Parkinson’s Disease Through the Lens of Metabolomics: A Targeted Systematic Review on Human Studies (2019–2024)
Abstract
1. Introduction
2. The Potential Role of Metabolomics for Parkinson’s Disease
3. Methods
3.1. Search Strategies and Selection Criteria
- Keywords: The databases were queried to look for specific keywords in their title, such as “Parkinson’s disease”, “NMR spectroscopy”, “mass spectrometry”, “metabolomics”. The exact search strings used for three databases were as follows:
- NIH PubMed query: (“Parkinson’s disease”[Title]) AND (“metabolomics”[Title] OR “NMR spectroscopy”[Title] OR “mass spectrometry”[Title] OR “metabolites”[Title]);
- Science Direct query: (“Parkinson’s disease”[Title]) AND (“metabolomics”[Title] OR “NMR spectroscopy”[Title] OR “mass spectrometry”[Title] OR “metabolites”[Title]);
- WOS query: TI = (“Parkinson’s disease”) AND TI = (“metabolomics” OR “NMR spectroscopy” OR “mass spectrometry” OR “metabolites”).
- Publication date: To ensure the relevance and timeliness of the present review, the initial screening included studies from the year 2000 onward. However, based on a preliminary bibliometric analysis of publication trends (described in the “Data Extraction and Analysis” section), the final selection was narrowed to studies published between 2019 and 2024, a period marked by a significant increase in PD metabolomics research.
- Relevance: The primary objective was to include papers that specifically investigate the role of metabolomics in PD. This criterion ensures that the selected studies directly contribute to the understanding of how metabolomics are implicated in the pathogenesis, diagnosis, or treatment of PD. Papers that do not focus on these specific aspects of PD were excluded.
- Study design: To maintain scientific rigor and reliability, only original research studies and clinical trials involving more than 50 patients with PD were included. This threshold was used to reduce the influence of underpowered analyses and enhance the reliability of reported metabolomic trends in the context of Parkinson’s disease.
3.2. Data Extraction and Analysis
3.3. Quality Assessment
4. Results
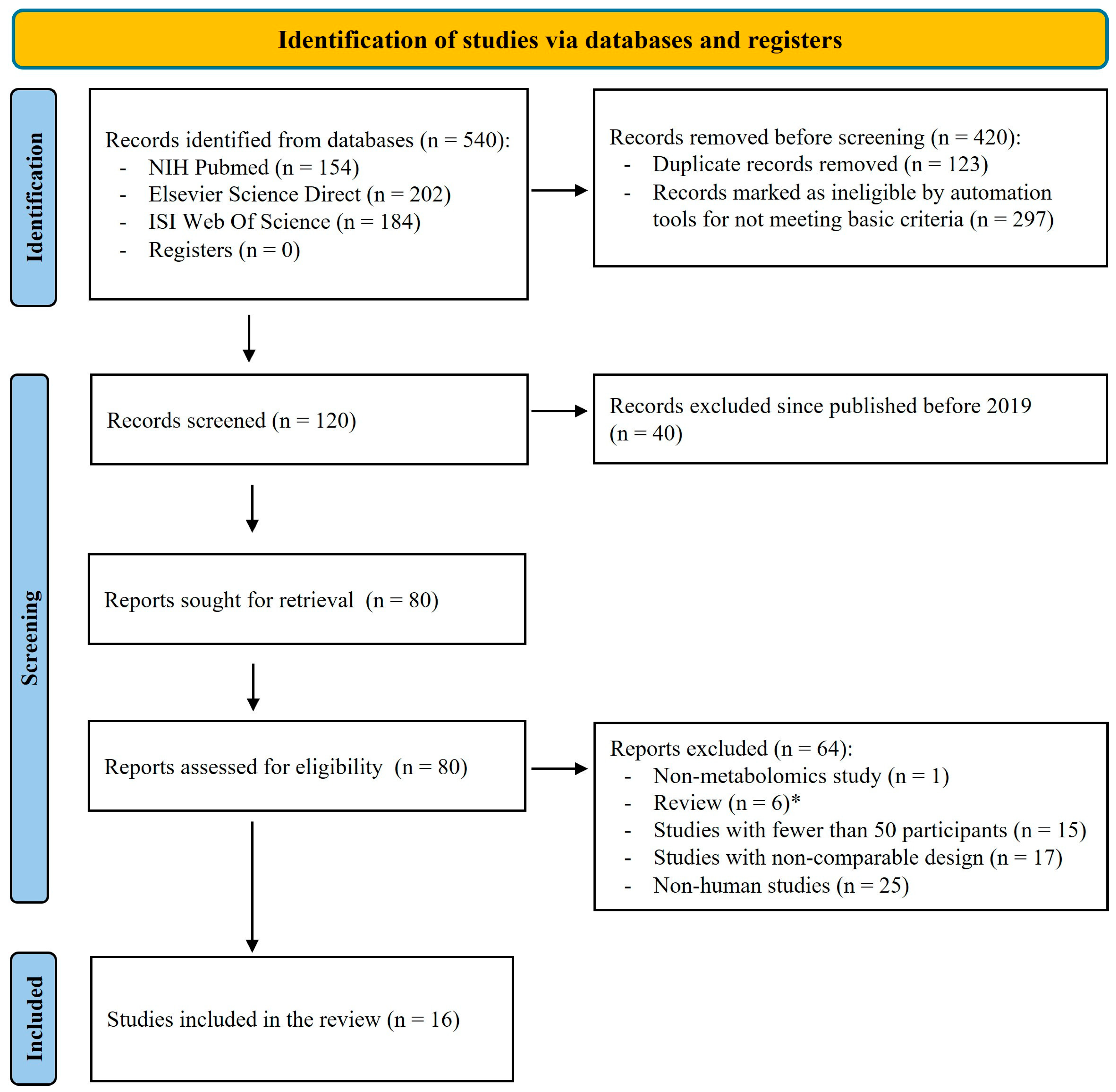
4.1. Study Selection
4.2. Characteristics of the Included Studies
4.3. Research Designs
4.4. Risk of Bias
4.5. Confounding Variables: Sex, Age, Medications, Diet
4.5.1. Sex and Age
4.5.2. Dopaminergic Therapies
4.5.3. Diet
4.6. Analytical Platforms and Techniques
4.7. Statistical Methods
4.8. Biofluid-Specific Metabolomics Signatures
4.8.1. Plasma: Systemic Indicators of Oxidative Stress and Lipid Dysregulation
4.8.2. Serum: Lipid Dysregulation and the Gut–Brain Axis
4.8.3. Cerebrospinal Fluid (CSF): Direct Insights into Neurochemical Alterations
4.8.4. Saliva: A Non-Invasive Avenue for Biomarker Discovery
