Pathogen-Reduced Low-Titer Group O Whole Blood for Managing Massive Blood Loss in Prehospital and Early Hospital Settings: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Product Preparation
2.2. Laboratory Investigations
2.3. Statistical Analysis
3. Results
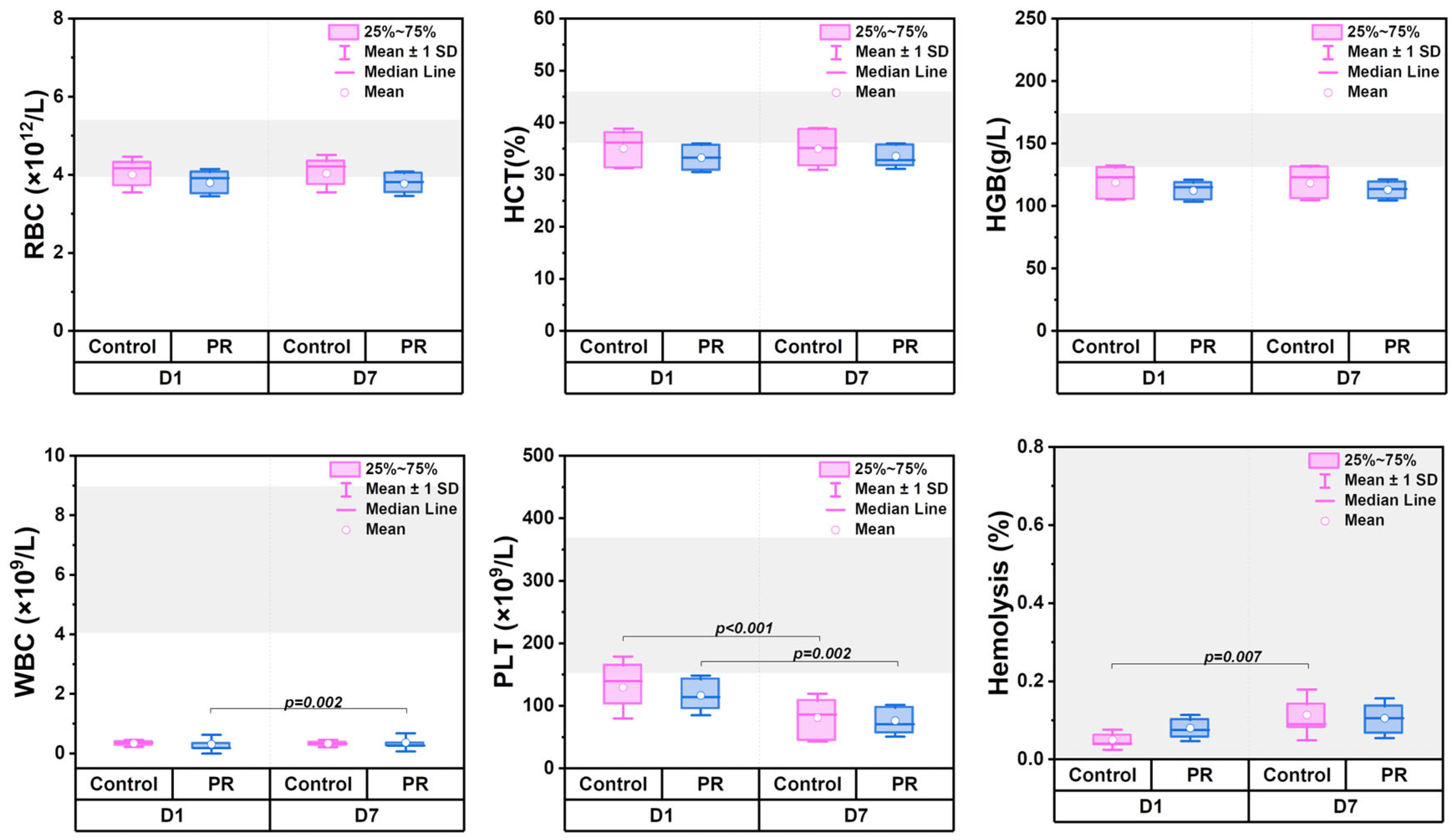
3.1. Changes in Hematologic Parameters
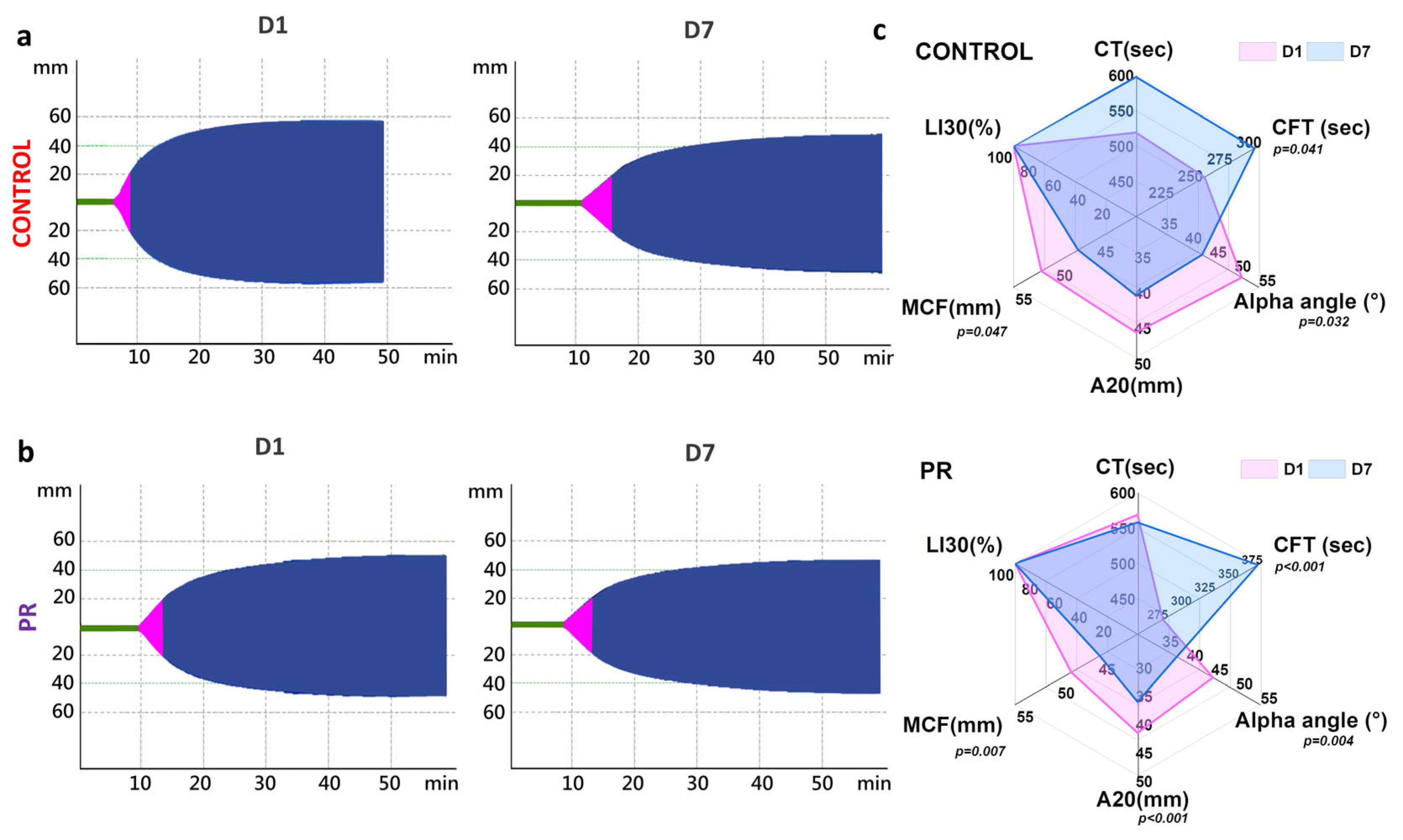
3.2. Measures of Coagulation Parameters
3.3. Erythrocyte Morphology and Young’s Modulus
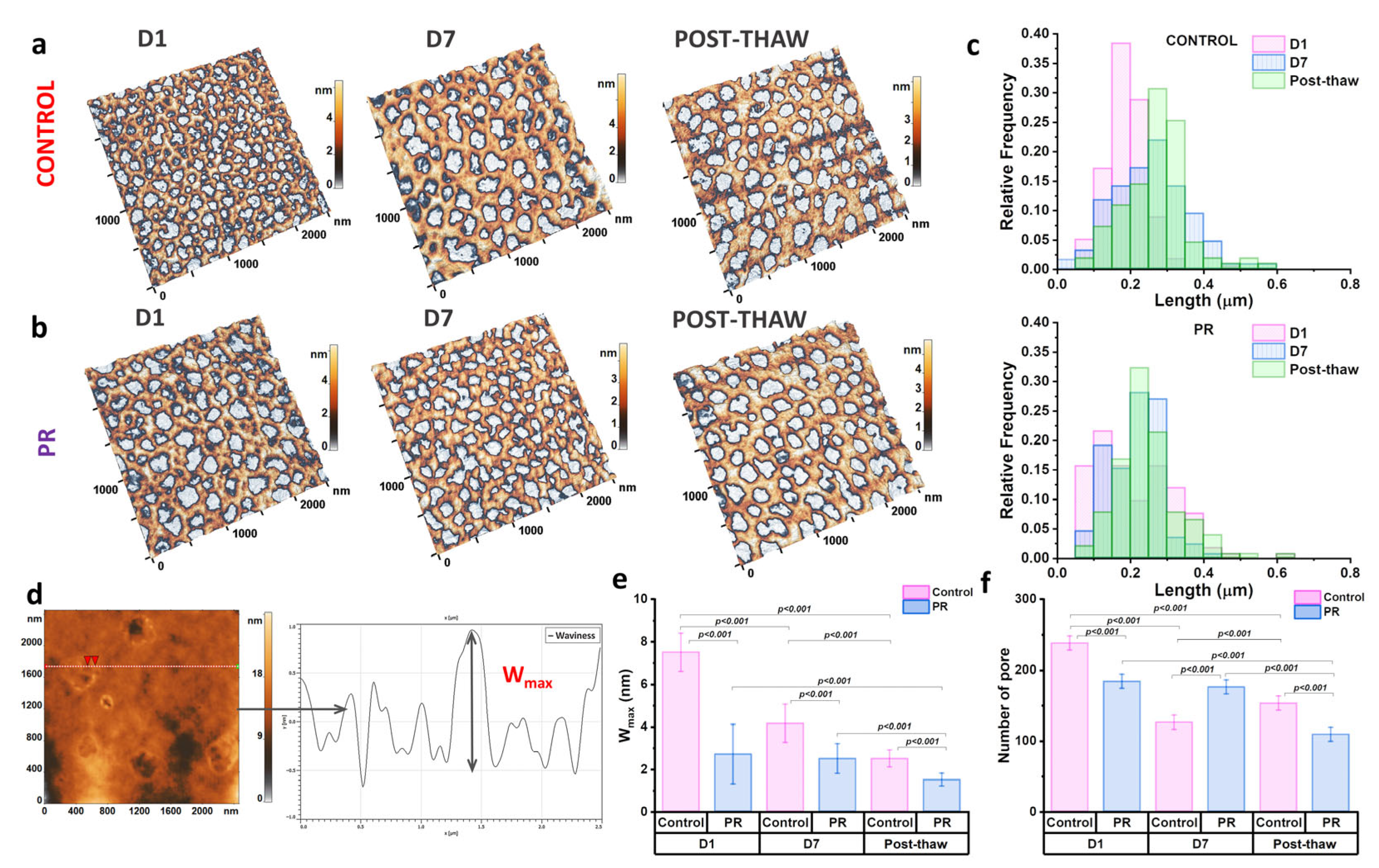
3.4. The Evaluation of the Cytoskeleton and Nanostructure of Erythrocyte Membranes
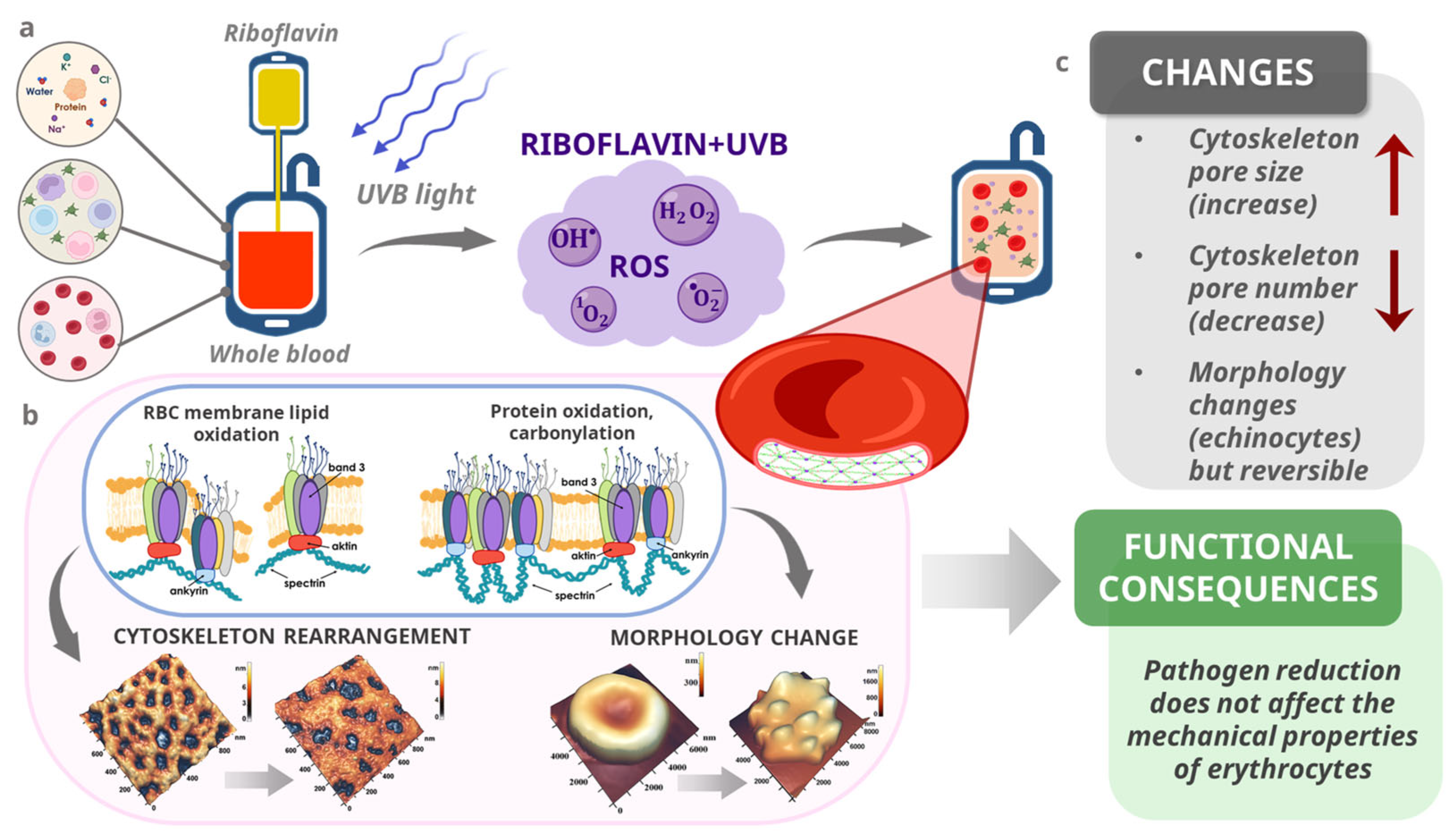
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| E | Young’s modulus |
| PR | Pathogen reduction |
| PLTs | Platelets |
| RBCs | Red blood cells |
| WBCs | White blood cells |
| WB | Whole blood |
References
- Daniel, Y.; Dufour-Gaume, F.; Vergnaud, A.; Denis, M.; Giaume, L.; Rozec, B.; Prat, N.; Lauzier, B. Adjuvant Therapies for Management of Hemorrhagic Shock: A Narrative Review. Crit. Care 2025, 29, 138. [Google Scholar] [CrossRef]
- Crombie, N.; Doughty, H.A.; Bishop, J.R.B.; Desai, A.; Dixon, E.F.; Hancox, J.M.; Herbert, M.J.; Leech, C.; Lewis, S.J.; Nash, M.R.; et al. Resuscitation with Blood Products in Patients with Trauma-Related Haemorrhagic Shock Receiving Prehospital Care (RePHILL): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Haematol. 2022, 9, e250–e261. [Google Scholar] [CrossRef]
- Meyer, D.E.; Vincent, L.E.; Fox, E.E.; O’Keeffe, T.; Inaba, K.; Bulger, E.; Holcomb, J.B.; Cotton, B.A. Every Minute Counts: Time to Delivery of Initial Massive Transfusion Cooler and Its Impact on Mortality. J. Trauma Acute Care Surg. 2017, 83, 19–24. [Google Scholar] [CrossRef]
- Christian, R.J.; McDavitt, C.; Nguyen, T.; Wong, T. Outcomes of Cold-Stored, Low-Titer Group O Whole Blood Transfusions in Nontrauma Massive Transfusion Protocol Activations. Arch. Pathol. Lab. Med. 2023, 147, 710–715. [Google Scholar] [CrossRef]
- Williams, J.; Merutka, N.; Meyer, D.; Bai, Y.; Prater, S.; Cabrera, R.; Holcomb, J.B.; Wade, C.E.; Love, J.D.; Cotton, B.A. Safety Profile and Impact of Low-Titer Group O Whole Blood for Emergency Use in Trauma. J. Trauma Acute Care Surg. 2020, 88, 87–93. [Google Scholar] [CrossRef]
