Pushing the DIEP Envelope: Where Are We Now?
Abstract
1. Introduction
2. Discussion
2.1. Optimizing Volume and Perfusion
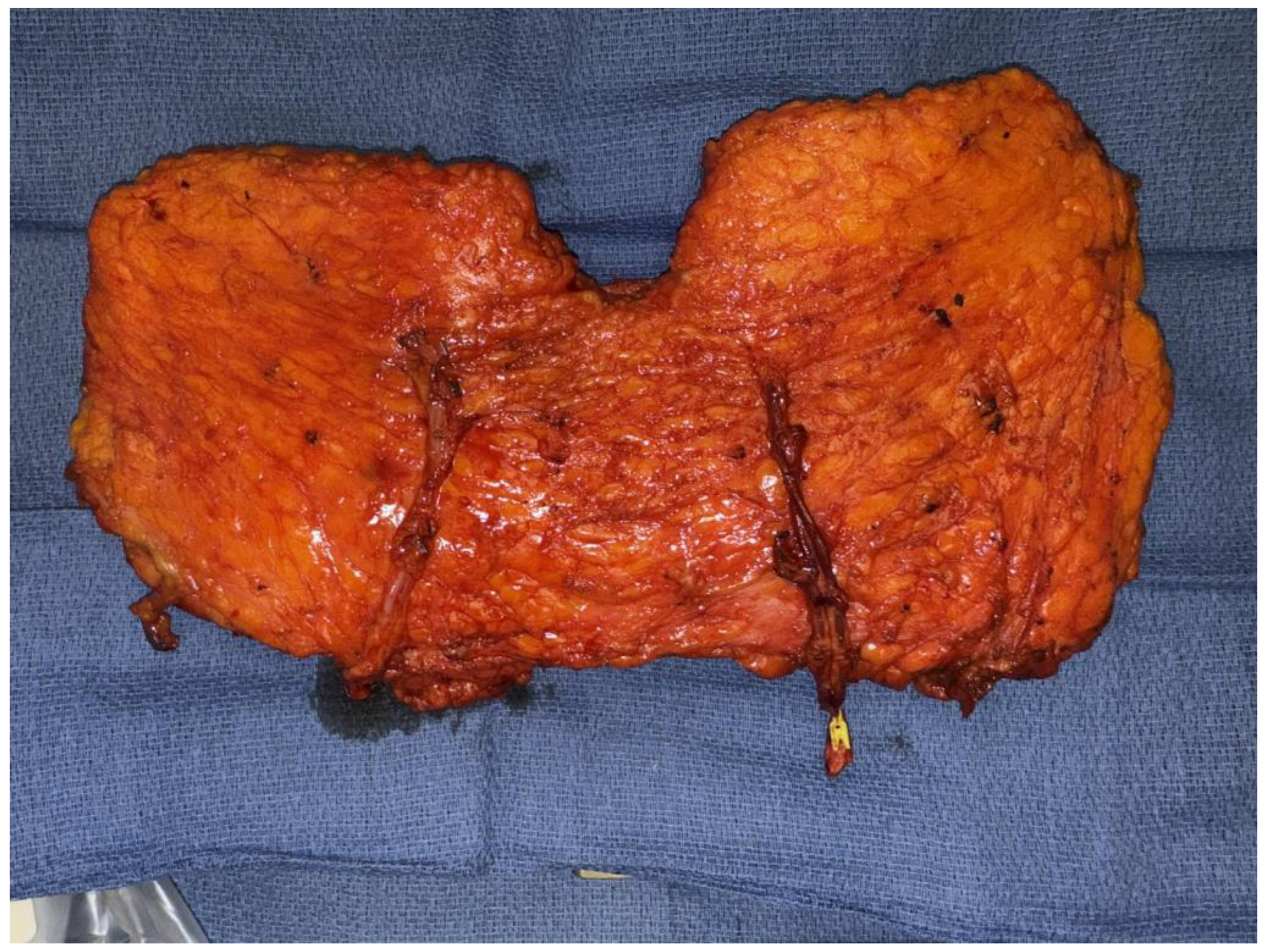
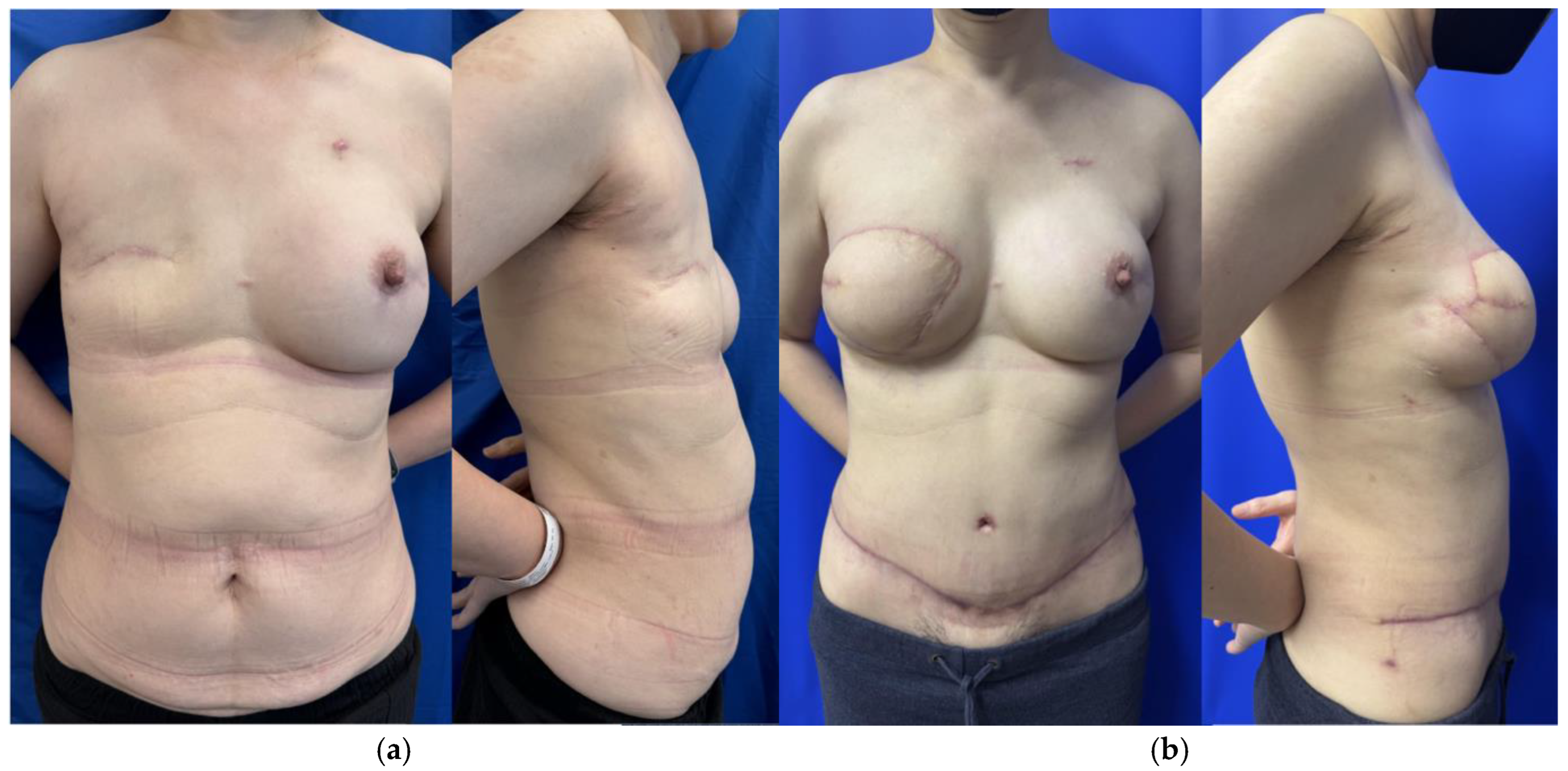
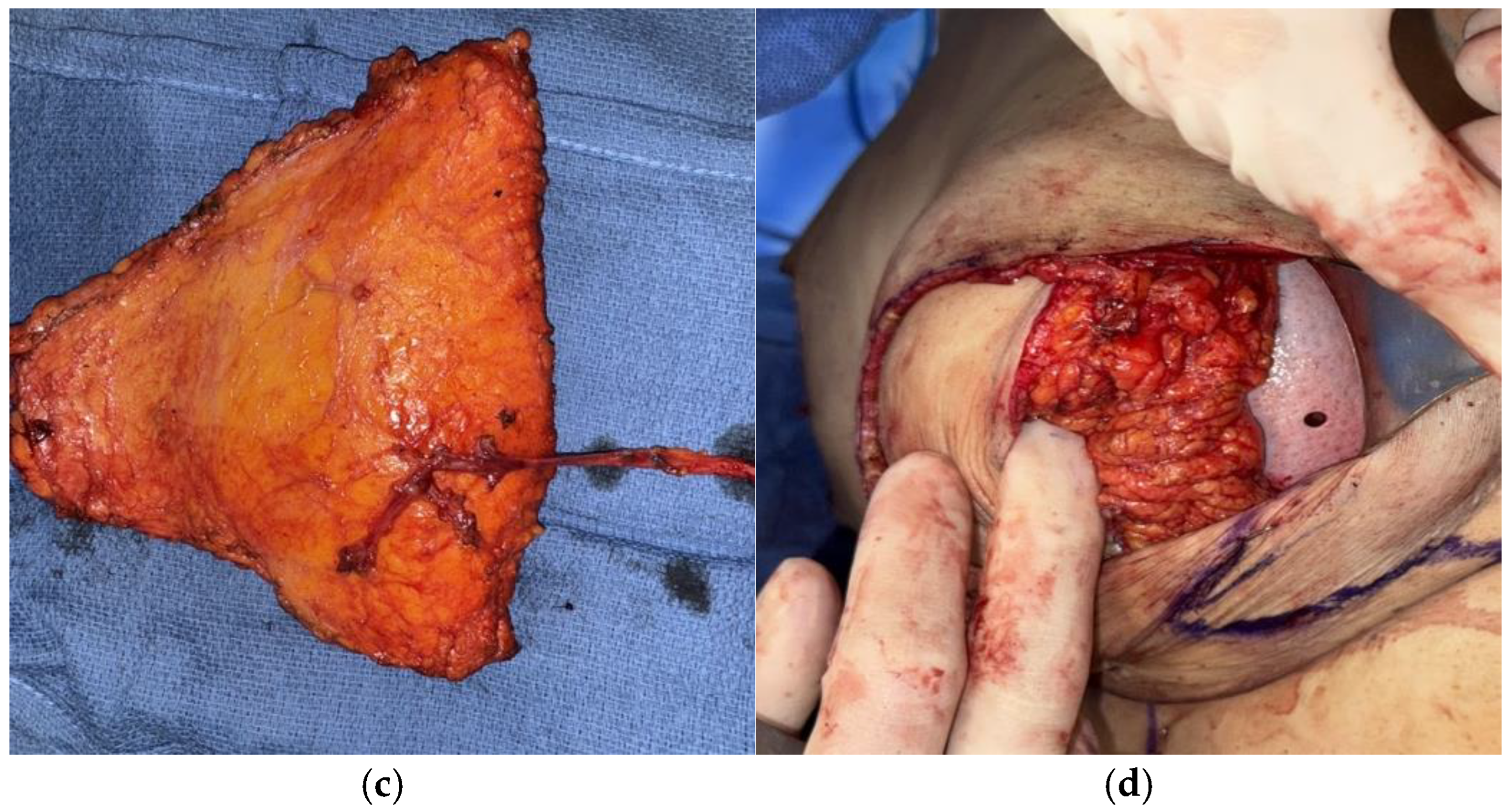
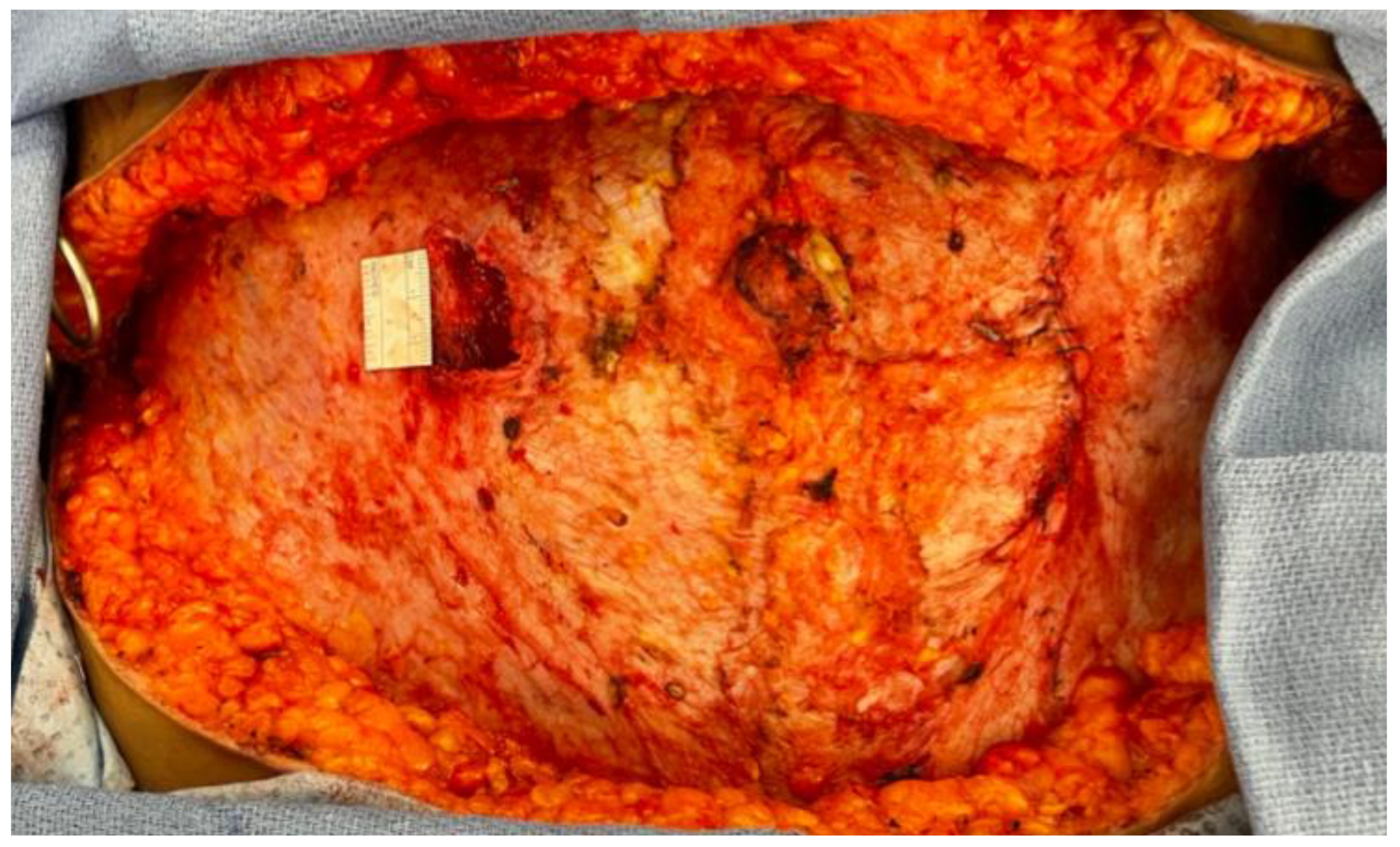
2.1.1. Stacked and Conjoined DIEP Flaps
2.1.2. Dual-Plane and Extended Flaps
2.1.3. Hybrid Autologous Techniques
2.1.4. DIEP Delay
2.2. Donor Site Optimization
2.2.1. Nerve-Sparing DIEP
2.2.2. Minimally-Invasive DIEP Flap Harvest Techniques
2.2.3. Limited Fascial Incision (LFI) Techniques
2.2.4. Laparoscopic Harvested DIEP Flap
2.2.5. Robotic DIEP Flap
2.2.6. APEX Flap
2.2.7. Mesh Reinforcement
2.3. Composite DIEP Flap
2.4. Optimizing Breast Aesthetics
2.4.1. Mastectomy Flap Considerations
2.4.2. Nipple Sparing Mastectomy Incisions
2.4.3. Staged Reduction and Mastopexy
2.4.4. Free Nipple Grafts
2.5. Sensory Restoration
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Steele, T.N.; Teotia, S.S.; Haddock, N.T. Multi-Flap Microsurgical Autologous Breast Reconstruction. J. Clin. Med. 2024, 13, 5324. [Google Scholar] [CrossRef]
