashi, N.; Yamamoto, S.; Tajiri, Y.; Yoshioka, N.; Masumoto, T.; Mori, H.; Abe, O.; Aoki, S.; Ohtomo, K. Brachial plexus injury: Clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics 2006, 26, S133–S143. [Google Scholar] [CrossRef]
- Felisaz, P.F.; Napolitano, A.; Terrani, S.; Parisi, C.; Toto-Brocchi, M.; Cè, M.; Alessandrino, F.; Oliva, G.; Cellina, M.; Gerevini, S. An Optimized 1.5 Tesla MRI Protocol of the Brachial Plexus. Neuroradiol. J. 2024, 37, 43–53. [Google Scholar] [CrossRef]
- Kwee, R.M.; Chhabra, A.; Wang, K.C.; Marker, D.R.; Carrino, J.A. Accuracy of MRI in Diagnosing Peripheral Nerve Disease: A Systematic Review of the Literature. Am. J. Roentgenol. 2014, 203, 1303–1309. [Google Scholar] [CrossRef]
- Brun-Vergara, M.L.; Reda, A.; Puac-Polanco, P.; Zakhari, N.; Shah, V.; Torres, C.H. MR Imaging of the Brachial Plexus: A Practical Review. Magn. Reson. Imaging Clin. N. Am. 2025, 33, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.G.; Whittam, A.; Teh, I.; Andersson, G.; Yeh, F.-C.; Wiberg, M.; Bourke, G. Diffusion Tensor Imaging of the Roots of the Brachial Plexus: A Systematic Review and Meta-Analysis of Normative Values. Clin. Transl. Imaging 2020, 8, 419–431. [Google Scholar] [CrossRef]
- Foesleitner, O.; Sulaj, A.; Sturm, V.; Kronlage, M.; Preisner, F.; Kender, Z.; Bendszus, M.; Szendroedi, J.; Heiland, S.; Schwarz, D. Diffusion tensor imaging in anisotropic tissues: Application of reduced gradient vector schemes in peripheral nerves. Eur. Radiol. Exp. 2024, 8, 37. [Google Scholar] [CrossRef]
- Kronlage, M.; Schwehr, V.; Schwarz, D.; Godel, T.; Uhlmann, L.; Heiland, S.; Bendszus, M.; Bäumer, P. Peripheral nerve diffusion tensor imaging (DTI): Normal values and demographic determinants in a cohort of 60 healthy individuals. Eur. Radiol. 2018, 28, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.S.; Jung, J.Y. Plexus and Peripheral Nerve MR Imaging: Advances and Applications: MR Neurography: Sequence Possibilities and Recent Advances. Magn. Reson. Imaging Clin. N. Am. 2025, 33, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Nwawka, O.K.; Adriaensen, M.; Andreisek, G.; Drakonaki, E.E.; Lee, K.S.; Lutz, A.M.; Martinoli, C.; Nacey, N.; Symanski, J.S. Imaging of Peripheral Nerves: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2025, 224, e2431064. [Google Scholar] [CrossRef]
- Pedrick, E.G.; Sneag, D.B.; Colucci, P.G.; Duong, M.; Tan, E.T. Three-dimensional mr neurography of the brachial plexus: Vascular suppression with low-dose ferumoxytol. Radiology 2023, 307, e221087. [Google Scholar] [CrossRef]
- Wang, K.C.; Salunkhe, A.R.; Morriso, J.J.; Lee, P.P.; Mejino, J.L.V.; Detwiler, L.T.; Brinkley, J.F.; Siegel, E.L.; Rubin, D.L.; Carrino, J.A. Ontology-based image navigation: Exploring 3.0-T MR neurography of the brachial plexus using AIM and radlex. Radiographics 2015, 35, 142–151. [Google Scholar] [CrossRef]
- Pušnik, L.; Radochová, B.; Janáček, J.; Saudek, F.; Serša, I.; Cvetko, E.; Umek, N.; Snoj, Ž. Fascicle differentiation of upper extremity nerves on high-resolution ultrasound with multimodal microscopic verification. Sci. Rep. 2025, 15, 557. [Google Scholar] [CrossRef]
- Thaploo, D.; Bhat, D.I.; Kulkarni, M.V.; Devi, B.I. Brachial Plexus Injury and Resting-State FMRI: Need for Consensus. Neurol. India 2019, 67, 679–683. [Google Scholar]
