Association Between Body Mass Index and Uterotonic Use in Postpartum Hemorrhage: A Retrospective Cohort Study
Abstract
1. Introduction
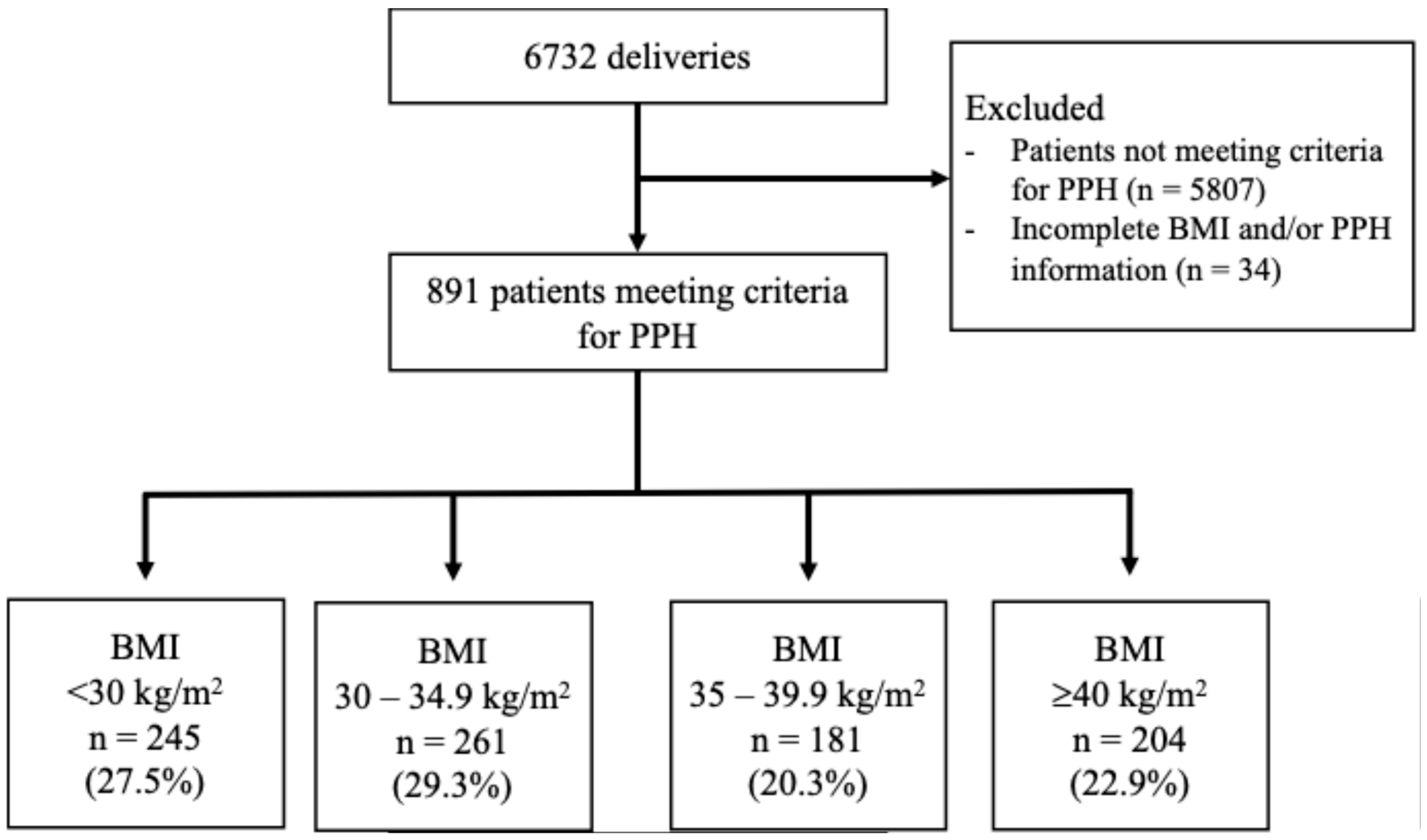
2. Materials and Methods
3. Results
3.1. Primary Outcome
3.2. Additional Findings
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Center for Disease Control and Prevention. Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/adult-obesity-facts/index.html (accessed on 26 March 2024).