- Stecco, C.; Azzena, G.P.; Macchi, V.; Porzionato, A.; Behr, A.; Rambaldo, A.; Tiengo, C.; De Caro, R. Rectus Abdominis Muscle Innervation: An Anatomical Study with Surgical Implications in Diep Flap Harvesting. Surg. Radiol. Anat. 2018, 40, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.; Allen, R. The Evolution of Perforator Flap Breast Reconstruction: Twenty Years after the First DIEP Flap. J. Reconstr. Microsurg. 2013, 30, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Beugels, J.; Levine, J.L.; Vasile, J.V.; Craigie, J.E.; Allen, R.J. The Delay Procedure in Deep Inferior Epigastric Artery Perforator Flap Breast Reconstruction. Plast. Reconstr. Surg. 2024, 153, 1063e–1072e. [Google Scholar] [CrossRef] [PubMed]
- Jeevan, R.; Browne, J.P.; Gulliver-Clarke, C.; Pereira, J.; Caddy, C.M.; Van Der Meulen, J.H.P.; Cromwell, D.A. Surgical Determinants of Patient-Reported Outcomes Following Postmastectomy Reconstruction in Women with Breast Cancer. Plast. Reconstr. Surg. 2017, 139, 1036e–1045e. [Google Scholar] [CrossRef]
- Malekpour, M.; Malekpour, F.; Wang, H.T.-H. Breast Reconstruction: Review of Current Autologous and Implant-Based Techniques and Long-Term Oncologic Outcome. World J. Clin. Cases 2023, 11, 2201–2212. [Google Scholar] [CrossRef]
