Effectiveness of Ultrasound-Guided Peritendinous Injection Treatment with Low Molecular Weight Hyaluronic Acid in Patients with Supraspinatus Tendinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- pain for at least 4 weeks
- diagnosis of tendinopathy, confirmed by magnetic resonance imaging (MRI) and/or standard ultrasound examination
- Visual Analogue Scale (VAS) of at least 2 points
- corticosteroid or local anti-inflammatory injections or were on oral corticosteroid therapy within four weeks before the treatment
- shockwave therapy six months before the treatment
- surgery in the previous twelve months
- acute post-traumatic symptoms
- advanced osteoarthritis
- calcific tendinopathy
- infections or skin diseases near the affected area
- hypersensitivity to the components of the drug
- uncontrolled diabetes mellitus, vasculopathy, peripheral neuropathy, neurological, and/or rheumatological diseases
2.2. Study Procedures and Evaluations
Follow-Ups
2.3. Primary Objectives
2.4. Secondary Objectives
2.5. Statistical Analysis
Sample Size Calculation
3. Results
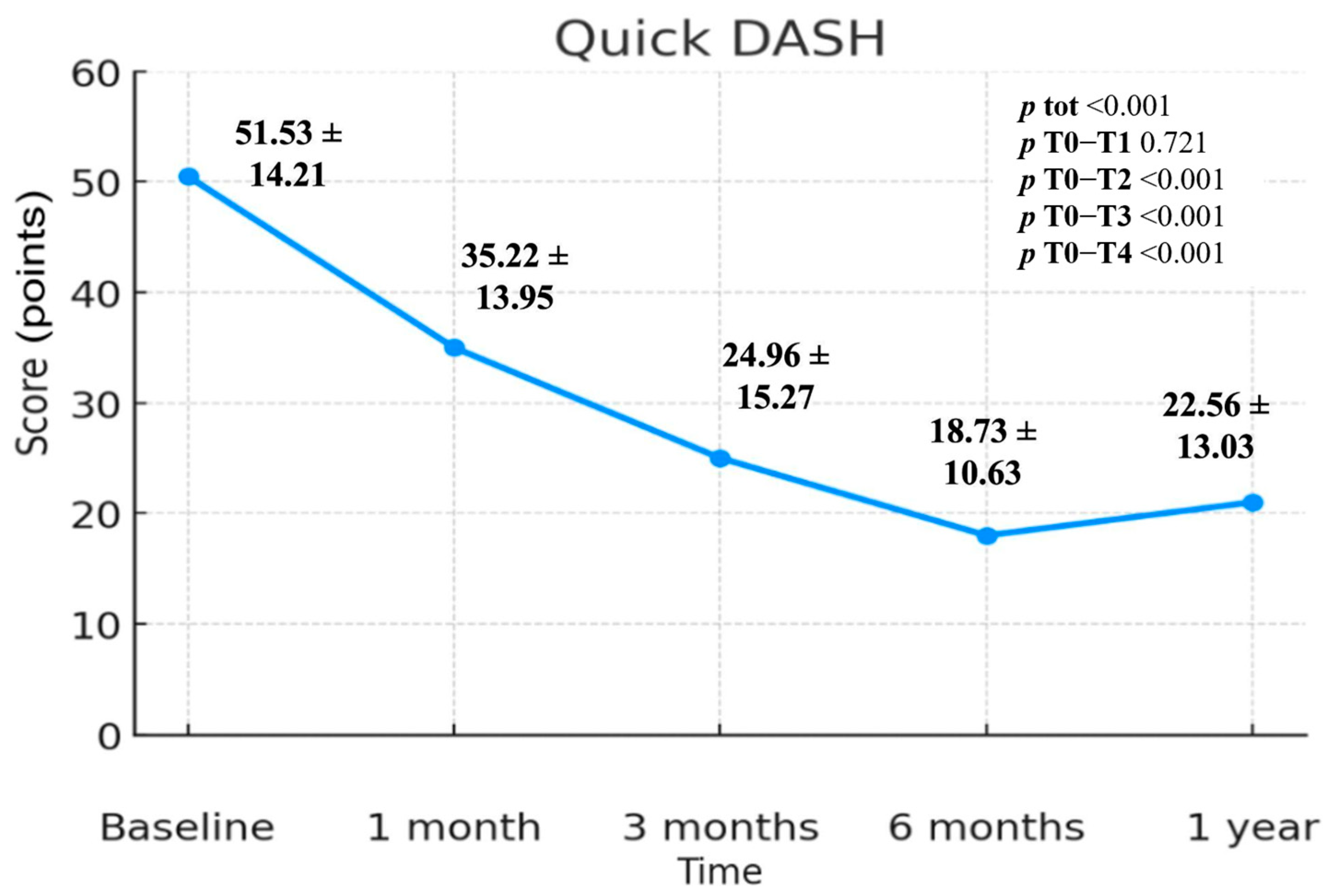
3.1. QuickDASH Scale
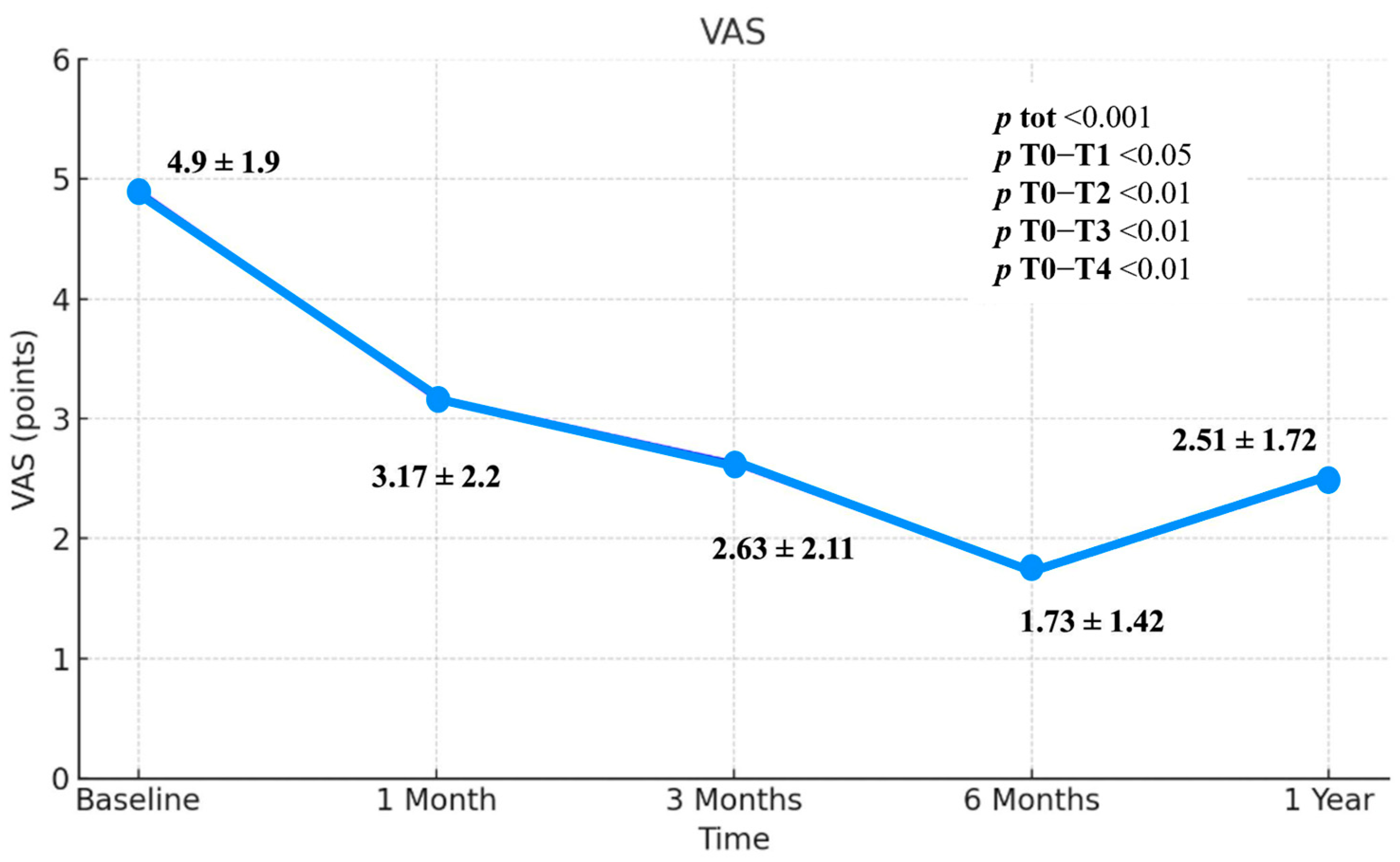
3.2. Visual Analogue Scale (VAS)
3.3. SF-12 Scale
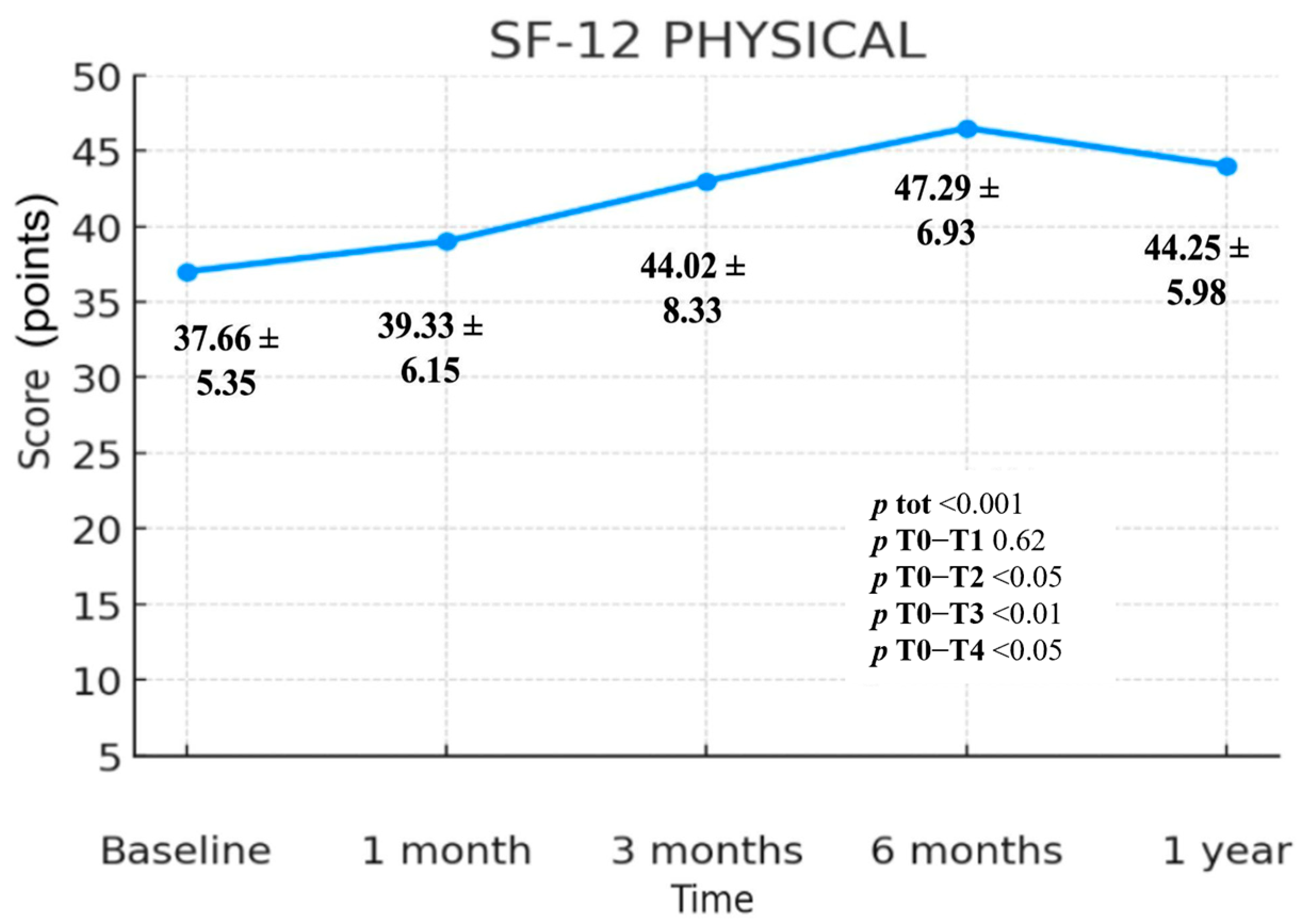
3.3.1. SF-12 Physical Component Summary (PCS)
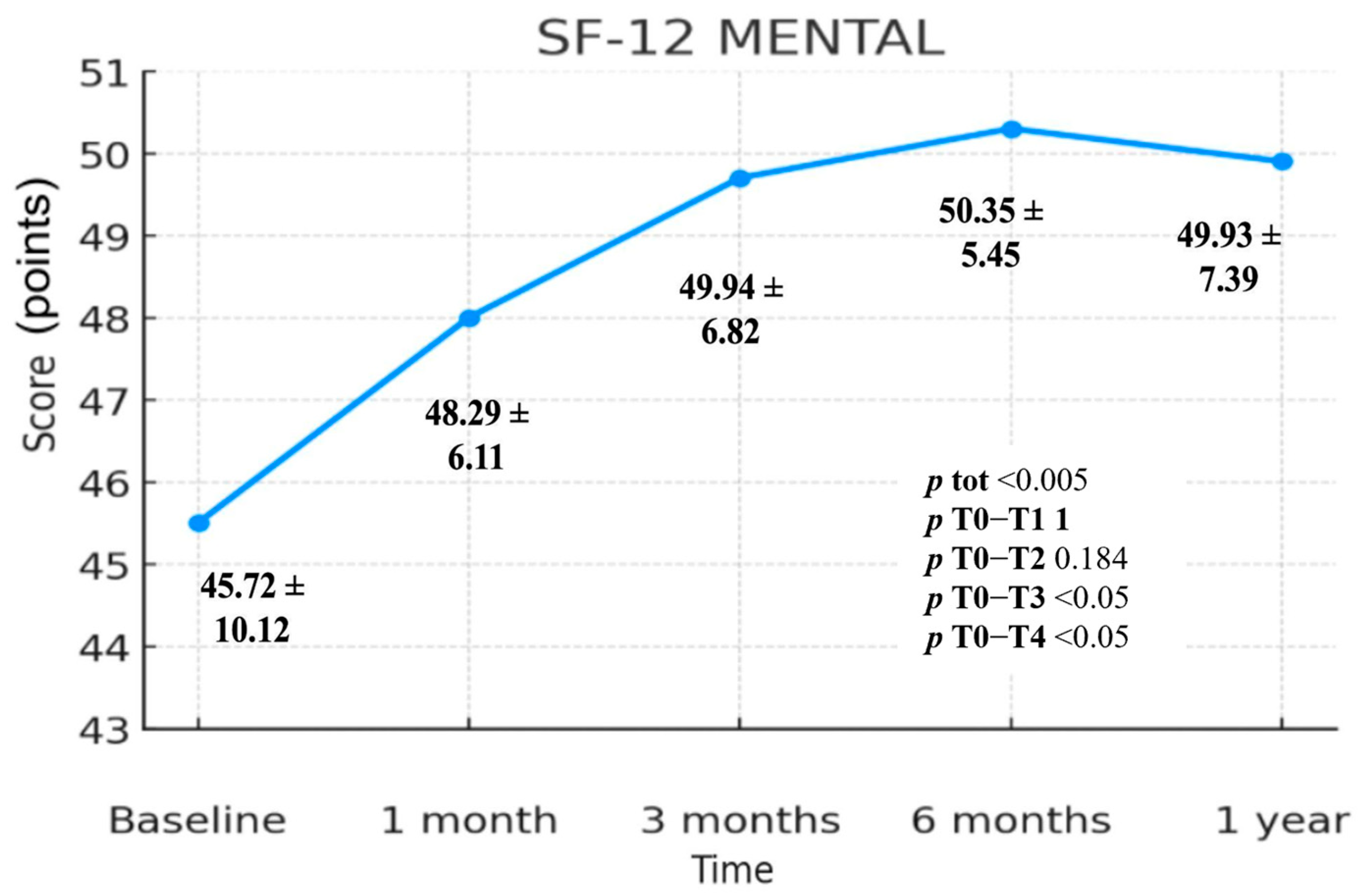
3.3.2. SF-12 Mental Component Summary (MCS)
3.3.3. Safety
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef]
- Maffulli, N.; Khan, K.M.; Puddu, G. Overuse tendon conditions: Time to change a confusing terminology. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 840–843. [Google Scholar] [CrossRef]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1, Erratum in Nat. Rev. Dis. Primers 2021, 7, 10. https://doi.org/10.1038/s41572-021-00251-8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, J.Y.; Lee, C.S.; Lee, S.J. Does hyaluronate injection work in shoulder