- Gerard, J.; Mueck, K.; Lubkin, D.; Hatton, G.; Brill, J.; Boukas, K.; Cox, C.; Wade, C.; Cotton, B. An Assessment of the Safety, Hemostatic Efficacy, and Clinical Impact of Low-Titer Group O Whole Blood in Children and Adolescents. J. Trauma Acute Care Surg. 2023, 95, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Susila, S.; Helin, T.; Lauronen, J.; Joutsi-Korhonen, L.; Ilmakunnas, M. In Vitro Comparison of Cold-stored Whole Blood and Reconstituted Whole Blood. Vox Sang. 2023, 118, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.M.; Mihalko, E.P.; Lu, L.; Thomas, K.A.; Schuerer, D.; Brown, J.B.; Bochicchio, G.V.; Spinella, P.C. Doing More with Less: Low-Titer Group O Whole Blood Resulted in Less Total Transfusions and an Independent Association with Survival in Adults with Severe Traumatic Hemorrhage. J. Thromb. Haemost. 2024, 22, 140–151. [Google Scholar] [CrossRef]
- Gaines, B.A.; Yazer, M.H.; Triulzi, D.J.; Sperry, J.L.; Neal, M.D.; Billiar, T.R.; Leeper, C.M. Low Titer Group O Whole Blood In Injured Children Requiring Massive Transfusion. Ann. Surg. 2023, 277, e919–e924. [Google Scholar] [CrossRef]
- Thomas, K.A.; Shea, S.M.; Yazer, M.H.; Spinella, P.C. Effect of Leukoreduction and Pathogen Reduction on the Hemostatic Function of Whole Blood. Transfusion 2019, 59, 1539–1548. [Google Scholar] [CrossRef]
- Siletz, A.E.; Blair, K.J.; Cooper, R.J.; Nguyen, N.C.; Lewis, S.J.; Fang, A.; Ward, D.C.; Jackson, N.J.; Rodriguez, T.; Grotts, J.; et al. A Pilot Study of Stored Low Titer Group O Whole Blood + Component Therapy versus Component Therapy Only for Civilian Trauma Patients. J. Trauma Acute Care Surg. 2021, 91, 655–662. [Google Scholar] [CrossRef]
- Haddaway, K.; Bloch, E.M.; Tobian, A.A.R.; Frank, S.M.; Sikorski, R.; Cho, B.C.; Zheng, G.; Jani, J.; Lokhandwala, P.M.; Lawrence, C.E.; et al. Hemostatic Properties of Cold-stored Whole Blood Leukoreduced Using a Platelet-sparing versus a Non-Platelet-sparing Filter. Transfusion 2019, 59, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Fadeyi, E.A.; Saha, A.K.; Naal, T.; Martin, H.; Fenu, E.; Simmons, J.H.; Jones, M.R.; Pomper, G.J. A Comparison between Leukocyte Reduced Low Titer Whole