- Liu, C.; Zhuang, Y.; Momeni, A.; Luan, J.; Chung, M.T.; Wright, E.; Lee, G.K. Quality of Life and Patient Satisfaction after Microsurgical Abdominal Flap versus Staged Expander/Implant Breast Reconstruction: A Critical Study of Unilateral Immediate Breast Reconstruction Using Patient-Reported Outcomes Instrument BREAST-Q. Breast Cancer Res. Treat. 2014, 146, 117–126. [Google Scholar] [CrossRef]
- Eltahir, Y.; Werners, L.L.C.H.; Dreise, M.M.; Zeijlmans Van Emmichoven, I.A.; Werker, P.M.N.; De Bock, G.H. Which Breast Is the Best? Successful Autologous or Alloplastic Breast Reconstruction: Patient-Reported Quality-of-Life Outcomes. Plast. Reconstr. Surg. 2015, 135, 43–50. [Google Scholar] [CrossRef]
- Zoccali, G.; Farhadi, J. Abdominal Perforator Exchange Flap (APEX): A Classification of Pedicle Rearrangements. Microsurgery 2021, 41, 607–614. [Google Scholar] [CrossRef]
- Salibian, A.A.; Bekisz, J.M.; Frey, J.D.; Nolan, I.T.; Kaoutzanis, C.; Yu, J.W.; Levine, J.P.; Karp, N.S.; Choi, M.; Thanik, V.D. Comparing Outcomes between Stacked/Conjoined and Non-stacked/Conjoined Abdominal Microvascular Unilateral Breast Reconstruction. Microsurgery 2021, 41, 240–249. [Google Scholar] [CrossRef]
- 2023 Procedural Statistics Release|Reconstructive Breast Procedures. 2023. Available online: https://www.plasticsurgery.org/documents/news/statistics/2023/plastic-surgery-statistics-report-2023.pdf (accessed on 1 March 2025).