disease in early stage? A multicenter, randomized, single blind and open comparative clinical study. J. Shoulder Elb. Surg. 2012, 21, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Smallcomb, M.; Khandare, S.M.; Vidt, M.E.; Simon, J.C. Therapeutic Ultrasound and Shockwave Therapy for Tendinopathy: A Narrative Review. Am. J. Phys. Med. Rehabilitation 2022, 101, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, A.; Oliva, F.; Vittadini, F.; Vetrano, M.; Bernetti, A.; Giordan, N.; Vulpiani, M.C.; Santilli, V.; Masiero, S.; Maffulli, N. Efficacy of ultrasound-guided hyaluronic acid injections in Achilles and patellar tendinopathies: A prospective multicentric clinical trial. Muscles Ligaments Tendons J. 2019, 9, 305–313. [Google Scholar] [CrossRef]
- Reinholz, A.K.; Till, S.E.; Arguello, A.M.; Barlow, J.D.; Okoroha, K.R.; Camp, C.L. Advances in the Treatment of Rotator Cuff Tears: Management of Rotator Cuff Tears in the Athlete. Clin. Sports Med. 2023, 42, 69–79. [Google Scholar] [CrossRef]
- Agostini, F.; De Sire, A.; Paoloni, M.; Finamore, N.; Ammendolia, A.; Mangone, M.; Bernetti, A. Effects of hyaluronic acid injections on pain and functioning in patients affected by tendinopathies: A narrative review. J. Back Musculoskelet. Rehabil. 2022, 35, 949–961. [Google Scholar] [CrossRef]
- Frizziero, A.; Vittadini, F.; Fusco, A.; Giombini, A.; Masiero, S. Efficacy of eccentric exercise in lower limb tendinopathies in athletes. J. Sports Med. Phys. Fit. 2016, 56, 1352–1358. [Google Scholar]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Bernetti, A.; Mangone, M.; Paolucci, T.; Santilli, V.; Verna, S.; Agostini, F.; Paoloni, M. Evaluation of the efficacy of intra-articular injective treatment with reticular hyaluronic acid (Mo.Re. Technology) in amateur athletes with over-use gonarthrosis. Medicina dello Sport 2020, 73, 127–139. [Google Scholar] [CrossRef]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheumatol. 1993, 39, 3–9. [Google Scholar]