Blood vs. Non-leukocyte Reduced Low Titer Whole Blood for Massive Transfusion Activation. Transfusion 2020, 60, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Sergunova, V.A.; Kuzovlev, A.N.; Onufrievich, A.D.; Inozemtsev, V.A.; Gudkova, O.E.; Sherstyukova, E.A. Conformational Disorders of RBC Membranes during Long-Term Storage. Russ. J. Hematol. Transfusiol. 2022, 67, 181–192. [Google Scholar] [CrossRef]
- Resolution of the Government of the Russian Federation of 22.06.2019 No. 797 (In Russian). Available online: http://government.ru/docs/all/122592/ (accessed on 4 December 2024).
- Sharma, R.; Marwaha, N. Leukoreduced Blood Components: Advantages and Strategies for Its Implementation in Developing Countries. Asian J. Transfus. Sci. 2010, 4, 3–8. [Google Scholar] [CrossRef]
- Bianchi, M.; Vaglio, S.; Pupella, S.; Marano, G.; Facco, G.; Liumbruno, G.M.; Grazzini, G. Leucoreduction of Blood Components: An Effective Way to Increase Blood Safety? Blood Transfus. 2016, 14, 214–227. [Google Scholar] [CrossRef]
- Morris, M.C.; Veile, R.; Friend, L.A.; Oh, D.; Pritts, T.A.; Dorlac, W.C.; Spinella, P.C.; Goodman, M.D. Effects of Whole Blood Leukoreduction on Platelet Function and Hemostatic Parameters. Transfus. Med. 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Remy, K.E.; Yazer, M.H.; Saini, A.; Mehanovic-Varmaz, A.; Rogers, S.R.; Cap, A.P.; Spinella, P.C. Effects of Platelet-Sparing Leukocyte Reduction and Agitation Methods on in Vitro Measures of Hemostatic Function in Cold-Stored Whole Blood. J. Trauma Acute Care Surg. 2018, 84, S104–S114. [Google Scholar] [CrossRef]
- Bubiński, M.; Gronowska, A.; Szykuła, P.; Woźniak, A.; Rodacka, A.; Santi, S.; Cardoso, M.; Lachert, E. Assessing Quality of Blood Components Derived from Whole Blood Treated with Riboflavin and Ultraviolet Light and Separated with a Fully Automated Device. Blood Transfus. 2022, 20, 395–403. [Google Scholar] [CrossRef]
- Allain, J.P.; Owusu-Ofori, A.K.; Assennato, S.M.; Marschner, S.; Goodrich, R.P.; Owusu-Ofori, S. Effect of Plasmodium Inactivation in Whole Blood on the Incidence of Blood Transfusion-Transmitted Malaria in Endemic Regions: The African Investigation of the Mirasol System (AIMS) Randomised Controlled Trial. Lancet 2016, 387, 1753–1761. [Google Scholar] [CrossRef]