- Martín-Noguerol, T.; Díaz-Angulo, C.; Luna, A.; Segovia, F.; Gómez-Río, M.; Górriz, J.M. Image-based AI tools in peripheral nerves assessment: Current status and integration strategies—A narrative review. Eur. J. Radiol. 2025, 190, 112255. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Du, X.; Zhang, Z.; Chen, G. The Value of Fast Dixon Combined with Deep Learning Technology in Contrast Agent-Free High-Resolution Magnetic Resonance Imaging of the Brachial Plexus. Front Neurol. 2025, 16, 1558927. [Google Scholar] [CrossRef]
- Qian, Y.; Alhaskawi, A.; Dong, Y.; Ni, J.; Abdalbary, S.; Lu, H. Transforming medicine: Artificial intelligence integration in the peripheral nervous system. Front. Neurol. 2024, 15, 1332048. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Howe, B.M.; Ramanathan, S.; Rhodes, N.G.; Korfiatis, P.; Amrami, K.K.; Spinner, R.J.; Kline, T.L. Non-Traumatic Brachial Plexopathy Identification from Routine MRIs: Retrospective Studies with Deep Learning Networks. Eur. J. Radiol. 2024, 181, 111744. [Google Scholar] [CrossRef]
- Belviso, I.; Palermi, S.; Sacco, A.M.; Romano, V.; Corrado, B.; Zappia, M.; Sirico, F. Brachial plexus injuries in sport medicine: Clinical evaluation, diagnostic approaches, treatment options, and rehabilitative interventions. J. Funct. Morphol. Kinesiol. 2020, 5, 22. [Google Scholar] [CrossRef]
- Singer, A.D.; Meals, C.; Kesner, V.; Boulis, N.; M Gonzalez, F.; Umpierrez, M.; Chhabra, A. The Multidisciplinary Approach to the Diagnosis and Management of Nonobstetric Traumatic Brachial Plexus Injuries. Am. J. Roentgenol. 2018, 211, 1319–1331. [Google Scholar] [CrossRef]
- Griffith, J.F. Don’t Be Perplexed by the Plexus! A Practical Approach to Brachial Plexus Ultrasound. BJR Open 2024, 7, tzaf003. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.G.; Tanner, S.F.; Teh, I.; Ridgway, J.P.; Shelley, D.; Chaka, B.; Rankine, J.J.; Andersson, G.; Wiberg, M.; Bourke, G. Diffusion Tensor Imaging for Diagnosing Root Avulsions in Traumatic Adult Brachial Plexus Injuries: A Proof-of-Concept Study. Front. Surg. 2020, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ding, Y.; Su, Y.; Wang, Y.; Liu, T.; Zhang, Z.; Liu, D.; Li, C.; Zheng, C.; Wang, L. Novel MRI Signs for Differentiating Neurogenic and Non-Neurogenic Peripheral Nerve Tumors: Insights from Contrast-Enhanced Magnetic Resonance Neurography. Eur. J. Radiol. 2025, 183, 111894. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Lin, Y.; Carrino, J.A. An Updated Review of Magnetic Resonance Neurography for Plexus Imaging. Korean J. Radiol. 2023, 24, 1114. [Google Scholar] [CrossRef]
- Nimura, C.A.; Milani, C.; Tan, E.T.; Sneag, D.B. Parsonage-Turner syndrome following monkeypox infection and vaccination. Skelet. Radiol. 2023, 52, 1781–1784. [Google Scholar] [CrossRef]
- Foesleitner, O.; Kirchner, M.; Preisner, F.; Kronlage, M.; Godel, T.; Jende, J.M.E.; Hilgenfeld, T.; Heiland, S.; Wick, W.; Bendszus, M.; et al. High-Resolution US versus MR Neurography for Diagnosis of Upper Extremity Peripheral Nerve Disorders. Radiology 2025, 314, e232063. [Google Scholar] [CrossRef]
- Wade, R.G.; Teh, I.; Shelley, D.; Bains, R.D.; Bedford, J.D.; Homer Newton, L.E.; Ng, C.Y.; Bourke, G. Diffusion Tensor Imaging at 3T for Diagnosing Root Avulsion in Adults with Acute Traumatic Brachial Plexus Injuries. Neuroimage Clin. 2025, 47, 103806. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Li, F.; Jiang, X.; Yu, D.; Wei, J.; Yuan, Y.; Xu, H. Comparison of Different Acceleration Factors of Artificial Intelligence-Compressed Sensing for Brachial Plexus MRI Imaging: Scanning Time and Image Quality. BMC Med. Imaging 2024, 24, 309. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Kawai, T.; Matsumoto, K.; Kawaguchi, T.; Urano, M.; Kitera, N.; Itoh, T.; Hiwatashi, A. Ultra-High-Resolution Photon-Counting Detector CT for Visualization of the Brachial Plexus. Eur. J. Radiol. 2024, 181, 111810. [Google Scholar] [CrossRef] [PubMed]