- Fitzsimons, K.J.; Modder, J.; Greer, I.A. Obesity in pregnancy: Risks and management. Obstet. Med. 2009, 2, 52–62. [Google Scholar] [CrossRef]
- ACOG. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet. Gynecol. 2017, 130, e168–e186. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Corbetta-Rastelli, C.M.; Friedman, A.M.; Sobhani, N.C.; Arditi, B.; Goffman, D.; Wen, T. Postpartum Hemorrhage Trends and Outcomes in the United States, 2000–2019. Obstet. Gynecol. 2023, 141, 152–161. [Google Scholar] [CrossRef]
- Menard, M.K.; Main, E.K.; Currigan, S.M. Executive summary of the reVITALize initiative: Standardizing obstetric data definitions. Obstet. Gynecol. 2014, 124, 150–153. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Quantitative Blood Loss in Obstetric Hemorrhage: Committee Opinion, Number 794. Obstet. Gynecol. 2019, 134, e150–e156. [Google Scholar] [CrossRef]
- Blomberg, M. Maternal obesity and risk of postpartum hemorrhage. Obstet. Gynecol. 2011, 118, 561–568. [Google Scholar] [CrossRef]
- Ende, H.B.; Lozada, M.J.; Chestnut, D.H.; Osmundson, S.S.; Walden, R.L.M.; Shotwell, M.S.; Bauchat, J.R. Risk Factors for Atonic Postpartum Hemorrhage: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2021, 137, 305–323. [Google Scholar] [CrossRef]
- Fyfe, E.M.; Thompson, J.M.; Anderson, N.H.; Groom, K.M.; McCowan, L.M. Maternal obesity and postpartum haemorrhage after vaginal and caesarean delivery among nulliparous women at term: A retrospective cohort study. BMC Pregnancy Childbirth 2012, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Vats, H.; Saxena, R.; Sachdeva, M.P.; Walia, G.K.; Gupta, V. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Whitley, J.; Dazelle, W.; Kripalani, S.; Ahmadzia, H. The association between body mass index and postpartum hemorrhage after cesarean delivery. Sci. Rep. 2023, 13, 11998. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; To, W. Risk factors for postpartum haemorrhage in twin pregnancies and haemorrhage severity. Hong Kong Med. J. 2023, 29, 295–300. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Body Mass Index (BMI). Available online: https://www.cdc.gov/bmi/adult-calculator/bmi-categories.html (accessed on 23 July 2025).
- Cook Medical. Resources: Bakri® Postpartum Balloon. Updated 2024. Available online: https://www.cookmedical.com/reproductive-health/resources-bakri-postpartum-balloon/ (accessed on 26 March 2024).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Polic, A.; Curry, T.L.; Louis, J.M. The Impact of Obesity on the Management and Outcomes of Postpartum Hemorrhage. Am. J. Perinatol. 2022, 39, 652–657. [Google Scholar] [CrossRef]
- Sutton, A.; Sanders, L.; Subramaniam, A.; Jauk, V.; Edwards, R. Abdominal Incision Selection for Cesarean Delivery of Women with Class III Obesity. Am. J. Perinatol. 2016, 33, 547–551. [Google Scholar] [CrossRef]