4.8.5. Sebum: A Novel Lipidomic Fingerprint for PD
4.8.6. Urine: A Metabolic Readout of Systemic Contributions to Neurodegeneration
4.9. Integration with Other Omics Approaches
5. Discussion
5.1. Amino Acid Metabolism
5.2. Lipid Metabolism
5.3. Energy Metabolism
5.4. Oxidative Stress
5.5. Neurotransmitter Metabolism
5.6. Gut Microbial Metabolites
5.7. Polyamine Metabolism
5.8. Metabolome Association with PD Stage and Duration
6. Strengths, Limitations, and Translational Outlook of Current Metabolomics Research in Parkinson’s Disease
6.1. Methodological Limitations and Confounding Factors
6.2. Need for Standardization and Harmonization
6.3. Translational Outlook and Future Directions
6.3.1. Strengths and Translational Potential of Current Metabolomics Studies
6.3.2. Metabolomics Perspective on Atypical Parkinsonian Syndromes
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fröhlich, F. Parkinson’s Disease. In Network Neuroscience, 1st ed.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 291–296. [Google Scholar] [CrossRef]
- Bergman, H.; Deuschl, G. Pathophysiology of Parkinson’s Disease: From Clinical Neurology to Basic Neuroscience and Back. Mov. Disord. 2002, 17, s28–s40. [Google Scholar] [CrossRef]
- Galvan, A.; Wichmann, T. Pathophysiology of Parkinsonism. Clin. Neurophysiol. 2008, 119, 1459–1474. [Google Scholar] [CrossRef]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.A.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Bohl, J.R.; Müller, C.M.; Rüb, U.; de Vos, R.A.I.; Del Tredici, K. Stanley Fahn Lecture 2005: The Staging Procedure for the Inclusion Body Pathology Associated with Sporadic Parkinson’s Disease Reconsidered. Mov. Disord. 2006, 21, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.G.; Bannur, B.M.; Chavan, M.D.; Saniya, K.; Sailesh, K.S.; Rajagopalan, A. Neuroanatomical Changes in Parkinson’s Disease in Relation to Cognition: An Update. J. Adv. Pharm. Technol. Res. 2016, 7, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Litvan, I. Mild Cognitive Impairment in Parkinson’s Disease. Minerva Med. 2011, 102, 441–459. [Google Scholar] [PubMed]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H.V. Non-Motor Symptoms of Parkinson’s Disease: Diagnosis and Management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Hughes, T.A.; Ross, H.F.; Mindham, R.H.S.; Spokes, E.G.S. Mortality in Parkinson’s Disease and Its Association with Dementia and Depression. Acta Neurol. Scand. 2004, 110, 118–123. [Google Scholar] [CrossRef]
- Levy, G.; Tang, M.X.; Louis, E.D.; Côté, L.J.; Alfaro, B.; Mejia, H.; Stern, Y.; Marder, K. The Association of Incident Dementia with Mortality in PD. Neurology 2002, 59, 1708–1713. [Google Scholar] [CrossRef]
- Parkinson Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 19 February 2025).