| Variant Type | Description | Clinical Relevance | Approximate Incidence |
|---|---|---|---|
| Prefixed plexus | Large C4 contribution (C4–C8) | Higher risk of upper root injury | 11% (6–17%) |
| Postfixed plexus | Involves T2 (C6–T2) | Risk of lower trunk avulsion | 1% (0–1%) |
| Absent musculocutaneous nerve | Fibers travel with median nerve | Impacts surgical dissection, block strategy | ~22.5% |
| Double musculocutaneous nerve | Two separate branches | May mimic neuroma/accessory branch | 5–7% |
| Median–musculocutaneous communication | LeMinor types II–III common | Alters electrodiagnostic findings | 5% (3–7%) |
| Crossing supraclavicular branch | Branch crosses over/lateral to clavicle | Important for interscalene block mapping | — |
| Sequence | FOV (mm) | TR/TE (ms) | Primary Purpose | Practical Notes & Pitfalls |
|---|---|---|---|---|
| Coronal 3D STIR | ~350 | 3000/194 | Multiplanar reformats, global plexus trajectory. | Ensure robust and uniform fat suppression; useful for MIP reconstructions; motion artifacts can degrade quality. |
| Coronal T1-weighted | ~320 | 647/6 | Defines fat planes, anatomical borders, mass encasement. | Good for anatomical landmarks and fat infiltration; align with STIR for nerve-to-fat contrast correlation. |
| Axial STIR | ~200 | Variable | Targets C5–T1 roots & cords; detects edema, denervation. | Small FOV boosts spatial resolution; watch for magic angle effect near oblique fibers. |
| Axial T1-weighted | ~200 | Variable | Complements STIR for root morphology and pseudomeningoceles. | Best plane for subtle root avulsions or CSF leaks; compare with coronal T1 for continuity and side-to-side differences. |
| Sagittal T2-weighted | ~240 | 4578/90 | Evaluates cervicobrachial cord lesions, root continuity. | Good for root tracking from foramina to trunks; use in combo with coronal and axial; CSF pulsation can obscure detail. |
| Post-contrast T1 FS | — | Variable | Enhances nerve–mass interface, tumor infiltration, fibrosis. | Delayed acquisitions (CE-MRI) improve conspicuity; always compare with pre-contrast baseline; assess for enhancement. |
| Grade | Classification | Definition | MR Neurography Findings | Treatment Implication |
|---|---|---|---|---|
| I | Neurapraxia | Temporary conduction block; axonal continuity intact | Normal or slight T2 hyperintensity; fascicles preserved | Spontaneous recovery |
| II | Axonotmesis | Axonal disruption; connective tissue sheaths intact | T2 hyperintense, nerve enlarged; loss of fascicular pattern; early muscle denervation | Possible spontaneous recovery; monitor |
| III | Advanced Axonotmesis | Axonal and partial fascicular damage | Marked T2 hyperintensity, fascicular disorganization; clear muscle denervation | Poorer prognosis; possible surgical repair |
| IV | Neuroma-in-Continuity (NIC) | Severe internal scarring within the nerve | Focal heterogeneous swelling; complex internal structure | Often requires surgical resection and nerve graft |
| V | Neurotmesis | Complete nerve transection | Complete discontinuity; end-bulb neuroma formation | Surgical repair or nerve grafting needed |
| Condition | US Findings | MRI Key Findings | CE-MRI Features | Distinguishing Clues |
|---|---|---|---|---|
| Preganglionic Trauma | Limited utility; deep root visualization is challenging | Pseudomeningoceles, root avulsion, spinal cord signal changes (edema, hemorrhage); root separation | Enhancement of intradural root stumps; better delineation of avulsed roots | Pseudomeningoceles (not pathognomonic), paraspinal muscle atrophy, most common at T1 root |