- Brocato, B.E.; Thorpe, E.M.; Gomez, L.M.; Wan, J.Y.; Mari, G. The effect of cesarean delivery skin incision approach in morbidly obese women on the rate of classical hysterotomy. J. Pregnancy 2013, 2013, 373–378. [Google Scholar] [CrossRef]
- Giugale, L.E.; Sakamoto, S.; Yabes, J.; Dunn, S.L.; Krans, E.E. Unintended hysterotomy extension during caesarean delivery: Risk factors and maternal morbidity. J. Obstet. Gynaecol. 2018, 38, 1048–1053. [Google Scholar] [CrossRef]
- Bligard, K.H.; Durst, J.K.; Stout, M.J.; Martin, S.; Cahill, A.G.; Macones, G.A.; Tuuli, M.G. Risk Factors and Maternal Morbidity Associated with Unintentional Hysterotomy Extension at the Time of Cesarean Delivery. Am. J. Perinatol. 2018, 36, 1054–1059. [Google Scholar] [CrossRef]
- Frisch, N.; Wessell, N.M.; Charters, M.; Peterson, E.; Cann, B.; Greenstein, A.; Silverton, C.D. Effect of Body Mass Index on Blood Transfusion in Total Hip and Knee Arthroplasty. Orthopedics 2016, 39, e844–e849. [Google Scholar] [CrossRef] [PubMed]
- Kuduvalli, M.; Oo, A.Y.; Newall, N.; Grayson, A.; Jackson, M.; Desmond, M.; Fabri, B.; Rashid, A. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 2005, 27, 592–598. [Google Scholar] [CrossRef]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Berton, M.; Bettonte, S.; Stader, F.; Battegay, M.; Marzolini, C. Repository Describing the Anatomical, Physiological, and Biological Changes in an Obese Population to Inform Physiologically Based Pharmacokinetic Models. Clin. Pharmacokinet. 2022, 61, 1251–1270. [Google Scholar] [CrossRef]
- Vricella, L.K.; Louis, J.M.; Chien, E.; Mercer, B.M. Blood volume determination in obese and normal-weight gravidas: The hydroxyethyl starch method. Am. J. Obstet. Gynecol. 2015, 213, 408.e1–408.e6. [Google Scholar] [CrossRef]
- Blokhin, I.O.; Lentz, S.R. Mechanisms of thrombosis in obesity. Curr. Opin. Hematol. 2013, 20, 437–444. [Google Scholar] [CrossRef]
- Zhu, T.; Tang, J.; Zhao, F.; Qu, Y.; Mu, D. Association between maternal obesity and offspring Apgar score or cord pH: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 18386. [Google Scholar] [CrossRef] [PubMed]
- Rougée, L.R.A.; Miyagi, S.J.; Collier, A.C. Obstetric Obesity Is Associated With Neonatal Hyperbilirubinemia With High Prevalence in Native Hawaiians and Pacific Island Women. Hawaii J. Med. Public Health 2016, 75, 373–374. [Google Scholar] [PubMed]
| Non-Obese BMI <30 kg/m2 (n = 245) | Class I: BMI 30–34.9 kg/m2 (n = 261) | Class II: BMI 35–39.9 kg/m2 (n = 181) | Class III: BMI ≥40 kg/m2 (n = 204) | p Value | |
|---|---|---|---|---|---|
| Age (years) | 28 (23, 32.8) | 29 (24, 34) | 29 (24, 35) | 30 (25, 35) | 0.04 |
| BMI (kg/m2) | 27.4 (25.4, 29) | 32.3 (31.2, 33.6) | 37.1 (36.1, 38.6) | 44.2 (42.2, 49.2) | <0.0001 |
| Self-Identified Race and Ethnicity | |||||
| Hispanic/LatinX | 171 (69.8) | 200 (76.6) | 146 (80.7) | 160 (78.4) | 0.45 |
| Non-Hispanic/LatinX | |||||