- Arirachakaran, A.; Boonard, M.; Yamaphai, S.; Prommahachai, A.; Kesprayura, S.; Kongtharvonskul, J. Extracorporeal shock wave therapy, ultrasound-guided percutaneous lavage, corticosteroid injection and combined treatment for the treatment of rotator cuff calcific tendinopathy: A network meta-analysis of RCTs. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 381–390. [Google Scholar] [CrossRef]
- Tagliafico, A.; Russo, G.; Boccalini, S.; Michaud, J.; Klauser, A.; Serafini, G.; Martinoli, C. Ultrasound-guided interventional procedures around the shoulder. Radiol. Med. 2014, 119, 318–326. [Google Scholar] [CrossRef]
- Liu, D.H.; Tsai, M.W.; Lin, S.H.; Chou, C.L.; Chiu, J.W.; Chiang, C.C.; Kao, C.L. Ultrasound-Guided Hyaluronic Acid Injections for Trigger Finger: A Double-Blinded, Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 2120–2127. [Google Scholar] [CrossRef]
- Khan, M.; Shanmugaraj, A.; Prada, C.; Patel, A.; Babins, E.; Bhandari, M. The Role of Hyaluronic Acid for Soft Tissue Indications: A Systematic Review and Meta-Analysis. Sports Health 2023, 15, 86–96. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Fogli, M.; Giordan, N.; Mazzoni, G. Efficacy and safety of hyaluronic acid (500-730kDa) Ultrasound-guided injections on painful tendinopathies: A prospective, open label, clinical study. Muscles Ligaments Tendons J. 2017, 7, 388–395. [Google Scholar] [CrossRef]
- Cipolletta, E.; Mashadi Mirza, R.; Di Matteo, A.; Di Carlo, M.; Grassi, W.; Filippucci, E. Clinical efficacy of ultrasound-guided hyaluronic acid injections in patients with supraspinatus tendon tear. Clin. Exp. Rheumatol. 2021, 39, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Meloni, F.; Milia, F.; Cavazzuti, M.; Doria, C.; Lisai, P.; Profili, S.; Meloni, G.B. Clinical evaluation of sodium hyaluronate in the treatment of patients with sopraspinatus tendinosis under echographic guide: Experimental study of periarticular injections. Eur. J. Radiol. 