- Farajzadeh, M.M.; Salibian, A.A. Optimizing Perfusion and Volume in Autologous Breast Reconstruction: Dual-Plane, Conjoined and Stacked Flaps. Plast. Aesthetic Res. 2024, 11, 32. [Google Scholar] [CrossRef]
- Parikh, J.A.; Bombardelli, J.; Doval, A.; Spiegel, A.J. Strategic Approaches to Intraflap Anastomosis: Navigating Conjoined DIEP Flap Reconstruction—A Comprehensive Roadmap. Plast. Reconstr. Surg.—Glob. Open 2024, 12, e5627. [Google Scholar] [CrossRef] [PubMed]
- Salibian, A.A.; Nolan, I.T.; Bekisz, J.M.; Frey, J.D.; Karp, N.S.; Choi, M.; Levine, J.P.; Thanik, V.D. A Systematic Review and Meta-Analysis of Microvascular Stacked and Conjoined-Flap Breast Reconstruction. J. Reconstr. Microsurg. 2021, 37, 631–642. [Google Scholar] [CrossRef] [PubMed]
- DellaCroce, F.J.; DellaCroce, H.C.; Blum, C.A.; Sullivan, S.K.; Trahan, C.G.; Wise, M.W.; Brates, I.G. Myth-Busting the DIEP Flap and an Introduction to the Abdominal Perforator Exchange (APEX) Breast Reconstruction Technique: A Single-Surgeon Retrospective Review. Plast. Reconstr. Surg. 2019, 143, 992–1008. [Google Scholar] [CrossRef]
- Tanna, N.; Barnett, S.L.; Robinson, E.L.; Smith, M.L. Hybrid Microsurgical Breast Reconstruction. Clin. Plast. Surg. 2023, 50, 337–346. [Google Scholar] [CrossRef]
- Evgeniou, E.; Teotia, S.S.; Haddock, N.T. Asymmetric Four Flap Breast Reconstruction with DIEP Flaps and PAP Flaps. Plast. Reconstr. Surg. 2022, 150, 1236e–1239e. [Google Scholar] [CrossRef]
- Sbitany, H.; Lentz, R.; Piper, M. The “Dual-Plane” DIEP Flap: Measuring the Effects of Superficial Arterial and Venous Flow Augmentation on Clinical Outcomes. J. Reconstr. Microsurg. 2019, 35, 411–416. [Google Scholar] [CrossRef]
- Jabbour, S.; Youn, R.; Kim, K.G.; Tirrell, A.R.; Harbour, P.W.; Dekker, P.K.; Fan, K.L.; Song, D.H. An Algorithmic Approach to Dual-System Venous Drainage for DIEP Flap Breast Reconstruction. Plast. Reconstr. Surg. 2023, 154, 1e–12e. [Google Scholar] [CrossRef]
- Beugels, J.; Vasile, J.V.; Tuinder, S.M.H.; Delatte, S.J.; St-Hilaire, H.; Allen, R.J.; Levine, J.L. The Stacked Hemiabdominal Extended Perforator Flap for Autologous Breast Reconstruction. Plast. Reconstr. Surg. 2018, 142, 1424–1434. [Google Scholar] [CrossRef]
- Kanchwala, S.; Momeni, A. Hybrid Breast Reconstruction—The Best of Both Worlds. Gland Surg. 2019, 8, 82–89. [Google Scholar] [CrossRef]
- Wang, C.; Roy, N.; Graziano, F.; Henderson, P. A Comparative Analysis Of Complications After Hybrid Versus Implant-Only Breast Reconstruction. Plast. Reconstr. Surg.—Glob. Open 2024, 12, 46. [Google Scholar] [CrossRef]
- Momeni, A.; Kanchwala, S.K. Improved Pocket Contr ol in Immediate Microsurgical Breast Reconstruction with Simultaneous Implant Placement through the Use of Mesh. Microsurgery 2018, 38, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, S.H.; Frank, S.G.; Cordeiro, P.G. Breast Reconstruction Following Conservative Mastectomies: Predictors of Complications and Outcomes. Gland Surg. 2015, 4, 484–496. [Google Scholar] [PubMed]
- Bach, A.D.; Morgenstern, I.H.; Horch, R.E. Secondary “Hybrid Reconstruction” Concept with Silicone Implants After Autologous Breast Reconstruction—Is It Safe and Reasonable? Med. Sci. Monit. 2020, 26, e921329. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Kanchwala, S. Hybrid Prepectoral Breast Reconstruction: A Surgical Approach That Combines the Benefits of Autologous and Implant-Based Reconstruction. Plast. Reconstr. Surg. 2018, 142, 1109–1115. [Google Scholar] [CrossRef]
- Blum, C.A.; DellaCroce, F.J.; Sullivan, S.K.; Trahan, C.; Wise, M.W. Creation of a Central Under Flap Pocket Allows Secondary Implant Augmentation of Perforator Flap Breast Reconstruction. Plast. Reconstr. Surg.—Glob. Open 2018, 6, e1734. [Google Scholar] [CrossRef]