| - White | 34 (13.9) | 31 (11.9) | 17 (9.4) | 17 (8.3) | 0.24 |
| - Black | 17 (6.9) | 11 (4.2) | 10 (5.5) | 21 (10.3) | 0.06 |
| - Asian | 17 (6.9) | 12 (4.6) | 2 (1.1) | 3 (1.5) | 0.003 |
| - Other/unknown | 6 (2.4) | 7 (2.7) | 6 (3.3) | 3 (1.5) | 0.64 |
| Nulliparous | 104 (42.4) | 106 (40.6) | 82 (45.3) | 80 (39.2) | 0.64 |
| EGA (weeks) | 39.1 (37.4, 40.1) | 39 (37.6, 40.1) | 39 (37.1, 39.9) | 38.6 (37, 39.4) | 0.0003 |
| Admission Hgb (g/dL) | 11.6 (10.4, 12.6) | 11.9 (10.8, 12.5) | 11.8 (10.7, 12.4) | 11.4 (10.6, 12.1) | 0.11 |
| Commercial insurance | 74 (39.2) | 70 (26.8) | 49 (27.1) | 50 (24.5) | 0.6 |
| Tobacco use | 8 (3.3) | 8 (3.1) | 4 (2.2) | 9 (4.4) | 0.69 |
| Anticoagulant use during pregnancy | 4 (1.6) | 4 (1.5) | 6 (3.3) | 10 (4.9) | 0.11 |
| Required induction or augmentation of labor | 133 (54.3) | 144 (55.2) | 112 (61.9) | 118 (57.8) | 0.74 |
| Pre-existing medical condition | |||||
| - Pre-gestational DM | 2 (0.8) | 12 (4.6) | 16 (8.8) | 23 (11.3) | <0.0001 |
| - Asthma | 5 (2) | 10 (3.8) | 8 (4.4) | 11 (5.4) | 0.3 |
| - Chronic HTN | 6 (2.4) | 18 (6.9) | 18 (9.9) | 31 (15.2) | <0.0001 |
| Pharmacologic Management | Non-Obese: BMI <30 kg/m2 (n = 245) | Class I: BMI 30–34.9 kg/m2 (n = 261) | Class II: BMI 35–39.9 kg/m2 (n = 181) | Class III: BMI ≥40 kg/m2 (n = 204) | p Value |
|---|---|---|---|---|---|
| Any uterotonic used | 236 (96.3) | 254 (97.3) | 176 (97.2) | 199 (97.6) | 0.87 |
| ≥2 uterotonics | 128 (52.2) | 160 (61.3) | 99 (54.7) | 93 (45.6) | 0.008 |
| Misoprostol | 50 (20.4) | 60 (23) | 30 (16.6) | 29 (14.2) | 0.08 |
| Carboprost | 88 (35.9) | 95 (36.3) | 69 (38.1) | 57 (27.9) | 0.14 |
| 1 dose | 56 (22.9) | 49 (18.8) | 37 (20.4) | 30 (14.7) | 0.18 |
| 2 doses | 20 (8.2) | 29 (11.1) | 21 (11.6) | 18 (8.8) | 0.55 |
| ≥3 doses | 12 (4.9) | 17 (6.5) | 11 (6.1) | 9 (4.4) | 0.72 |
| Methylergonovine | 111 (45.3) | 121 (46.4) | 61 (33.7) | 43 (21.1) | <0.0001 |
| Tranexamic acid | 130 (53.1) | 160 (61.3) | 112 (61.9) | 110 (53.9) | 0.11 |
| Non-Obese: BMI <30 kg/m2 (n = 245) | Class I: BMI 30–34.9 kg/m2 (n = 261) | Class II: BMI 35–39.9 kg/m2 (n = 181) | Class III: BMI ≥40 kg/m2 (n = 204) | p Value | |
|---|---|---|---|---|---|
| Mode of delivery | |||||
| - Cesarean Delivery | 119 (48.6) | 143 (54.8) | 107 (59.1) | 137 (67.2) | 0.0009 |
| Uterine incision | |||||
| - Low transverse | 103 (42) | 123 (47.1) | 100 (55.2) | 108 (52.9) | 0.027 |
| - Classical | 14 (5.7) | 12 (4.6) | 6 (3.3) | 20 (9.8) | 0.034 |
| - Other | 3 (1.2) | 9 (3.4) | 2 (1.1) | 8 (3.9) | 0.13 |
| Reason for PPH | |||||
| - Atony | 107 (43.7) | 130 (49.8) | 85 (47) | 82 (40.2) | 0.19 |
| - Hysterotomy extension | 26 (10.6) | 24 (9.2) | 32 (17.7) | 45 (22) | <0.0001 |
| - Retained POCs | 2 (0.8) | 3 (1.1) | 3 (1.6) | 3 (1.5) | 0.89 |
| - 3rd or 4th degree perineal laceration | 10 (4.1) | 7 (2.7) | 2 (1.1) | 4 (2) | 0.52 |