2008, 68, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Gummesson, C.; Ward, M.M.; Atroshi, I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): Validity and reliability based on responses within the full-length DASH. BMC Musculoskelet. Disord. 2006, 7, 44. [Google Scholar] [CrossRef]
- Sung, Y.T.; Wu, J.S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. Methods 2018, 50, 1694–1715. [Google Scholar] [CrossRef] [PubMed]
- Kodraliu, G.; Mosconi, P.; Groth, N.; Carmosino, G.; Perilli, A.; Gianicolo, E.A.; Rossi, C.; Apolone, G. Subjective health status assessment: Evaluation of the Italian version of the SF-12 Health Survey. Results from the MiOS Project. J. Epidemiol. Biostat. 2001, 6, 305–316. [Google Scholar] [CrossRef]
- Cipolletta, E.; Filippucci, E.; Incorvaia, A.; Schettino, M.; Smerilli, G.; Di Battista, J.; Tesei, G.; Cosatti, M.A.; Di Donato, E.; Tardella, M.; et al. Ultrasound-Guided Procedures in Rheumatology Daily Practice: Feasibility, Accuracy, and Safety Issues. J. Clin. Rheumatol. 2021, 27, 226–231. [Google Scholar] [CrossRef]
- Messina, C.; Banfi, G.; Orlandi, D.; Lacelli, F.; Serafini, G.; Mauri, G.; Secchi, F.; Silvestri, E.; Sconfienza, L.M. Ultrasound-guided interventional procedures around the shoulder. Br. J. Radiol. 2016, 89, 20150372. [Google Scholar] [CrossRef]
- Sherman, T.; Ferguson, J.; Davis, W.; Russo, M.; Argintar, E. Does the use of ultrasound affect contamination of musculoskeletal injections sites? Clin. Orthop. Relat. Res. 2015, 473, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Budtz, C.R.; Andersen, J.H.; de Vos Andersen, N.B.; Christiansen, D.H. Responsiveness and minimal important change for the quick-DASH in patients with shoulder disorders. Health Qual. Life Outcomes 2018, 16, 226. [Google Scholar] [CrossRef] [PubMed]