- Lee Mosley, R.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress, and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut–Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 13, 782082. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, H.; Ilyas, I.; Mahmood, A.; Hou, L. Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson’s Disease. Brain Sci. 2023, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Callegari, S.; Kirk, N.S.; Gan, Z.Y.; Dite, T.; Cobbold, S.A.; Leis, A.; Dagley, L.F.; Glukhova, A.; Komander, D. Structure of Human PINK1 at a Mitochondrial TOM-VDAC Array. Science 2025, 388, 303–310. [Google Scholar] [CrossRef]
- Uitti, R.J.; Baba, Y.; Wszolek, Z.K.; Putzke, D.J. Defining the Parkinson’s Disease Phenotype: Initial Symptoms and Baseline Characteristics in a Clinical Cohort. Park. Relat. Disord. 2005, 11, 139–145. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Omberg, L.; Waddell, E.; Adams, J.L.; Adams, R.; Ali, M.R.; Amodeo, K.; Arky, A.; Augustine, E.F.; Dinesh, K.; et al. Deep Phenotyping of Parkinson’s Disease. J. Park. Dis. 2020, 10, 855–873. [Google Scholar] [CrossRef]
- Alster, P.; Madetko, N.; Koziorowski, D.; Friedman, A. Progressive Supranuclear Palsy—Parkinsonism Predominant (PSP-P)—A Clinical Challenge at the Boundaries of PSP and Parkinson’s Disease (PD). Front. Neurol. 2020, 11, 180. [Google Scholar] [CrossRef]
- Tolosa, E.; Wenning, G.; Poewe, W. The Diagnosis of Parkinson’s Disease. Lancet Neurol. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Jankovic, J.; Rajput, A.; McDermott, M.; Perl, D. The Evolution of Diagnosis in Early Parkinson Disease. Arch. Neurol. 2000, 57, 369–372. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Urazgildeeva, G.R.; Abaimov, D.A.; Fedotova, E.Y.; Poleschuk, V.V.; Illarioshkin, S.N.; Lokhov, P.G. Parkinson’s Disease: Available Clinical and Promising Omics Tests for Diagnostics, Disease Risk Assessment, and Pharmacotherapy Personalization. Diagnostics 2020, 10, 339. [Google Scholar] [CrossRef]
- Krach, F.; Bogiongko, M.E.; Winner, B. Decoding Parkinson’s Disease—IPSC-Derived Models in the OMICs Era. Mol. Cell. Neurosci. 2020, 106, 103501. [Google Scholar] [CrossRef]
- Kori, M.; Aydln, B.; Unal, S.; Arga, K.Y.; Kazan, D. Metabolic Biomarkers and Neurodegeneration: A Pathway Enrichment Analysis of Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. OMICS J. Integr. Biol. 2016, 20, 645–661. [Google Scholar] [CrossRef]
- Tan, A.H.; Chong, C.W.; Lim, S.Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An Emerging but Powerful Tool for Precision Medicine. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, J.; Uppal, K.; Chandler, J.D.; Lili, L.N.; Hu, X.; Go, Y.-M.; Jones, D.P. Integration of Multi-Omics Data Reveal Dynamic Oxidative Stress Responses to Manganese in Human SH-SY5Y Neuroblastoma Cells. Free Radic. Biol. Med. 2016, 100, S160. [Google Scholar] [CrossRef]
- Nie, S.; Wang, J.; Deng, Y.; Ye, Z.; Ge, Y. Inflammatory Microbes and Genes as Potential Biomarkers of Parkinson’s Disease. NPJ Biofilms Microbiomes 2022, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M.; Kamel, F.; Ross, G.W.; Jewell, S.A.; Marras, C.; Hoppin, J.A.; Umbach, D.M.; Bhudhikanok, G.S.; Meng, C.; Korell, M.; et al. Peptidoglycan Recognition Protein Genes and Risk of Parkinson’s Disease. Mov. Disord. 2014, 29, 1171–1180. [Google Scholar] [CrossRef]
- Dossi, G.; Squarcina, L.; Rango, M. In Vivo Mitochondrial Function in Idiopathic and Genetic Parkinson’s Disease. Metabolites 2020, 10, 19. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Mo, C.; Liu, X.; Li, J.; Yan, Z.; Qian, Y.; Lai, Y.; Xu, S.; Yang, X.; et al. Association Between Microbial Tyrosine Decarboxylase Gene and Levodopa Responsiveness in Patients With Parkinson Disease. Neurology 2022, 99, e2443–e2453. [Google Scholar] [CrossRef]
- Crooks, S.A.; Bech, S.; Halling, J.; Christiansen, D.H.; Ritz, B.; Petersen, M.S. Carnitine Levels and Mutations in the SLC22A5 Gene in Faroes Patients with Parkinson’s Disease. Neurosci. Lett. 2018, 675, 116–119. [Google Scholar] [CrossRef]
- Anandhan, A.; Lei, S.; Levytskyy, R.; Pappa, A.; Panayiotidis, M.I.; Cerny, R.L.; Khalimonchuk, O.; Powers, R.; Franco, R. Glucose Metabolism and AMPK Signaling Regulate Dopaminergic Cell Death Induced by Gene (α-Synuclein)-Environment (Paraquat) Interactions. Mol. Neurobiol. 2017, 54, 3825–3842. [Google Scholar] [CrossRef]
- Vishweswaraiah, S.; Akyol, S.; Yilmaz, A.; Ugur, Z.; Gordevičius, J.; Oh, K.J.; Brundin, P.; Radhakrishna, U.; Labrie, V.; Graham, S.F. Methylated Cytochrome P450 and the Solute Carrier Family of Genes Correlate With Perturbations in Bile Acid Metabolism in Parkinson’s Disease. Front. Neurosci. 2022, 16, 804261. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yang, H.; Liu, Y. Review of Metabolomics-Based Biomarker Research for Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 1041–1057. [Google Scholar] [CrossRef]
