Empowering Early Recovery: The Role of Impella 5.5 in Takotsubo Cardiomyopathy Complicated by Cardiogenic Shock
Abstract
1. Background
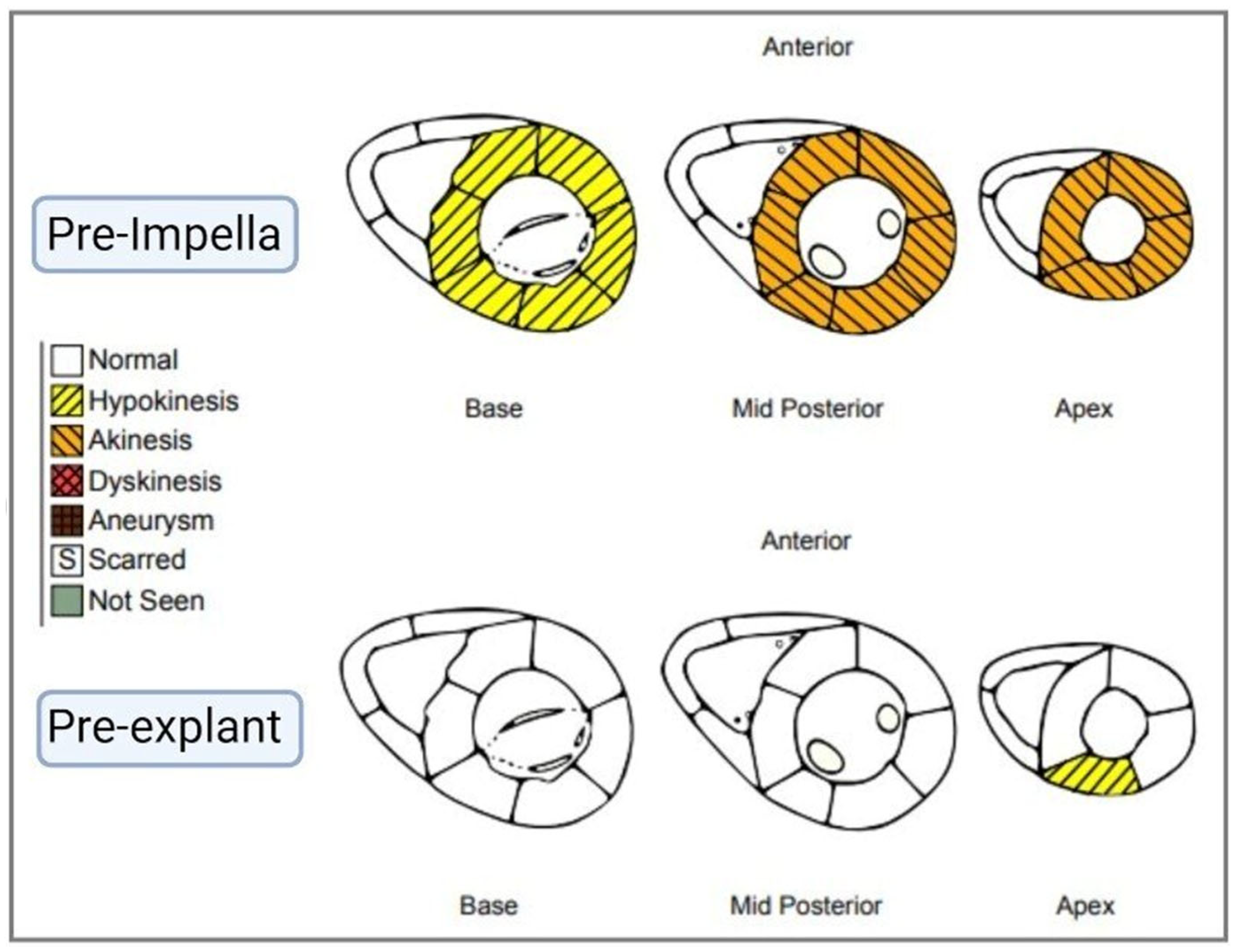
2. Case Presentation
3. Discussion
3.1. Presentation and Diagnostic Criteria for TCM
- Transient dyskinesis of the LV midsegments with or without apical involvement; regional wall motion abnormalities beyond a single epicardial vascular distribution.