- Chester, R.; Jerosch-Herold, C.; Lewis, J.; Shepstone, L. The SPADI and QuickDASH Are Similarly Responsive in Patients Undergoing Physical Therapy for Shoulder Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 538–547. [Google Scholar] [CrossRef]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef]
- Ozgen, M.; Fırat, S.; Sarsan, A.; Topuz, O.; Ardıç, F.; Baydemir, C. Short- and long-term results of clinical effectiveness of sodium hyaluronate injection in supraspinatus tendinitis. Rheumatol. Int. 2012, 32, 137–144. [Google Scholar] [CrossRef]
- Mohebbi, R.; Rezasoltani, Z.; Mir, M.; Mohebbi, M.; Vatandoost, S.; Esmaily, H. High- Versus Low-Molecular-Weight Hyaluronic Acid for the Treatment of Rotator Cuff Tendinopathy: A Triple-Blind Randomized Comparative Trial. Ann. Pharmacother. 2021, 55, 1203–1214. [Google Scholar] [CrossRef]
- Frizziero, A.; Vittadini, F.; Barazzuol, M.; Gasparre, G.; Finotti, P.; Meneghini, A.; Maffulli, N.; Masiero, S. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non-calcific rotator cuff tendinopathies: Preliminary results. J. Sports Med. Phys. Fit. 2017, 57, 1162–1168. [Google Scholar] [CrossRef]
- Muneta, T.; Koga, H.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Hyaluronan injection therapy for athletic patients with patellar tendinopathy. J. Orthop. Sci. 2012, 17, 425–431. [Google Scholar] [CrossRef]
- Frizziero, A.; Vittadini, F.; Bigliardi, D.; Costantino, C. Low Molecular Weight Hyaluronic Acid (500-730 Kda) Injections in Tendinopathies—A Narrative Review. J. Funct. Morphol. Kinesiol. 2021, 7, 3. [Google Scholar] [CrossRef]
- Lavigne, A.; Sandouk, E.; Hiett, A.; Chang, M.C.; Bélanger, V.; Lamontagne, M.; Khadavi, M. Current evidence on hyaluronic acid injections for rotator cuff tendinopathy: A scoping review. Shoulder Elb. 2025, 34, 1131–1145. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, A.; Fini, M.; Salamanna, F.; Veicsteinas, A.; Maffulli, N.; Marini, M. Effect of training and sudden detraining on the patellar tendon and its enthesis in rats. BMC Musculoskelet. Disord. 