- Ciobanu, A.M.; Ionita, I.; Buleandra, M.; David, I.G.; Popa, D.E.; Ciucu, A.A.; Budisteanu, M. Current Advances in Metabolomic Studies on Non-Motor Psychiatric Manifestations of Parkinson’s Disease (Review). Exp. Ther. Med. 2021, 22, 1010. [Google Scholar] [CrossRef]
- Troisi, J.; Landolfi, A.; Cavallo, P.; Marciano, F.; Barone, P.; Amboni, M. Metabolomics in Parkinson’s Disease. Adv. Clin. Chem. 2021, 104, 107–149. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Balck, A.; Klein, C.; Fleming, R.M.T. Identification of Metabolites Reproducibly Associated with Parkinson’s Disease via Meta-Analysis and Computational Modelling. NPJ Park. Dis. 2024, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Glaab, E.; Trezzi, J.P.; Greuel, A.; Jäger, C.; Hodak, Z.; Drzezga, A.; Timmermann, L.; Tittgemeyer, M.; Diederich, N.J.; Eggers, C. Integrative Analysis of Blood Metabolomics and PET Brain Neuroimaging Data for Parkinson’s Disease. Neurobiol. Dis. 2019, 124, 555–562. [Google Scholar] [CrossRef]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Escobar Galvis, M.L.; Brundin, P.; Huang, X.; et al. Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 2020, 35, 2028–2037. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, V.; Kumaran, S.S.; Dwivedi, S.N.; Srivastava, A.; Jagannathan, N.R. Quantitative Metabolomics of Saliva Using Proton NMR Spectroscopy in Patients with Parkinson’s Disease and Healthy Controls. Neurol. Sci. 2020, 41, 1201–1210. [Google Scholar] [CrossRef]
- Kremer, T.; Taylor, K.I.; Siebourg-Polster, J.; Gerken, T.; Staempfli, A.; Czech, C.; Dukart, J.; Galasko, D.; Foroud, T.; Chahine, L.M.; et al. Longitudinal Analysis of Multiple Neurotransmitter Metabolites in Cerebrospinal Fluid in Early Parkinson’s Disease. Mov. Disord. 2021, 36, 1972–1978. [Google Scholar] [CrossRef]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of Sebum Reveals Lipid Dysregulation in Parkinson’s Disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive Metabolic Profiling of Parkinson’s Disease by Liquid Chromatography-Mass Spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef]
- Mallet, D.; Dufourd, T.; Decourt, M.; Carcenac, C.; Bossù, P.; Verlin, L.; Fernagut, P.O.; Benoit-Marand, M.; Spalletta, G.; Barbier, E.L.; et al. A Metabolic Biomarker Predicts Parkinson’s Disease at the Early Stages in Patients and Animal Models. J. Clin. Investig. 2022, 132, e146400. [Google Scholar] [CrossRef] [PubMed]
- Dahabiyeh, L.A.; Nimer, R.M.; Rashed, M.; Wells, J.D.; Fiehn, O. Serum-Based Lipid Panels for Diagnosis of Idiopathic Parkinson’s Disease. Metabolites 2023, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Li, J.; Wu, K.H.; Lu, M. Diagnostic Metabolomic Profiling of Parkinson’s Disease Biospecimens. Neurobiol. Dis. 2023, 177, 105962. [Google Scholar] [CrossRef]
- Paul, K.C.; Zhang, K.; Walker, D.I.; Sinsheimer, J.; Yu, Y.; Kusters, C.; Del Rosario, I.; Folle, A.D.; Keener, A.M.; Bronstein, J.; et al. Untargeted Serum Metabolomics Reveals Novel Metabolite Associations and Disruptions in Amino Acid and Lipid Metabolism in Parkinson’s Disease. Mol. Neurodegener. 2023, 18, 100. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Yan, J.; Xu, J.; Hu, D.; Ji, F.; Zeng, T.; Wang, F.; Wang, B.; Fang, J.; et al. Urine Biomarkers Discovery by Metabolomics and Machine Learning for Parkinson’s Disease Diagnoses. Chin. Chem. Lett. 2023, 34, 108230. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Nimer, R.M.; Wells, J.D.; Abu-rish, E.Y.; Fiehn, O. Diagnosing Parkinson’s Disease and Monitoring Its Progression: Biomarkers from Combined GC-TOF MS and LC-MS/MS Untargeted Metabolomics. Heliyon 2024, 10, e30452. [Google Scholar] [CrossRef]
- de Lope, E.G.; Loo, R.T.J.; Rauschenberger, A.; Ali, M.; Pavelka, L.; Marques, T.M.; Gomes, C.P.C.; Krüger, R.; Glaab, E.; Acharya, G.; et al. Comprehensive Blood Metabolomics Profiling of Parkinson’s Disease Reveals Coordinated Alterations in Xanthine Metabolism. NPJ Park. Dis. 2024, 10, 68. [Google Scholar] [CrossRef]
- Hu, W.; Wang, W.; Liao, H.; Bulloch, G.; Zhang, X.; Shang, X.; Huang, Y.; Hu, Y.; Yu, H.; Yang, X.; et al. Metabolic Profiling Reveals Circulating Biomarkers Associated with Incident and Prevalent Parkinson’s Disease. NPJ Park. Dis. 2024, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Huang, Y.-J.; Wei, Y.-D.; Li, T.; Ke, W.; Chen, G.-H.; Dong, M.-X. Plasma Metabolomics Profiles Indicate Sex Differences of Lipid Metabolism in Patients with Parkinson’s Disease. Sci. Rep. 2024, 14, 31262. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Ji, F.; Yan, J.; Fang, J.; Zhang, D.; Xu, J.; Ji, J.; Hao, X.; Luan, H.; et al. Discovery of Plasma Biomarkers for Parkinson’s Disease Diagnoses Based on Metabolomics and Lipidomics. Chin. Chem. Lett. 2024, 35, 109653. [Google Scholar] [CrossRef]
- Cochrane. Cochrane Handbook for Systematic Reviews of Interventions (Current Version). Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current (accessed on 20 June 2025).