- Shakir, S.; Spencer, A.B.; Kozak, G.M.; Jablonka, E.M.; Kanchwala, S.K. Make Your Own Deep Inferior Epigastric Artery Perforator Flap: Perforator Delay Improves Deep Inferior Epigastric Artery Perforator Flap Reliability. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2478. [Google Scholar] [CrossRef]
- Alves, A.S.; Tan, V.; Scampa, M.; Kalbermatten, D.F.; Oranges, C.M. Complications of Immediate versus Delayed DIEP Reconstruction: A Meta-Analysis of Comparative Studies. Cancers 2022, 14, 4272. [Google Scholar] [CrossRef]
- Rozen, W.M.; Tran, T.M.N.; Ashton, M.W.; Barrington, M.J.; Ivanusic, J.J.; Taylor, G.I. Refining the Course of the Thoracolumbar Nerves: A New Understanding of the Innervation of the Anterior Abdominal Wall. Clin. Anat. 2008, 21, 325–333. [Google Scholar] [CrossRef]
- Woodburne, R.; Burkel, W. Essentials of Human Anatomy, 8th ed.; Oxfird Univeristy Press: New York, NY, USA, 1988. [Google Scholar]
- Gosling, J.; Harris, O.; Humpherson, J.; Whitmore, I.; Willan, P. Atlas of Human Anatomy with Integrated Test; Gower Medical Publishing: London, UK, 1985. [Google Scholar]
- Duchateau, J.; Declety, A.; Lejour, M. Innervation of the Rectus Abdominis Muscle: Implications for Rectus Flaps. Plast. Reconstr. Surg. 1988, 82, 223–228. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Tsangaris, T.; Momen, B. Breast Reconstruction with the DIEP Flap or the Muscle-Sparing (MS-2) Free TRAM Flap: Is There a Difference? Plast. Reconstr. Surg. 2005, 115, 436–444. [Google Scholar] [CrossRef]
- Rozen, W.M.; Ashton, M.W.; Murray, A.C.A.; Taylor, G.I. Avoiding Denervation of Rectus Abdominis in DIEP Flap Harvest: The Importance of Medial Row Perforators. Plast. Reconstr. Surg. 2008, 122, 710–716. [Google Scholar] [CrossRef]
- Lee, B.T.; Chen, C.; Nguyen, M.; Lin, S.J.; Tobias, A.M. A New Classification System for Muscle and Nerve Preservation in DIEP Flap Breast Reconstruction. Microsurgery 2010, 30, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.B.; Salavati, S.; Feng, L.; Butler, C.E. Abdominal Donor-Site Outcomes for Medial versus Lateral Deep Inferior Epigastric Artery Branch Perforator Harvest. Plast. Reconstr. Surg. 2011, 127, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Hilven, P.H.; Vandevoort, M.; Bruyninckx, F.; De Baerdemaeker, R.; Dupont, Y.; Peeters, Q.; Nanhekhan, L.; Fabre, G. Limiting the Fascia Incision Length in a DIEP Flap: Repercussion on Abdominal Wall Morbidity. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1108–1116. [Google Scholar] [CrossRef]
- Martinez, C.A.; Boutros, S.G. Abdominoplasty and Breast Augmentation with Outpatient Cosmetic Deep Inferior Epigastric Perforator Flaps. Plast. Reconstr. Surg. 2023, 151, 234e–240e. [Google Scholar] [CrossRef] [PubMed]
- Colohan, S.; Maia, M.; Langevin, C.J.; Donfrancesco, A.; Shirvani, A.; Trussler, A.P.; Saint-Cyr, M. The Short- and Ultrashort-Pedicle Deep Inferior Epigastric Artery Perforator Flap in Breast Reconstruction. Plast. Reconstr. Surg. 2012, 129, 331–340. [Google Scholar] [CrossRef]
- Martinez, C.A.; Reis, S.M.; Rednam, R.; Boutros, S.G. The Outpatient DIEP: Safety and Viability Following a Modified Recovery Protocol. Plast. Reconstr. Surg.—Glob. Open 2018, 6, e1898. [Google Scholar] [CrossRef]
- Shakir, S.; Spencer, A.B.; Piper, M.; Kozak, G.M.; Soriano, I.S.; Kanchwala, S.K. Laparoscopy Allows the Harvest of the DIEP Flap with Shorter Fascial Incisions as Compared to Endoscopic Harvest: A Single Surgeon Retrospective Cohort Study. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1203–1212. [Google Scholar] [CrossRef]