2011, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Berardi, A.C.; Gissi, C.; Cataldi, A.; Osti, L. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J. Drug Target. 2020, 28, 212–224. [Google Scholar] [CrossRef]
- Gallorini, M.; Antonetti Lamorgese Passeri, C.; Cataldi, A.; Berardi, A.C.; Osti, L. Hyaluronic Acid Alleviates Oxidative Stress and Apoptosis in Human Tenocytes via Caspase 3 and 7. Int. J. Mol. Sci. 2022, 23, 8817. [Google Scholar] [CrossRef]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; de Sire, A.; Mezian, K.; Curci, C.; Perrero, L.; Turco, A.; Andaloro, S.; Ammendolia, A.; Fusco, N.; Invernizzi, M. Impact of exercise training on muscle mitochondria modifications in older adults: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Osti, L.; Berardocco, M.; Di Giacomo, V.; Di Bernardo, G.; Oliva, F.; Berardi, A.C. Hyaluronic acid increases tendon derived cell viability and collagen type I expression in vitro: Comparative study of four different hyaluronic acid preparations by molecular weight. BMC Musculoskelet. Disord. 2015, 16, 284. [Google Scholar] [CrossRef]
- Agostini, F.; Bressanin, E.; de Sire, A.; Finamore, N.; Alviti, F.; Santilli, V.; Bernetti, A.; Paoloni, M.; Mangone, M. The effect of intra-articular injections of hyaluronic acid for the treatment of trapezio-metacarpal joint osteoarthritis. J. Pers. Med. 2024, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; Ferrillo, M.; Turco, A.; Folli, A.; Moalli, S.; Refati, F.; Perrero, L.; Ammendolia, A.; de Sire, A.; Invernizzi, M. Multidisciplinary rehabilitation after hyaluronic acid injections for elderly with knee, hip, shoulder, and temporomandibular joint osteoarthritis. Medicina 2023, 59, 2047. [Google Scholar] [CrossRef]