- Ottawa Hospital. Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 June 2025).
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Geetha, T.; Burnett, D.; Babu, J.R. The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients 2022, 14, 4472. [Google Scholar] [CrossRef]
- Emwas, A.H.M. The Strengths and Weaknesses of NMR Spectroscopy and Mass Spectrometry with Particular Focus on Metabolomics Research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar] [CrossRef]
- Pan, Z.; Raftery, D. Comparing and Combining NMR Spectroscopy and Mass Spectrometry in Metabolomics. Anal. Bioanal. Chem. 2007, 387, 525–527. [Google Scholar] [CrossRef]
- Sotnikova, T.D.; Beaulieu, J.M.; Espinoza, S.; Masri, B.; Zhang, X.; Salahpour, A.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. The Dopamine Metabolite 3-Methoxytyramine Is a Neuromodulator. PLoS ONE 2010, 5, e13452. [Google Scholar] [CrossRef]
- Sharma, N.; Khurana, N.; Muthuraman, A.; Utreja, P. Pharmacological Evaluation of Vanillic Acid in Rotenone-Induced Parkinson’s Disease Rat Model. Eur. J. Pharmacol. 2021, 903, 174112. [Google Scholar] [CrossRef]
- Carrillo, F.; Ghirimoldi, M.; Fortunato, G.; Palomba, N.P.; Ianiro, L.; De Giorgis, V.; Khoso, S.; Giloni, T.; Pietracupa, S.; Modugno, N.; et al. Multiomics approach identifies dysregulated lipidomic and proteomic networks in Parkinson’s disease patients mutated in TMEM175. NPJ Park. Dis. 2025, 11, 23. [Google Scholar] [CrossRef]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Dong, M.X.; Huang, Y.L.; Lu, C.Q.; Qian, Q.; Zhang, C.C.; Xu, X.M.; Liu, Y.; Chen, G.H.; Wei, Y.D. Integrated Metabolomics and Proteomics Analysis Reveals Plasma Lipid Metabolic Disturbance in Patients with Parkinson’s Disease. Front. Mol. Neurosci. 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine Pathway in Parkinson’s Disease—An Update. eNeurologicalSci 2020, 21, 100270. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.N.; Umans, J.; Swarovski, M.S.; Minhas, P.S.; Mendiola, J.H.; Midttun, Ø.; Ulvik, A.; Shahid-Besanti, M.; Linortner, P.; Mhatre, S.D.; et al. Parkinson’s Disease Is Characterized by Vitamin B6-Dependent Inflammatory Kynurenine Pathway Dysfunction. NPJ Park. Dis. 2025, 11, 96. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Fu, P.; Zhang, Z.; Lin, K.; Ko, J.K.S.; Yung, K.K.L. Roles of Glutamate Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4391. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-Induced Excitotoxicity in Parkinson’s Disease: The Role of Glial Cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Yoo, H.S.; Shanmugalingam, U.; Smith, P.D. Potential Roles of Branched-Chain Amino Acids in Neurodegeneration. Nutrition 2022, 103–104, 111762. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Haidar, M.; Kooij, G.; Hendriks, J.J.A. Fatty Acid Metabolism in the Progression and Resolution of CNS Disorders. Adv. Drug Deliv. Rev. 2020, 159, 198–213. [Google Scholar] [CrossRef]
- Toomey, C.E.; Heywood, W.E.; Evans, J.R.; Lachica, J.; Pressey, S.N.; Foti, S.C.; Al Shahrani, M.; D’Sa, K.; Hargreaves, I.P.; Heales, S.; et al. Mitochondrial Dysfunction Is a Key Pathological Driver of Early Stage Parkinson’s. Acta Neuropathol. Commun. 2022, 10, 134. [Google Scholar] [CrossRef]
- Okarmus, J.; Havelund, J.F.; Ryding, M.; Schmidt, S.I.; Bogetofte, H.; Heon-Roberts, R.; Wade-Martins, R.; Cowley, S.A.; Ryan, B.J.; Færgeman, N.J.; et al. Identification of Bioactive Metabolites in Human IPSC-Derived Dopaminergic Neurons with PARK2 Mutation: Altered Mitochondrial and Energy Metabolism. Stem Cell Rep. 2021, 16, 1510–1526. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, Y.; Zhang, W.; Xie, F.; Deng, C.; Zheng, W.; Zhu, S.; Wang, Q. Causal Effect of Gut-Microbiota-Derived Metabolite Trimethylamine N-Oxide on Parkinson’s Disease: A Mendelian Randomization Study. Eur. J. Neurol. 2023, 30, 3451–3461. [Google Scholar] [CrossRef]