- Shakir, S.; Spencer, A.B.; Kozak, G.M.; Nathan, S.L.; Soriano, I.S.; Kanchwala, S.K. Laparoscopically Assisted DIEP Flap Harvest Minimizes Fascial Incision in Autologous Breast Reconstruction. Plast. Reconstr. Surg. 2020, 146, 265e–275e. [Google Scholar] [CrossRef]
- Bishop, S.N.; Asaad, M.; Liu, J.; Chu, C.K.; Clemens, M.W.; Kapur, S.S.; Largo, R.D.; Selber, J.C. Robotic Harvest of the Deep Inferior Epigastric Perforator Flap for Breast Reconstruction: A Case Series. Plast. Reconstr. Surg. 2022, 149, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Selber, J.C. The Robotic DIEP Flap. Plast. Reconstr. Surg. 2020, 145, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, S.Y.; Park, H.S.; Kim, C.H.; Kim, J.Y.; Lew, D.H.; Roh, T.S.; Lee, D.W. Robotic DIEP Flap Harvest through a Totally Extraperitoneal Approach Using a Single-Port Surgical Robotic System. Plast. Reconstr. Surg. 2021, 148, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Wormer, B.; Clavin, N.; Lefaivre, J.-F.; Korn, J.; Teng, E.; Aukskalnis, A.; Robinson, J. Reducing Postoperative Abdominal Bulge Following Deep Inferior Epigastric Perforator Flap Breast Reconstruction with Onlay Monofilament Poly-4-Hydroxybutyrate Biosynthetic Mesh. J. Reconstr. Microsurg. 2016, 33, 8–18. [Google Scholar] [CrossRef]
- Parmeshwar, N.; Lem, M.; Dugan, C.L.; Piper, M. Evaluating Mesh Use for Abdominal Donor Site Closure after Deep Inferior Epigastric Perforator Flap Breast Reconstruction: A Systematic Review and Meta-analysis. Microsurgery 2023, 43, 855–864. [Google Scholar] [CrossRef]
- Schmauss, D.; Machens, H.-G.; Harder, Y. Breast Reconstruction after Mastectomy. Front. Surg. 2016, 2, 71. [Google Scholar] [CrossRef]
- Dancey, A.; Nassimizadeh, A.; Nassimizadeh, M.; Warner, R.M.; Waters, R. A Chimeric Vascularised Groin Lymph Node Flap and DIEP Flap for the Management of Lymphoedema Secondary to Breast Cancer. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 735–737. [Google Scholar] [CrossRef]
- Winters, H.; Tielemans, H.J.P.; Hummelink, S.; Slater, N.J.; Ulrich, D.J.O. DIEP Flap Breast Reconstruction Combined with Vascularized Lymph Node Transfer for Patients with Breast Cancer-Related Lymphedema. Eur. J. Surg. Oncol. 2022, 48, 1718–1722. [Google Scholar] [CrossRef]
- Di Taranto, G.; Coleman, G.J.; Hardwicke, J.; Wallis, K.L.; Skillman, J. A Comparative Study between Deep Inferior Epigastric Artery Perforator Flap Breast Reconstruction and DIEP Flap Breast Reconstruction Coupled with Vascularized Lymph Node Transfer: Improving the Quality of Life of Patients with Breast Cancer Related Lymphedema without Affecting Donor Site Outcomes. Microsurgery 2023, 43, 213–221. [Google Scholar] [CrossRef]
- Forte, A.J.; Cinotto, G.; Boczar, D.; Huayllani, M.T.; Lu, X.; Manrique, O.J.; McLaughlin, S.A. Lymph Node Transfer Combined with Deep Inferior Epigastric Perforators and Transverse Rectus Abdominis Myocutaneous Procedures: A Systematic Review. Gland Surg. 2020, 9, 521–527. [Google Scholar] [CrossRef]
- Demiri, E.; Dionyssiou, D.; Kyriazidis, I.; Drougou, A.; Tsimponis, A. Predesigned Chimeric Deep Inferior Epigastric Perforator and Inguinal Lymph Node Flap for Combined Breast and Lymphedema Reconstruction: A Comprehensive Algorithmic Approach. JPRAS Open 2024, 40, 1–18. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Chang, E.I. Combined Deep Inferior Epigastric Artery Perforator Flap with Vascularized Groin Lymph Node Transplant for Treatment of Breast Cancer-Related Lymphedema. Gland Surg. 2021, 10, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Ramaut, L.; De Baerdemaeker, R.; Zeltzer, A. Decreasing Donor Site Morbidity after Groin Vascularized Lymph Node Transfer with Lessons Learned from a 12-Year Experience and Review of the Literature. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, P.; Manrique, O.J.; Bustos, S.S.; Vargas, M.I.; Reynaga, C.; Agko, M.; Huang, T.C.T.; Benites, E.F.; Mayer, H.F.; Forte, A.J. Combined Microvascular Breast and Lymphatic Reconstruction with Deep Inferior Epigastric Perforator Flap and Gastroepiploic Vascularized Lymph Node Transfer for Postmastectomy Lymphedema Patients. Gland Surg. 2020, 9, 512–520. [Google Scholar] [CrossRef]
- Chang, E.; Masià, J.; Smith, M. Combining Autologous Breast Reconstruction and Vascularized Lymph Node Transfer. Semin. Plast. Surg. 2018, 32, 36–41. [Google Scholar] [CrossRef]
- Choi, M.; Frey, J.D. Optimizing Aesthetic Outcomes in Breast Reconstruction After Nipple-Sparing Mastectomy. Aesthetic Surg. J. 2020, 40 (Suppl. S2), S13–S21. [Google Scholar] [CrossRef]
- Salibian, A.A.; Frey, J.D.; Choi, M.; Karp, N.S. Optimizing the Mastectomy Flap to Improve Aesthetic Outcomes. Aesthetic Surg. J. 2020, 40 (Suppl. S2), S1–S12. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Choi, M.; Karp, N.S. The Importance of Tissue Perfusion in Reconstructive Breast Surgery. Plast. Reconstr. Surg. 2019, 144, 21S–29S. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Choi, M.; Karp, N.S. Optimizing Outcomes in Nipple-Sparing Mastectomy: Mastectomy Flap Thickness Is Not One Size Fits All. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2103. [Google Scholar] [CrossRef]
- Salibian, A.A.; Bekisz, J.M.; Frey, J.D.; Thanik, V.D.; Levine, J.P.; Karp, N.S.; Choi, M. Comparing Incision Choices in Immediate Microvascular Breast Reconstruction after Nipple-Sparing Mastectomy: Unique Considerations to Optimize Outcomes. Plast. Reconstr. Surg. 2021, 148, 1173–1185. [Google Scholar] [CrossRef]
- Kronowitz, S.J.; Hunt, K.K.; Kuerer, H.M.; Babiera, G.; McNeese, M.D.; Buchholz, T.A.; Strom, E.A.; Robb, G.L. Delayed-Immediate Breast Reconstruction. Plast. Reconstr. Surg. 2004, 113, 1617–1628. [Google Scholar] [CrossRef]
- Barnes, L.L.; Patterson, A.; Lem, M.; Holland, M.C.; Lentz, R.; Sbitany, H.; Piper, M.L. Immediate Versus Delayed-Immediate Autologous Breast Reconstruction After Nipple-Sparing Mastectomy. Ann. Plast. Surg. 2023, 90, 432–436. [Google Scholar] [CrossRef]
- Wu Young, M.Y.; Garza, R.M.; Chang, D.W. “Immediate versus Delayed Autologous Breast Reconstruction in Patients Undergoing Post-mastectomy Radiation Therapy: A Paradigm Shift”. J. Surg. Oncol. 2022, 126, 949–955. [Google Scholar] [CrossRef]
- Momeni, A.; Kanchwala, S.; Sbitany, H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast. Reconstr. Surg. 2020, 145, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Awaida, C.J.; Bernier, C.; Bou-Merhi, J.S.; Trabelsi, N.O.; Gagnon, A.; El-Khatib, A.; Harris, P.G.; Odobescu, A. Staged Mastopexy Before Nipple-Sparing Mastectomy: Improving Safety and Appearance in Implant-Based and Autologous Breast Reconstruction. Plast. Reconstr. Surg. 2023, 153, 864e–872e. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.L.; Rottman, S.J.; Seiboth, L.A.; Hannan, C.M. Breast Reconstruction Using a Staged Nipple-Sparing Mastectomy Following Mastopexy or Reduction. Plast. Reconstr. Surg. 2012, 129, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Doval, A.; Burns, H.R.; Kaplan, J.; Ellsworth, W.A.; Chevray, P.M.; Spiegel, A.J.; Friedman, J.D. Staged Breast Reconstruction Utilizing Primary Nipple Repositioning Surgery Prior to Nipple-Sparing Mastectomy. J. Plast. Reconstr. Aesthetic Surg. 2024, 91, 249–257. [Google Scholar] [CrossRef]