- Cancelas, J.A.; Slichter, S.J.; Rugg, N.; Pratt, P.G.; Nestheide, S.; Corson, J.; Pellham, E.; Huntington, M.; Goodrich, R.P. Red Blood Cells Derived from Whole Blood Treated with Riboflavin and Ultraviolet Light Maintain Adequate Survival in Vivo after 21 Days of Storage. Transfusion 2017, 57, 1218–1225. [Google Scholar] [CrossRef]
- Assen, S.; Cardenas, J.; George, M.; Wang, Y.W.; Wade, C.E.; Meyer, D.; Cotton, B.A. Hemostatic Potential of Cold-Stored Non-Leukoreduced Whole Blood over Time: An Assessment of Platelet Function and Thrombin Generation for Optimal Shelf Life. J. Trauma Acute Care Surg. 2020, 89, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Karapati, E.; Valsami, S.; Sokou, R.; Pouliakis, A.; Tsaousi, M.; Sulaj, A.; Iliodromiti, Z.; Iacovidou, N.; Boutsikou, T. Hemostatic Profile of Intrauterine Growth-Restricted Neonates: Assessment with the Use of NATEM Assay in Cord Blood Samples. Diagnostics 2024, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, A.A.P.; Kim, T.-Y. The Role of Evidence-Based Algorithms for Rotational Thromboelastometry-Guided Bleeding Management. Russ. J. Hematol. Transfusiol. 2023, 68, 241–270. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- EDQM. Guide to the Preparation, Use and Quality Assurance of Blood Components, 21st ed.; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2023; ISBN 978-92-871-9359-9. [Google Scholar]
- Henkelman, S.; Noorman, F.; Badloe, J.F.; Lagerberg, J.W.M. Utilization and Quality of Cryopreserved Red Blood Cells in Transfusion Medicine. Vox Sang. 2015, 108, 103–112. [Google Scholar] [CrossRef]
- Lagerberg, J.W. Frozen Blood Reserves. Methods Mol. Biol. 2021, 2180, 523–538. [Google Scholar]
- Sergunova, V.; Leesment, S.; Kozlov, A.; Inozemtsev, V.; Platitsina, P.; Lyapunova, S.; Onufrievich, A.; Polyakov, V.; Sherstyukova, E. Investigation of Red Blood Cells by Atomic Force Microscopy. Sensors 2022, 22, 2055. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Osorio, D.; Martí-Carvajal, A.J.; Arevalo-Rodriguez, I. Leukoreduction for the Prevention of Adverse Reactions from Allogeneic Blood Transfusion. Cochrane Database Syst. Rev. 2015, 2015, CD009745. [Google Scholar] [CrossRef]