- Voigt, R.M.; Wang, Z.; Brown, J.M.; Engen, P.A.; Naqib, A.; Goetz, C.G.; Hall, D.A.; Metman, L.V.; Shaikh, M.; Forsyth, C.B.; et al. Gut Microbial Metabolites in Parkinson’s Disease: Association with Lifestyle, Disease Characteristics, and Treatment Status. Neurobiol. Dis. 2022, 170, 105780. [Google Scholar] [CrossRef]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson’s Disease: Balancing Between Neurotoxicity and Neuroprotection. Annu. Rev. Biochem. 2023, 92, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Wee, H.L.; Chan, Y.H.; Seah, S.H.; Au, W.L.; Lau, P.N.; Pica, E.C.; Li, S.C.; Luo, N.; Tan, L.C. Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Mov. Disord. 2010, 25, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Géhin, C.; Tokarska, J.; Fowler, S.J.; Barran, P.E.; Trivedi, D.K. No skin off your back: The sampling and extraction of sebum for metabolomics. Metabolomics 2023, 19, 21. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl. Biochem. Biotechnol. 2012, 168, 1718–1727. [Google Scholar] [CrossRef]
- Long, N.P.; Nghi, T.D.; Kang, Y.P.; Anh, N.H.; Kim, H.M.; Park, S.K.; Kwon, S.W. Toward a Standardized Strategy of Clinical Metabolomics for the Advancement of Precision Medicine. Metabolites 2020, 10, 51. [Google Scholar] [CrossRef]
- Goh, Y.Y.; Saunders, E.; Pavey, S.; Rushton, E.; Quinn, N.; Houlden, H.; Chelban, V. Multiple System Atrophy. Pract. Neurol. 2023, 23, 208–221. [Google Scholar] [CrossRef]
- Wenning, G.K.; Ben-Shlomo, Y.; Hughes, A.; Daniel, S.E.; Lees, A.; Quinn, N.P. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J. Neurol. Neurosurg. Psychiatry 2000, 68, 434–440. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Ben-Shlomo, Y.; Lees, A.J. The Accuracy of Diagnosis of Parkinsonian Syndromes in a Specialist Movement Disorder Service. Brain 2002, 125, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Ishikawa, K.I.; Saiki, S.; Hatano, T.; Oji, Y.; Okuzumi, A.; Fujimaki, M.; Koinuma, T.; Ueno, S.I.; Imamichi, Y.; et al. Plasma Metabolite Biomarkers for Multiple System Atrophy and Progressive Supranuclear Palsy. PLoS ONE 2019, 14, e0223113. [Google Scholar] [CrossRef]
- Plewa, S.; Poplawska-Domaszewicz, K.; Florczak-Wyspianska, J.; Klupczynska-Gabryszak, A.; Sokol, B.; Miltyk, W.; Jankowski, R.; Kozubski, W.; Kokot, Z.J.; Matysiak, J. The Metabolomic Approach Reveals the Alteration in Human Serum and Cerebrospinal Fluid Composition in Parkinson’s Disease Patients. Pharmaceuticals 2021, 14, 935. [Google Scholar] [CrossRef]
- Pathan, M.; Wu, J.; Lakso, H.-Å.; Forsgren, L.; Öhman, A. Plasma Metabolite Markers of Parkinson’s Disease and Atypical Parkinsonism. Metabolites 2021, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Przewodowska, D.; Alster, P.; Madetko-Alster, N. Role of the Intestinal Microbiota in the Molecular Pathogenesis of Atypical Parkinsonian Syndromes. Int. J. Mol. Sci. 2025, 26, 3928. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef]
- De Pablo-Fernandez, E.; Gebeyehu, G.G.; Flain, L.; Slater, R.; Frau, A.; Ijaz, U.Z.; Warner, T.; Probert, C. The faecal metabolome and mycobiome in Parkinson’s disease. Park. Relat. Disord. 2022, 95, 65–69. [Google Scholar] [CrossRef]
| Authors’ and Patients’ Nationality | Study Design | Participants a | Gender b | Age (Years) c | Sample Type | Experimental Platform d | Statistical Analysis of Metabolomics Data e |
|---|---|---|---|---|---|---|---|