| Postganglionic Trauma | Swelling, discontinuity, neuroma, segmental enlargement, hourglass constrictions; highly sensitive for terminal branches | Thickened nerves, high T2 signal, neuroma-in-continuity, muscle denervation | May enhance neuromas and detect perineural fibrosis | Denervation in target muscles, correlation with EMG, useful for surgical planning |
| Benign PNST | Well-defined, hypoechoic mass, posterior enhancement, preserved nerve continuity | T1 iso, T2 hyper with target sign, intense enhancement | Target and tail signs | No invasion, mobile, ‘split-fat sign’ |
| MPNST | Ill-defined, irregular, possible invasion | Heterogeneous T2, large, infiltrative | Nerve-effacing sign, no target | NF1, post-radiation, rapid growth |
| Metastasis | Discrete, hypervascular, lower trunks | T1 hypo, T2 hyper, enhances | Asymmetric, intense enhancement | Coexisting nodal disease |
| Pancoast Tumor | Limited | Apex mass invading triangle | Loss of interscalene fat pad | Shoulder pain, Horner’s, unresectable if fat pad lost |
| Radiation Plexopathy | Symmetric thickening, avascular | Diffuse symmetric T2 hyperintensity, mild enhancement | Homogeneous enhancement | Uniformity, late onset |
| Post-Radiation Recurrence | Irregular mass, possible vascularity | Focal, asymmetric, nodular enhancement | Asymmetric, nodular | Favors tumor if nonuniform |
| Neuralgic Amyotrophy (PTS) | Suprascapular nerve CSA > 4.2 mm2, fascicular disorganization, hourglass constriction | Hourglass constrictions, focal fascicular narrowing, muscle edema | — | CSA cutoff, dynamic fascicle changes |
| CIDP/MMN | Symmetric/multifocal root/median nerve enlargement, intraneural hypervascularity (SMI) | ‘Onion bulb’ pattern in hereditary forms, diffuse root enlargement | — | Hypervascularity supports inflammation |
| Thoracic Outlet Syndrome (TOS) | Lower trunk indentation/swelling, ‘wedge-sickle sign’, dynamic Doppler with provocative maneuvers | Fibrous bands, vessel impingement, dynamic compression on positional MRI | — | Provocative maneuvers key for diagnosis |
| Post-Surgical/Iatrogenic | Nerve discontinuity, swelling, or perineural fibrosis at surgical site | Fascicular disruption, perineural scarring, muscle denervation | — | Post-surgical history, fibrosis pattern |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacella, G.; Natella, R.; Bruno, F.; Bruno, M.; Franco, D.; Romano, D.G.; Zappia, M. Brachial Plexopathies: A Comprehensive Radiologic Method Integrating Ultrasound and MRI. J. Clin. Med. 2025, 14, 6311. https://doi.org/10.3390/jcm14176311
Pacella G, Natella R, Bruno F, Bruno M, Franco D, Romano DG, Zappia M. Brachial Plexopathies: A Comprehensive Radiologic Method Integrating Ultrasound and MRI. Journal of Clinical Medicine. 2025; 14(17):6311. https://doi.org/10.3390/jcm14176311
Chicago/Turabian StylePacella, Giulia, Raffaele Natella, Federico Bruno, Michela Bruno, Donatella Franco, Daniele Giuseppe Romano, and Marcello Zappia. 2025. "Brachial Plexopathies: A Comprehensive Radiologic Method Integrating Ultrasound and MRI" Journal of Clinical Medicine 14, no. 17: 6311. https://doi.org/10.3390/jcm14176311
APA StylePacella, G., Natella, R., Bruno, F., Bruno, M., Franco, D., Romano, D. G., & Zappia, M. (2025). Brachial Plexopathies: A Comprehensive Radiologic Method Integrating Ultrasound and MRI. Journal of Clinical Medicine, 14(17), 6311. https://doi.org/10.3390/jcm14176311