- Chien, S.H.; Huang, H.Y.; Chen, Y.J.; Tsai, Y.C.; Lu, S.H.; Lee, L.H.; Liu, H.M.; Chen, W.C.; Liu, Y.C.; Lin, T.A.; et al. Comparing Transfusion Reactions between Pre-Storage and Post-Storage Leukoreduced Apheresis Platelets: An Analysis Using Propensity Score Matching. Ann. Hematol. 2024, 103, 1389–1396. [Google Scholar] [CrossRef]
- Sundararajan, S.; Dodhy, S.C.; Pittman, R.N.; Lewis, S.J. Effects of Non-Leukocyte-Reduced and Leukocyte-Reduced Packed Red Blood Cell Transfusions on Oxygenation of Rat Spinotrapezius Muscle. Microvasc. Res. 2014, 91, 30–36. [Google Scholar] [CrossRef][Green Version]
- Khan, A.I.; Patidar, G.K.; Lakshmy, R.; Makhija, N.; Talwar, S.; Hazarika, A. Effect of Leukoreduction on Transfusion-related Immunomodulation in Patients Undergoing Cardiac Surgery. Transfus. Med. 2020, 30, 497–504. [Google Scholar] [CrossRef]
- Nayeri, N.D.; Nadali, J.; Divani, A.; Hatefimoadab, N. Ways To Enhance Blood Transfusion Safety: A Systematic Review. Florence Nightingale J. Nurs. 2022, 30, 288. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, J.; Braathen, H.; Lunde, T.H.F.; Kristoffersen, E.K.; Hervig, T.; Strandenes, G.; Apelseth, T.O. Cold-Stored Leukoreduced CPDA-1 Whole Blood: In Vitro Quality and Hemostatic Properties. Transfusion 2020, 60, 1042–1049. [Google Scholar] [CrossRef]
- Makarevich, D.G.; Grebenchikov, O.A.; Yadgarov, M.Y.; Berikashvili, L.B.; Kadantseva, K.K.; Likhvantsev, V.V. Achieving and Maintaining Effective Plasma Concentration of Lithium After Oral Administration. Gen. Reanimatol. 2023, 19, 27–33. [Google Scholar] [CrossRef]
- Goroncharovskaya, I.V.; Khvatov, V.B.; Evseev, A.K.; Shabanov, A.K.; Goldin, M.M.; Petrikov, S.S. Monitoring of the Blood Plasma Redox Potential During Plasma Quarantining (Preliminary Report). Gen. Reanimatol. 2019, 15, 47–53. [Google Scholar] [CrossRef]
- Juárez-Vela, R.; Andrés-Esteban, E.M.; Santolalla-Arnedo, I.; Ruiz de Viñaspre-Hernández, R.; Benito-Puncel, C.; Serrano-Lázaro, A.; Marcos-Neira, P.; López-Fernández, A.; Tejada-Garrido, C.I.; Sánchez-González, J.L.; et al. Epidemiology and Associated Factors in Transfusion Management in Intensive Care Unit. J. Clin. Med. 2022, 11, 3532. [Google Scholar] [CrossRef]