| Glaab et al. (2019) [40] Germany | Integrative analysis | PD (60) Ct (15) | 19 M/41 F (PD) 8 M/7 F (Ct) | 65.7 ± 9.0 (PD) 65.1 ± 8.4 (Ct) | Plasma | GC-MS | Welch’s t test, SVM, RF, ROC analysis |
| Heilman et al. (2020) [41] USA | Case-control study | PD (97) Ct (90) | 49 M/48 F (PD) 43 M/47 F (Ct) | 66.9 ± 8.3 (PD) 66.6 ± 10.3 (Ct) | Plasma, CSF | HPLC | Mann–Whitney U-test, PLS-DA, OPLS-DA, ROC curve analysis |
| Kumari et al. (2020) [42] North India | Case-control study | PD (76) Ct (37) | 14 M/23 F (PD) 56 M/17 F (Ct) | 54.9 ± 7.8 (PD) 53.0 ± 8.6 (Ct) | Saliva | NMR | Mann–Whitney U-test, PLS-DA, OPLS-DA, ROC curve analysis |
| Kremer et al. (2021) [43] Germany, USA | Longitudinal analysis | PD (157) Ct (105) | 106 M/51 F (PD) 70 M/35 F (Ct) | 63.2 ± 9.6 (PD) 64.1 ± 8.9 (Ct) | CSF | LC–MS/MS | Linear regression, linear mixed-effect model, ROC curve analysis |
| Sinclair et al. (2021) [44] UK | Observational study | PD (218) Ct (56) | 137 M/81 F (PD) 26 M/30 F (Ct) | 70.0 ± 8.8 (PD) 54.3 ± 14.4 (Ct) | Sebum (from skin) | LC-MS | PLS-DA, ROC curve analysis, pathway enrichment analysis |
| Shao et al. (2021) [45] China | Case-control study | PD (223) Ct (169) | 124 M/99 F (PD) 94 M/75 F (Ct) | 66.3 ± 1.3 (PD) 6.2 ± 1.3 (Ct) | Plasma | LC-HRMS | Mann–Whitney U test, PCA, OPLS-DA, hierarchical cluster analysis, binary logistic regression analysis, ROC curve analysis |
| Mallet et al. (2022) [46] Italy, USA | Translational study | PD (129) Ct (53) | 63 M/66 F (PD) 28 M/25 F (Ct) | 61.4 ± 9.1 (PD) 62.4 ± 8.3 (Ct) | Serum | NMR | ANOVA, OPLS-DA, ROC curve analysis |
| Dahabiyeh et al. (2023) [47] Jordan | Case-control study | PD (50) Ct (45) | 31 M/19 F (PD) 23 F/22 M (Ct) | 64.2 ± 13.3 (PD) 59.4 ± 10.4 (Ct) | Serum | LC-MS/MS | Student’s t-test, ANOVA, PLS-DA |
| LeWitt et al. (2023) [48] USA | Case-control study | PD (50) Ct (50) | 30 M/20 F (PD) 21 M/29 F (Ct) | 69.5 ± 6.6 (PD) 66.0 ± 6.9 (Ct) | Serum, CSF | UHPLC-MS/MS | Student’s t-test, SVM, PLS, LASSO regression, pathway enrichment analysis |
| Paul et al.(2023) [49] USA | Metabolome-wide association study (MWAS) | PD (642) Ct (277) | 406 M/236 F (PD) 129 M/154 F (Ct) | 66.8 ± 10.4 (PD) 65.6 ± 12.8 (Ct) | Serum | LC-HRMS | Linear regression, logistic regression |
| Wang et al. (2023) [50] China | Case-control study | PD (104) Ct (111) | 65 M/39 F (PD) 60 M/51 F (Ct) | 59.4 ± 12.1 (PD) 57.2 ± 9.1 (Ct) | Urine | LC-MS | PLS-DA, RF, XGBoost, LASSO regression, ridge regression, ROC curve analysis |
| Dahabiyeh et al. (2024) [51] Jordan | Case-control study | PD (50) Ct (45) | 31 M/19 F (PD) 22M/23 F (Ct) | 64.2 ± 13.3 (PD) 59.4 ± 10.4 (Ct) | Serum | GC-TOF MS LC-MS/MS | ANOVA, PLS-DA, ChemRICH analysis |
| de Lope et al. (2024) [52] Luxembourg | Cohort-wide profiling | PD (549) Ct (590) | 360 M/189 F (PD) 384 M/206 F (Ct) | 66.0 ± 10.7 (PD) 61.7 ± 11.7 (Ct) | Plasma | LC-MS/MS | Linear regression, SVM, ROC curve analysis, pathway enrichment analysis |
| Hu et al. (2024) [53] UK | Prospective cohort study | PD (639) Ct (109.146) | 409 M/235 F (PD) 50.308 M/58.838 F (Ct) | 62.7 ± 5.6 (PD) 56.5 ± 8.1 (Ct) | Plasma | NMR | PCA, Cox proportional hazard model, logistic regression, ROC curve analysis |
| Hu et al. (2024) [54] China | Observational study | PD (75) Ct (31) | 37 M/38 F (PD) 16 M/15 F (Ct) | 66.1 ± 6.3 (PD) 64.1 ± 8.7 (Ct) | Plasma | LC-MS | PCA, OPLS-DA |
| Wang et al. (2024) [55] China | Case-control study | PD (99) Ct (91) | 54 M/45 F (PD) 46 M/45 F (Ct) | 60.9 ± 10.1 (PD) 61.7 ± 11.2 (Ct) | Plasma | LC-MS | Student’s t-test, Mann–Whitney U test, PLS-DA, KNN, RF, LASSO, SVM, ROC curve analysis |
| Study | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|
| Glaab et al. 2019 [40] | ●●●● | ●● | ●●● | 9/9 |
| Heilman et al. 2020 [41] | ●●● | ● | ●● | 6/9 |