- Frizziero, A.; Salamanna, F.; Giavaresi, G.; Ferrari, A.; Martini, L.; Marini, M.; Veicsteinas, A.; Maffulli, N.; Masiero, S.; Fini, M. Hyaluronic acid injections protect patellar tendon from detraining-associated damage. Histol. Histopathol. 2015, 30, 1079–1088. [Google Scholar] [CrossRef]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef]
- Gorelick, L.; Rozano-Gorelick, A.; Saab, A.; Ram, E. Single Hyaluronate Injection in the Management of Insertional Achilles Tendinopathy in Comparison to Corticosteroid Injections and Non-invasive Conservative Treatments. Sch. Bull. 2015, 1, 16–20. [Google Scholar]
- Pellegrino, R.; Brindisino, F.; Barassi, G.; Sparvieri, E.; Di Iorio, A.; de Sire, A.; Ruosi, C. Combined ultrasound guided peritendinous hyaluronic acid (500–730 KDa) injection with extracorporeal shock waves therapy vs. extracorporeal shock waves therapy-only in the treatment of shoulder pain due to rotator cuff tendinopathy: A randomized clinical trial. J. Sports Med. Phys. Fit. 2022, 62, 1211–1218. [Google Scholar] [CrossRef]
- Bernetti, A.; Agostini, F.; Finamore, N.; Dal Borgo, M.; Mangone, M.; Ammendolia, A.; Paoloni, M.; de Sire, A. Effectiveness of ultrasound-guided hip injections on pain and functioning in patients with hip osteoarthritis: A systematic review. J. Back Musculoskelet. Rehabil. 2025, 38, 19–47. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Participants (n = 24) |
|---|---|
| Sex, M/F (%M) | 14/10 (58.33%) |
| Age (Mean ± Sd) | 59.7 ± 11.1 Years |
| BMI (Mean ± Sd) | 26.95 ± 3.51 kg/m2 |
| Side Affected, Left/Right (%Left) | 8/16 (33.33%) |
| Dominant Side, Left/Right (%Left) | 5/19 (20.83%) |
| T0 | T1 | T2 | T3 | T4 | p tot | p T0–T1 | p T0–T2 | p T0–T3 | p T0–T4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Quick-DASH | 51.53 ± 14.21 | 35.22 ± 13.95 | 24.96 ± 15.27 | 18.73 ± 10.63 | 22.56 ± 13.03 | <0.001 | 0.721 | <0.001 | <0.001 | <0.001 |
| VAS | 4.9 ± 1.9 | 3.17 ± 2.2 | 2.63 ± 2.11 | 1.73 ± 1.42 | 2.51 ± 1.72 | <0.001 | <0.05 | <0.01 | <0.01 | <0.01 |