- Klein, H.G. Pathogen Inactivation Technology: Cleansing the Blood Supply. J. Intern. Med. 2005, 257, 224–237. [Google Scholar] [CrossRef]
- Rezvany, M.R.; Moradi Hasan-Abad, A.; Sobhani-Nasab, A.; Esmaili, M.A. Evaluation of Bacterial Safety Approaches of Platelet Blood Concentrates: Bacterial Screening and Pathogen Reduction. Front. Med. 2024, 11, 1325602. [Google Scholar] [CrossRef]
- Alabdullatif, M.; Osman, I.E.; Alrasheed, M.; Ramirez-Arcos, S.; Alyousef, M.; Althawadi, S.; Alhumiadan, H. Evaluation of Riboflavin and Ultraviolet Light Treatment against Klebsiella Pneumoniae in Whole Blood-Derived Platelets: A Pilot Study. Transfusion 2021, 61, 1562–1569. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Chander, J.; Nirankari, V.S. In Vitro Antimicrobial Efficacy of Riboflavin, Ultraviolet-A Radiation, and Combined Riboflavin/Ultraviolet-A Radiation on Ocular Pathogens. Taiwan J. Ophthalmol. 2021, 13, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Ohlsson, S.; Andersson, T.N.; Watz, E.; Larsson, S.; Sandgren, P.; Uhlin, M. Pathogen Reduced Red Blood Cells as an Alternative to Irradiated and Washed Components with Potential for up to 42 Days Storage. Blood Transfus. 2024, 22, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Jobes, D.; Wolfe, Y.; O’Neill, D.; Calder, J.; Jones, L.; Sesok-Pizzini, D.; Zheng, X.L. Toward a Definition of “Fresh” Whole Blood: An in Vitro Characterization of Coagulation Properties in Refrigerated Whole Blood for Transfusion. Transfusion 2011, 51, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.; Okhota, S.; Avtaeva, Y.; Melnikov, I.; Matroze, E.; Gabbasov, Z. Von Willebrand Factor in Diagnostics and Treatment of Cardiovascular Disease: Recent Advances and Prospects. Front. Cardiovasc. Med. 2022, 9, 1038030. [Google Scholar] [CrossRef]
- Makris, M.; Gavriilaki, E.; Ztriva, E.; Evangelidis, P.; Lefkou, E.; Vlachaki, E.; Bountola, S.; Perifanis, V.; Matsagkas, M.; Savopoulos, C.; et al. Prospective Study of ADAMTS13 and von Willebrand Factor’s Role in the Prediction of Outcomes in Acute Ischemic Stroke. J. Clin. Med. 2022, 14, 2470. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, G.; Li, N.; Hou, T.; Shao, X.; Tang, W.; Wang, F.; Luan, J.; Zhu, P. An in Vitro Study of Coagulation Properties in Refrigerated Whole Blood and Reconstituted Whole Blood. Vox Sang. 2019, 114, 694–700. [Google Scholar] [CrossRef]