| Kumari et al. 2020 [42] | ●●● | ● | ●● | 6/9 |
| Kremer et al. 2021 [43] | ●●● | ●● | ●●● | 8/9 |
| Sinclair et al. 2021 [44] | ●●● | ●● | ●●● | 8/9 |
| Shao et al. 2021 [45] | ●●●● | ●● | ●●● | 9/9 |
| Mallet et al. 2022 [46] | ●●● | ●● | ●● | 7/9 |
| Dahabiyeh et al. 2023 [47] | ●●●● | ● | ●●● | 8/9 |
| LeWitt et al. 2023 [48] | ●●●● | ●● | ●●● | 9/9 |
| Paul et al. 2023 [49] | ●●●● | ● | ●●● | 8/9 |
| Wang et al. 2023 [50] | ●●● | ●● | ●● | 7/9 |
| Dahabiyeh et al. 2024 [51] | ●●●● | ● | ●●● | 8/9 |
| de Lope et al. 2024 [52] | ●●●● | ●● | ●●● | 9/9 |
| Hu et al. 2024 [53] | ●●●● | ●● | ●●● | 9/9 |
| Hu et al. 2024 [54] | ●●● | ●● | ●●● | 8/9 |
| Wang et al. 2024 [55] | ●●● | ● | ●● | 6/9 |
| Metabolic Disregulation | Metabolites a | Biofluids |
|---|---|---|
| Amino acid metabolism | N-acetylglutamate (NAG) ↑, alanine ↑, aspartate ↓, g-aminobutyric acid (GABA) ↑, glycine ↑, glutamate ↑, histidine ↑, isoleucine ↑, phenylacetyl-L-glutamine ↑, phenylalanine ↑, pyroglutamic acid ↑, serine ↑, threonine ↑, tryptophan ↓, tyrosine ↑, valine ↑ | plasma [41,53], serum [46,49], saliva [42] |
| Energy metabolism | acetoacetate ↑, acetoin ↑, alanine ↑, citric acid ↑, 3,3-dimethylglutaric acid ↓, β-hydroxybutyrate ↑, orotic acid ↑, oxoglutaric acid ↑, pantothenic acid ↓, pyruvate ↑ | plasma [55], saliva [42], serum [46,49], urine [50] |
| Gut microbiota-derived metabolites | acetate ↑, butyrate ↑, p-cresol ↑, p-cresol glucuronide ↑, p-cresol sulfate ↑, phenylacetyl-L-glutamine ↑, propionate ↑, TMAO ↑ | plasma [45,52,53], saliva [42], serum [49] |
| Lipid metabolism |
| plasma [40,45,53,54,55], sebum [44], serum [47,49,51] |
| Neurotransmitter metabolism | Tryptophan–kynurenine pathway: N-acetylglutamate (NAG) ↑, g-aminobutyric acid (GABA) ↑, glycine ↑, 3-hydroxyanthranilic acid ↓, 3-hydroxykynurenine ↑, indolelactic acid ↓, kynurenic acid ↓, kynurenine ↓, quinolinic acid ↑ Dopamine metabolism: 3-methoxytyramine ↑, N-acetyl-l-tyrosine ↑, 3,4-dihydroxyphenylacetic acid (DOPAC) ↓, homovanillic acid (HVA) ↓ | CSF [41], plasma [41,45], saliva [42], serum [49] CSF [43], urine [50] |
| Oxidative stress | threonic acid ↑, uric acid ↑, vanillic acid ↑, xanthine and derivatives ↑ | plasma [40,52], serum [51], urine [50] |
| Polyamine metabolism | N-acetylputrescine ↑, N-acetylcadaverine ↑, N8-acetylspermidine ↑, L-ornithine ↑ | CSF [48], serum [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannas, F.; Kopeć, K.K.; Zuddas, N.; Cesare Marincola, F.; Arcara, G.; Loi, M.; Mussap, M.; Fanos, V. Parkinson’s Disease Through the Lens of Metabolomics: A Targeted Systematic Review on Human Studies (2019–2024). J. Clin. Med. 2025, 14, 6277. https://doi.org/10.3390/jcm14176277
Cannas F, Kopeć KK, Zuddas N, Cesare Marincola F, Arcara G, Loi M, Mussap M, Fanos V. Parkinson’s Disease Through the Lens of Metabolomics: A Targeted Systematic Review on Human Studies (2019–2024). Journal of Clinical Medicine. 2025; 14(17):6277. https://doi.org/10.3390/jcm14176277
Chicago/Turabian StyleCannas, Federico, Karolina Krystyna Kopeć, Natalia Zuddas, Flaminia Cesare Marincola, Giorgio Arcara, Michele Loi, Michele Mussap, and Vassilios Fanos. 2025. "Parkinson’s Disease Through the Lens of Metabolomics: A Targeted Systematic Review on Human Studies (2019–2024)" Journal of Clinical Medicine 14, no. 17: 6277. https://doi.org/10.3390/jcm14176277
APA StyleCannas, F., Kopeć, K. K., Zuddas, N., Cesare Marincola, F., Arcara, G., Loi, M., Mussap, M., & Fanos, V. (2025). Parkinson’s Disease Through the Lens of Metabolomics: A Targeted Systematic Review on Human Studies (2019–2024). Journal of Clinical Medicine, 14(17), 6277. https://doi.org/10.3390/jcm14176277