- Absence of obstructive coronary artery disease or acute plaque rupture.
- New electrocardiographic abnormalities or modest troponin elevation.
- Absence of pheochromocytoma and myocarditis.
3.2. Current Guidelines for Treatment of TCM
- Avoidance of the use of catecholamines which can mimic the pathogenesis of TCM, avoidance of milrinone which showed similar effects in pre-clinical models via an increase in cAMP, and favor the use of levosimendan, a calcium sensitizer and cardiac stimulant.
- Early initiation of MCS to reduce the need for inotrope and pressor support while allowing LV recovery and providing a window for decision-making.
- Early beta blocker therapy after hemodynamic stabilization.
3.3. Current State of Impella 5.5 in Takotsubo Cardiomyopathy
3.4. Extended Support for Recovery After Impella 5.5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | cardiogenic shock |
| GDMT | guideline directed medical therapy |
| LDH | lactate dehydrogenase |
| LV | left ventricle |
| LVEF | left ventricular ejection fraction |
| LVOTO | left ventricular outflow tract obstruction |
| MCOT | mobile cardiac outpatient telemetry |
| MCS | mechanical circulatory support |
| RV | right ventricle |
| TCM | takotsubo cardiomyopathy |
| TCM-CS | takotsubo cardiomyopathy related cardiogenic shock |
References
- Amin, H.Z.; Amin, L.Z.; Pradipta, A. Takotsubo Cardiomyopathy: A Brief Review. J. Med. Life 2020, 13, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Brito, D.; Khalid, N.; Ibrahim, M.A. Takotsubo Cardiomyopathy. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Eftychiou, S.; Kalakoutas, A.; Proudfoot, A. The role of temporary mechanical circulatory support in de novo heart failure syndromes with cardiogenic shock: A contemporary review. J. Intensive Med. 2023, 3, 89–103. [Google Scholar] [CrossRef] [PubMed]
- von Mackensen, J.K.R.; Shazly, A.E.; Schoenrath, F.; Kempfert, J.; Starck, C.T.; Potapov, E.V.; Jacobs, S.; Falk, V.; Wert, L. Successful treatment of cardiogenic shock due to Takotsubo syndrome with implantation of a temporary microaxial left ventricular assist device in transaxillary approach. J. Cardiothorac. Surg. 2023, 18, 343. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.P.; Antohi, E.L.; Arrigo, M.; Ben Gal, T.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock–a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sharma, S.; Luce, C.; Ruiz, J.; Goswami, R. Case Report: Unmasking sustainable left ventricular recovery in chronic heart failure with axillary temporary mechanical circulatory support. Front. Cardiovasc. Med. 2024, 11, 1407552. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, P.; Pitsillidi, A.; Pyrpyris, N.; Fragkoulis, C.; Leontsinis, I.; Koutsopoulos, G.; Mantzouranis, E.; Soulaidopoulos, S.; Kasiakogias, A.; Dimitriadis, K.; et al. Pregnancy-Associated Takotsubo Syndrome: A Narrative Review of the Literature. J. Clin. Med. 2025, 14, 2356. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Solh, T. Takotsubo cardiomyopathy: Review of broken heart syndrome. J. Am. Acad. Physician Assist. 2020, 33, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Frangieh, A.H.; Obeid, S.; Ghadri, J.R.; Imori, Y.; D’Ascenzo, F.; Kovac, M.; Ruschitzka, F.; Lüscher, T.F.; Duru, F.; Templin, C. ECG Criteria to Differentiate Between Takotsubo (Stress) Cardiomyopathy and Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003418. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, T.; Hano, T.; Kasamatsu, K.; Yamamoto, K.; Tsuruo, Y.; Nishio, I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J. Cardiovasc. Pharmacol. 