- McRae, H.L.; Kara, F.; Milito, C.; Cahill, C.; Blumberg, N.; Refaai, M.A. Whole Blood Haemostatic Function throughout a 28-day Cold Storage Period: An in Vitro Study. Vox Sang. 2021, 116, 190–196. [Google Scholar] [CrossRef]
- Pidcoke, H.F.; McFaul, S.J.; Ramasubramanian, A.K.; Parida, B.K.; Mora, A.G.; Fedyk, C.G.; Valdez-Delgado, K.K.; Montgomery, R.K.; Reddoch, K.M.; Rodriguez, A.C.; et al. Primary Hemostatic Capacity of Whole Blood: A Comprehensive Analysis of Pathogen Reduction and Refrigeration Effects over Time. Transfusion 2013, 53, 137–149. [Google Scholar] [CrossRef]
- Barns, S.; Balanant, M.A.; Sauret, E.; Flower, R.; Saha, S.; Gu, Y. Investigation of Red Blood Cell Mechanical Properties Using AFM Indentation and Coarse-Grained Particle Method. Biomed. Eng. Online 2017, 16, 140. [Google Scholar] [CrossRef]
- Himbert, S.; Rheinstädter, M.C. Structural and Mechanical Properties of the Red Blood Cell’s Cytoplasmic Membrane Seen through the Lens of Biophysics. Front. Physiol. 2022, 13, 953257. [Google Scholar] [CrossRef]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarska, M.; Grosicki, M.; Bulat, K.; Mardyla, M.; Szczesny-Malysiak, E.; Blat, A.; Dybas, J.; Sacha, T.; Marzec, K.M. Temporal Sequence of the Human RBCs’ Vesiculation Observed in Nano-Scale with Application of AFM and Complementary Techniques. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zheng, Y.; Wang, X.; Shehata, N.; Wang, C.; Sun, Y. Stiffness Increase of Red Blood Cells during Storage. Microsyst. Nanoeng. 2018, 4, 17103. [Google Scholar] [CrossRef]
- Martínez-Vieyra, I.; Hernández-Rojo, I.; Rosales-García, V.H.; Chávez-Piña, A.E.; Cerecedo, D. Oxidative Stress and Cytoskeletal Reorganization in Hypertensive Erythrocytes. Antioxidants 2024, 14, 5. [Google Scholar] [CrossRef]
- Sel, S.; Nass, N.; Pötzsch, S.; Trau, S.; Simm, A.; Kalinski, T.; Duncker, G.I.; Kruse, F.E.; Auffarth, G.U.; Brömme, H.-J. UVA Irradiation of Riboflavin Generates Oxygen-Dependent Hydroxyl Radicals. Redox Rep. 2014, 19, 72–79. [Google Scholar] [CrossRef]