| SF-12 Mental | 45.72 ± 10.12 | 48.29 ± 6.11 | 49.94 ± 6.82 | 50.35 ± 5.45 | 49.93 ± 7.39 | <0.005 | 1 | 0.184 | <0.05 | <0.05 |
| SF-12 Physical | 37.66 ± 5.35 | 39.33 ± 6.15 | 44.02 ± 8.33 | 47.29 ± 6.93 | 44.25 ± 5.98 | <0.001 | 0.62 | <0.05 | <0.01 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostini, F.; de Sire, A.; Savina, A.; Iudicelli, G.; Fisicaro, A.; Camponogara, G.; Narciso, M.; Fricano, A.; Conti, M.; Longo, U.G.; et al. Effectiveness of Ultrasound-Guided Peritendinous Injection Treatment with Low Molecular Weight Hyaluronic Acid in Patients with Supraspinatus Tendinopathy. J. Clin. Med. 2025, 14, 6291. https://doi.org/10.3390/jcm14176291
Agostini F, de Sire A, Savina A, Iudicelli G, Fisicaro A, Camponogara G, Narciso M, Fricano A, Conti M, Longo UG, et al. Effectiveness of Ultrasound-Guided Peritendinous Injection Treatment with Low Molecular Weight Hyaluronic Acid in Patients with Supraspinatus Tendinopathy. Journal of Clinical Medicine. 2025; 14(17):6291. https://doi.org/10.3390/jcm14176291
Chicago/Turabian StyleAgostini, Francesco, Alessandro de Sire, Alessio Savina, Giovanni Iudicelli, Andrea Fisicaro, Giacomo Camponogara, Marco Narciso, Alessio Fricano, Marco Conti, Umile Giuseppe Longo, and et al. 2025. "Effectiveness of Ultrasound-Guided Peritendinous Injection Treatment with Low Molecular Weight Hyaluronic Acid in Patients with Supraspinatus Tendinopathy" Journal of Clinical Medicine 14, no. 17: 6291. https://doi.org/10.3390/jcm14176291
APA StyleAgostini, F., de Sire, A., Savina, A., Iudicelli, G., Fisicaro, A., Camponogara, G., Narciso, M., Fricano, A., Conti, M., Longo, U. G., Santilli, V., Ammendolia, A., Mangone, M., & Paoloni, M. (2025). Effectiveness of Ultrasound-Guided Peritendinous Injection Treatment with Low Molecular Weight Hyaluronic Acid in Patients with Supraspinatus Tendinopathy. Journal of Clinical Medicine, 14(17), 6291. https://doi.org/10.3390/jcm14176291