2003, 42 (Suppl. S1), S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Santoro, F.; Eitel, C.; Graf, T.; Möller, C.; Tarantino, N.; Guastafierro, F.; Di Biase, M.; Thiele, H.; Brunetti, N.D.; et al. Prevalence and prognostic relevance of atrial fibrillation in patients with Takotsubo syndrome. Int. J. Cardiol. 2017, 245, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Westenfeld, R.; Møller, J.E.; Pappalardo, F.; Ibrahim, K.; Bonello, L.; Wilkins, C.; Pershad, A.; Mannino, S.F.; Schreiber, T.L.; et al. Impella Mechanical Circulatory Support for Takotsubo Syndrome With Shock: A Retrospective Multicenter Analysis. Cardiovasc. Revasc Med. 2022, 40, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, R.; Nagano, N.; Kokubu, N.; Hashimoto, K.; Nakata, J.; Kishiue, N.; Takahashi, R.; Otomo, S.; Tsuchihashi, K.; Yano, T. Favorable Effects of Impella on Takotsubo Syndrome Complicated with Cardiogenic Shock. Int. Heart J. 2021, 62, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.M.; Jarmi, T.; Sareyyupoglu, B.; Nativi, J.; Patel, P.C.; Leoni, J.C.; Landolfo, K.; Pham, S.; Yip, D.S.; Goswami, R.M. Axillary mechanical circulatory support improves renal function prior to heart transplantation in patients with chronic kidney disease. Sci. Rep. 2023, 13, 19671. [Google Scholar] [CrossRef] [PubMed]
| Pre-Impella 5.5 | Pre-Explant | 2-Month Outpatient Follow-Up | |
|---|---|---|---|
| Vitals | |||
| Height (cm) | 170.2 | 170.2 | 170.2 |
| Weight (kg) | 65.8 | 64.5 | 59.7 |
| Body Mass Index (BMI) (kg/cm2) | 22.7 | 22.3 | 20.7 |
| Hemodynamics | |||
| Heart Rate (bpm) | 119 | 61 | 110 |
| Blood Pressure (mmHg) | 98/70 | 105/55 | 125/72 |
| Mean Arterial Pressure (mmHg) | 79 | 72 | 90 |
| Left Ventricular Ejection Fraction (%) | 24 | 59 | 59 |
| Right Atrial Pressure (mmHg) | 20 | 15 | 5 |
| Mean Pulmonary Artery Pressure (mmHg) | 48 | 45 | - |
| Pulmonary Capillary Wedge Pressure (mmHg) | 19 | - | - |
| Fick Cardiac Output (L/min) | 4.4 | 6.6 | - |
| Fick Cardiac Index (L/min/m2) | 2.5 | 3.65 | - |
| Laboratory values | |||
| Hemoglobin (g/dL) | 10.6 | 8.7 | 10.6 |
| Platelets (109/L) | 228 | 246 | 654 |
| Lactate Dehydrogenase (U/L) | 1776 | 314 | - |
| Mixed Venous Saturation | 67 | 53 | - |
| Serum Lactate (mmol/L) | 18.7 | 1.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, A.; Ruiz, J.; Shapiro, A.; Klingbeil, R.; Martin, A.; Goswami, R. Empowering Early Recovery: The Role of Impella 5.5 in Takotsubo Cardiomyopathy Complicated by Cardiogenic Shock. J. Clin. Med. 2025, 14, 6278. https://doi.org/10.3390/jcm14176278
Desai A, Ruiz J, Shapiro A, Klingbeil R, Martin A, Goswami R. Empowering Early Recovery: The Role of Impella 5.5 in Takotsubo Cardiomyopathy Complicated by Cardiogenic Shock. Journal of Clinical Medicine. 2025; 14(17):6278. https://doi.org/10.3390/jcm14176278
Chicago/Turabian StyleDesai, Aarti, Jose Ruiz, Anna Shapiro, Rebecca Klingbeil, Archer Martin, and Rohan Goswami. 2025. "Empowering Early Recovery: The Role of Impella 5.5 in Takotsubo Cardiomyopathy Complicated by Cardiogenic Shock" Journal of Clinical Medicine 14, no. 17: 6278. https://doi.org/10.3390/jcm14176278
APA StyleDesai, A., Ruiz, J., Shapiro, A., Klingbeil, R., Martin, A., & Goswami, R. (2025). Empowering Early Recovery: The Role of Impella 5.5 in Takotsubo Cardiomyopathy Complicated by Cardiogenic Shock. Journal of Clinical Medicine, 14(17), 6278. https://doi.org/10.3390/jcm14176278