- Goroncharovskaya, I.V.; Evseev, A.K.; Shabanov, A.K.; Kulabukhov, V.V.; Kuzovlev, A.N.; Popugaev, K.A.; Petrikov, S.S. Effect of Fresh Frozen Plasma Transfusion on Electrochemical Parameters of Blood Plasma in Patients with Severe Combined Trauma. Gen. Reanimatol. 2020, 16, 4–13. [Google Scholar] [CrossRef]
- Alshalani, A.; Uhel, F.; Cremer, O.L.; Schultz, M.J.; de Vooght, K.M.K.; van Bruggen, R.; Acker, J.P.; Juffermans, N.P. Donor-Recipient Sex Is Associated with Transfusion-Related Outcomes in Critically Ill Patients. Blood Adv. 2022, 6, 3260–3267. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherstyukova, E.; Semenova, J.; Kandrashina, S.; Bogdanova, A.; Vinogradov, I.; Inozemtsev, V.; Shvedov, M.; Grechko, A.; Dokukin, M.; Kuzovlev, A.; et al. Pathogen-Reduced Low-Titer Group O Whole Blood for Managing Massive Blood Loss in Prehospital and Early Hospital Settings: An In Vitro Study. J. Clin. Med. 2025, 14, 6292. https://doi.org/10.3390/jcm14176292
Sherstyukova E, Semenova J, Kandrashina S, Bogdanova A, Vinogradov I, Inozemtsev V, Shvedov M, Grechko A, Dokukin M, Kuzovlev A, et al. Pathogen-Reduced Low-Titer Group O Whole Blood for Managing Massive Blood Loss in Prehospital and Early Hospital Settings: An In Vitro Study. Journal of Clinical Medicine. 2025; 14(17):6292. https://doi.org/10.3390/jcm14176292
Chicago/Turabian StyleSherstyukova, Ekaterina, Julia Semenova, Snezhanna Kandrashina, Alina Bogdanova, Ilya Vinogradov, Vladimir Inozemtsev, Mikhail Shvedov, Alexander Grechko, Maxim Dokukin, Artem Kuzovlev, and et al. 2025. "Pathogen-Reduced Low-Titer Group O Whole Blood for Managing Massive Blood Loss in Prehospital and Early Hospital Settings: An In Vitro Study" Journal of Clinical Medicine 14, no. 17: 6292. https://doi.org/10.3390/jcm14176292
APA StyleSherstyukova, E., Semenova, J., Kandrashina, S., Bogdanova, A., Vinogradov, I., Inozemtsev, V., Shvedov, M., Grechko, A., Dokukin, M., Kuzovlev, A., Klychnikova, E., Bulanov, A., Kostin, A., & Sergunova, V. (2025). Pathogen-Reduced Low-Titer Group O Whole Blood for Managing Massive Blood Loss in Prehospital and Early Hospital Settings: An In Vitro Study. Journal of Clinical Medicine, 14(17), 6292. https://doi.org/10.3390/jcm14176292