| Nonpharmacologic Management of PPH | |||||
| - Vaginal packing | 15 (6.1) | 10 (3.8) | 7 (3.9) | 5 (2.5) | 0.26 |
| - Bakri | 13 (5.3) | 22 (8.4) | 9 (5) | 9 (4.4) | 0.24 |
| - UAE | 5 (2) | 3 (1.1) | 0 (0) | 2 (1) | 0.27 |
| - Move to OR 1 | 7 (5.7) | 9 (7.6) | 6 (3.3) | 3 (4.4) | 0.77 |
| - D&C | 2 (0.8) | 4 (1.5) | 2 (1.1) | 1 (0.5) | 0.77 |
| - B-lynch | 2 (0.8) | 1 (0.4) | 2 (1.1) | 3 (1.5) | 0.64 |
| - O-Leary | 17 (6.9) | 6 (2.3) | 10 (5.5) | 12 (5.9) | 0.1 |
| - Hysterectomy | 11 (4.5) | 5 (1.9) | 4 (2.2) | 3 (1.5) | 0.22 |
| QBL at delivery (mL) | 1168 (1000, 1431) | 1152 (1003, 1393) | 1116 (1006, 1434) | 1245 (1031, 1507) | 0.053 |
| Total QBL at 24 h (mL) | 1312 (1137, 1624) | 1310 (1130, 1568) | 1272 (1130, 1618) | 1384 (1142, 1665) | 0.59 |
| Required blood transfusion | 86 (35.1) | 64 (24.5) | 45 (24.9) | 40 (19.6) | 0.002 |
| Non-Obese: BMI <30 kg/m2 (n = 245) | Class I: BMI 30–34.9 kg/m2 (n = 261) | Class II: BMI 35–39.9 kg/m2 (n = 181) | Class III: BMI ≥40 kg/m2 (n = 204) | p Value | |
|---|---|---|---|---|---|
| Neonatal birthweight | 3334 (2865, 3665) | 3380 (2950, 3780) | 3374 (2983, 3780) | 3440 (2933, 3790) | 0.18 |
| Apgar Scores | |||||
| - 1 min < 7 | 39 (15.9) | 36 (13.8) | 25 (13.8) | 51 (24.5) | 0.009 |
| - 5 min < 7 | 15 (6.1) | 9 (3.4) | 6 (3.3) | 19 (9.3) | 0.02 |
| NICU admission | 51 (20.8) | 56 (21.5) | 40 (22.1) | 51 (25) | 0.62 |
| Hyperbilirubinemia requiring phototherapy | 44 (18) | 39 (14.9) | 39 (21.5) | 58 (28.4) | 0.003 |
| Neonate LOS (days) | 12.5 (4.75, 34.5) | 12 (5, 23) | 12.5 (4.75, 32.75) | 18 (5, 37) | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Munter, B.T.; Lee, M.Y.; Sundjaja, C.D.; Paul, N.D.; Klausmeyer, M.M.; Yammine, N.A.; Ramsey, P.S.; Byrne, J.J. Association Between Body Mass Index and Uterotonic Use in Postpartum Hemorrhage: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 6283. https://doi.org/10.3390/jcm14176283
Cheng C, Munter BT, Lee MY, Sundjaja CD, Paul ND, Klausmeyer MM, Yammine NA, Ramsey PS, Byrne JJ. Association Between Body Mass Index and Uterotonic Use in Postpartum Hemorrhage: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(17):6283. https://doi.org/10.3390/jcm14176283
Chicago/Turabian StyleCheng, CeCe, Bryce T. Munter, Michaela Y. Lee, Claire D. Sundjaja, Natasha D. Paul, Margaret M. Klausmeyer, Nastassia A. Yammine, Patrick S. Ramsey, and John J. Byrne. 2025. "Association Between Body Mass Index and Uterotonic Use in Postpartum Hemorrhage: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 17: 6283. https://doi.org/10.3390/jcm14176283
APA StyleCheng, C., Munter, B. T., Lee, M. Y., Sundjaja, C. D., Paul, N. D., Klausmeyer, M. M., Yammine, N. A., Ramsey, P. S., & Byrne, J. J. (2025). Association Between Body Mass Index and Uterotonic Use in Postpartum Hemorrhage: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(17), 6283. https://doi.org/10.3390/jcm14176283