- Lu Wang, M.; Qin, N.; Chadab, T.M.; Chen, Y.; Huang, H.; Ellison, A.; Otterburn, D.M. A Pilot Study Comparing Sensation in Buried Versus Nonburied Deep Inferior Epigastric Perforator Flaps. Ann. Plast. Surg. 2023, 90, S574–S577. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Levine, J.P.; Karp, N.S.; Choi, M. Incision Choices in Nipple-Sparing Mastectomy: A Comparative Analysis of Outcomes and Evolution of a Clinical Algorithm. Plast. Reconstr. Surg. 2018, 142, 826e–835e. [Google Scholar] [CrossRef]
- Colwell, A.S.; Tessler, O.; Lin, A.M.; Liao, E.; Winograd, J.; Cetrulo, C.L.; Tang, R.; Smith, B.L.; Austen, W.G., Jr. Breast Reconstruction Following Nipple-Sparing Mastectomy: Predictors of Complications, Reconstruction Outcomes, and 5-Year Trends. Breast Dis. A Year Book Q. 2014, 25, 259–261. [Google Scholar] [CrossRef]
- Daar, D.A.; Abdou, S.A.; Rosario, L.; Rifkin, W.J.; Santos, P.J.; Wirth, G.A.; Lane, K.T. Is There a Preferred Incision Location for Nipple-Sparing Mastectomy? A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2019, 143, 906e–919e. [Google Scholar] [CrossRef]
- Raghavan, S.; Peled, A.W.; Hansen, S.L.; Esserman, L.J.; Sbitany, H. Approaches to Microvascular Breast Reconstruction After Total Skin-Sparing Mastectomy: A Comparison of Techniques. Ann. Plast. Surg. 2015, 74 (Suppl. S1), S46–S51. [Google Scholar] [CrossRef]
- Egan, K.G.; Cullom, M.; Nazir, N.; Butterworth, J.A. Patient Satisfaction Increases with Nipple Reconstruction Following Autologous Breast Reconstruction. Plast. Reconstr. Surg. 2021, 148, 177e–184e. [Google Scholar] [CrossRef]
- Egan, K.G.; Lai, E.; Holding, J.; Butterworth, J.A. A Comparative Analysis of Outcomes of Free Nipple Areolar Grafting in Autologous Breast Reconstruction. J. Reconstr. Microsurg. 2021, 37, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Linder, S. Autologous Breast Reconstruction with Free Nipple–Areola Graft after Circumareolar (Skin Reducing) Mastectomy. J. Pers. Med. 2022, 12, 1588. [Google Scholar] [CrossRef]
- Bubberman, J.M.; Van Rooij, J.A.F.; Van Der Hulst, R.R.W.J.; Tuinder, S.M.H. Sensory Recovery and the Role of Innervated Flaps in Autologous Breast Reconstruction—A Narrative Review. Gland Surg. 2023, 12, 1094–1109. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.J.; Menn, Z.K.; Eldor, L.; Kaufman, Y.; Dellon, A.L. Breast Reinnervation: DIEP Neurotization Using the Third Anterior Intercostal Nerve. Plast. Reconstr. Surg.—Glob. Open 2013, 1, e72. [Google Scholar] [CrossRef] [PubMed]
- Beugels, J.; Bijkerk, E.; Lataster, A.; Heuts, E.M.; Van Der Hulst, R.R.W.J.; Tuinder, S.M.H. Nerve Coaptation Improves the Sensory Recovery of the Breast in DIEP Flap Breast Reconstruction. Plast. Reconstr. Surg. 2021, 148, 273–284. [Google Scholar] [CrossRef]
- Xia, T.Y.; Scomacao, I.; Djohan, R.; Moreira, A.; Gurunian, R.; Schwarz, G.S. Neurotization Does Not Prolong Operative Time in Free Flap Breast Reconstruction. Aesthetic Plast. Surg. 2022, 46, 2159–2163. [Google Scholar] [CrossRef]
- Bubberman, J.M.; Brandts, L.; Van Kuijk, S.M.J.; Van Der Hulst, R.R.W.J.; Tuinder, S.M.H. The Efficacy of Sensory Nerve Coaptation in DIEP Flap Breast Reconstruction—Preliminary Results of a Double-Blind Randomized Controlled Trial. The Breast 2024, 74, 103691. [Google Scholar] [CrossRef]
- Santanelli, F.; Longo, B.; Angelini, M.; Laporta, R.; Paolini, G. Prospective Computerized Analyses of Sensibility in Breast Reconstruction with Non-Reinnervated DIEP Flap. Plast. Reconstr. Surg. 2011, 127, 1790–1795. [Google Scholar] [CrossRef]

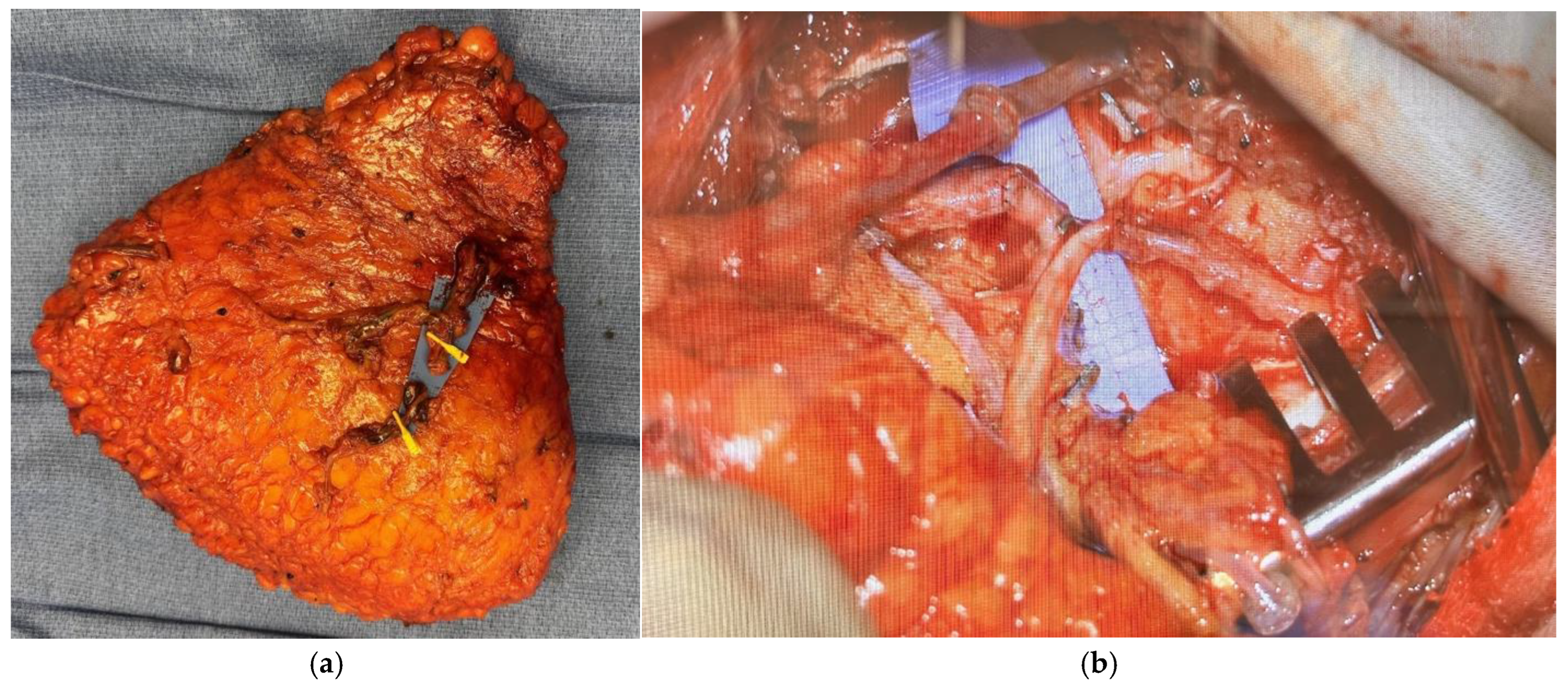
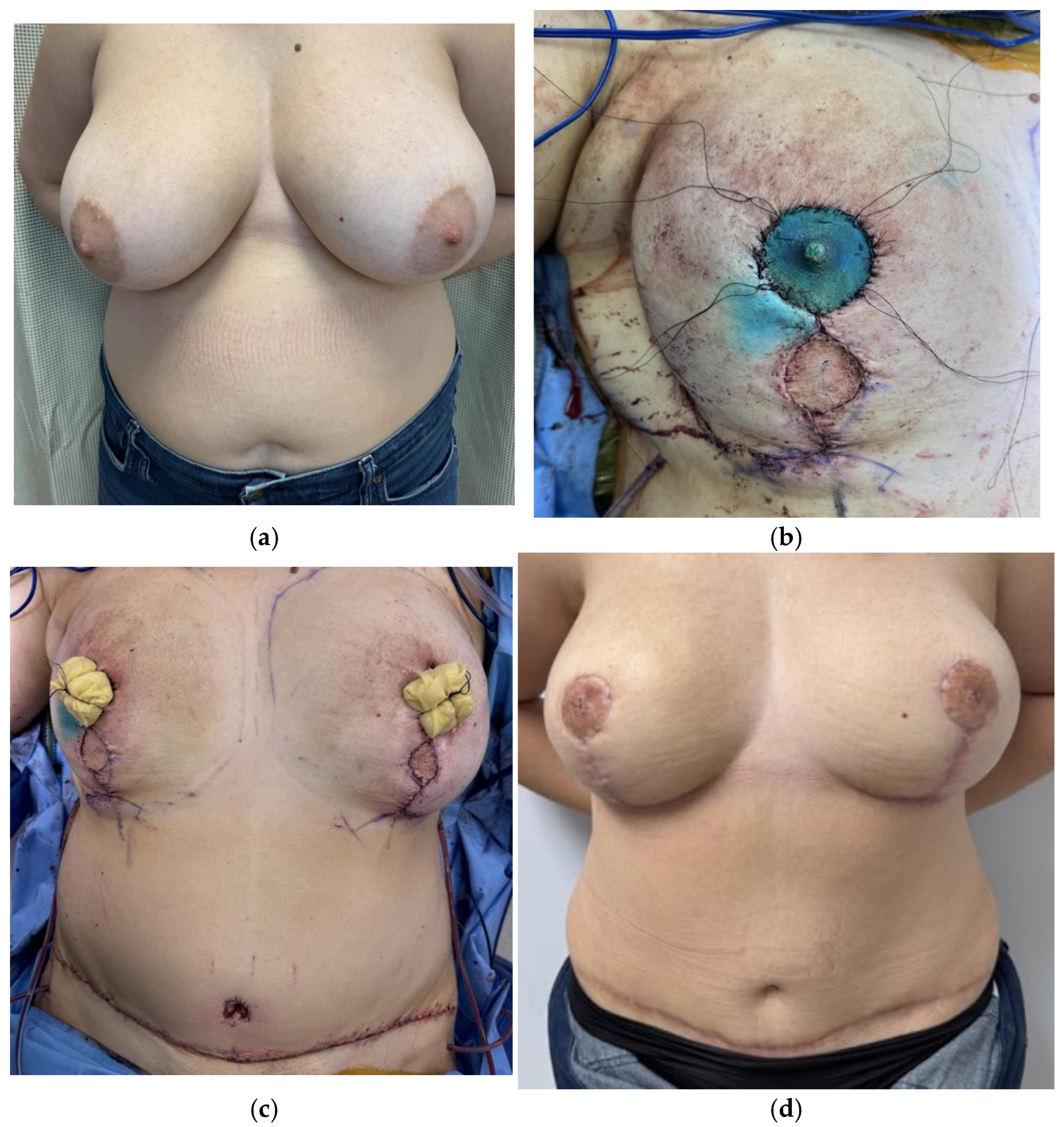
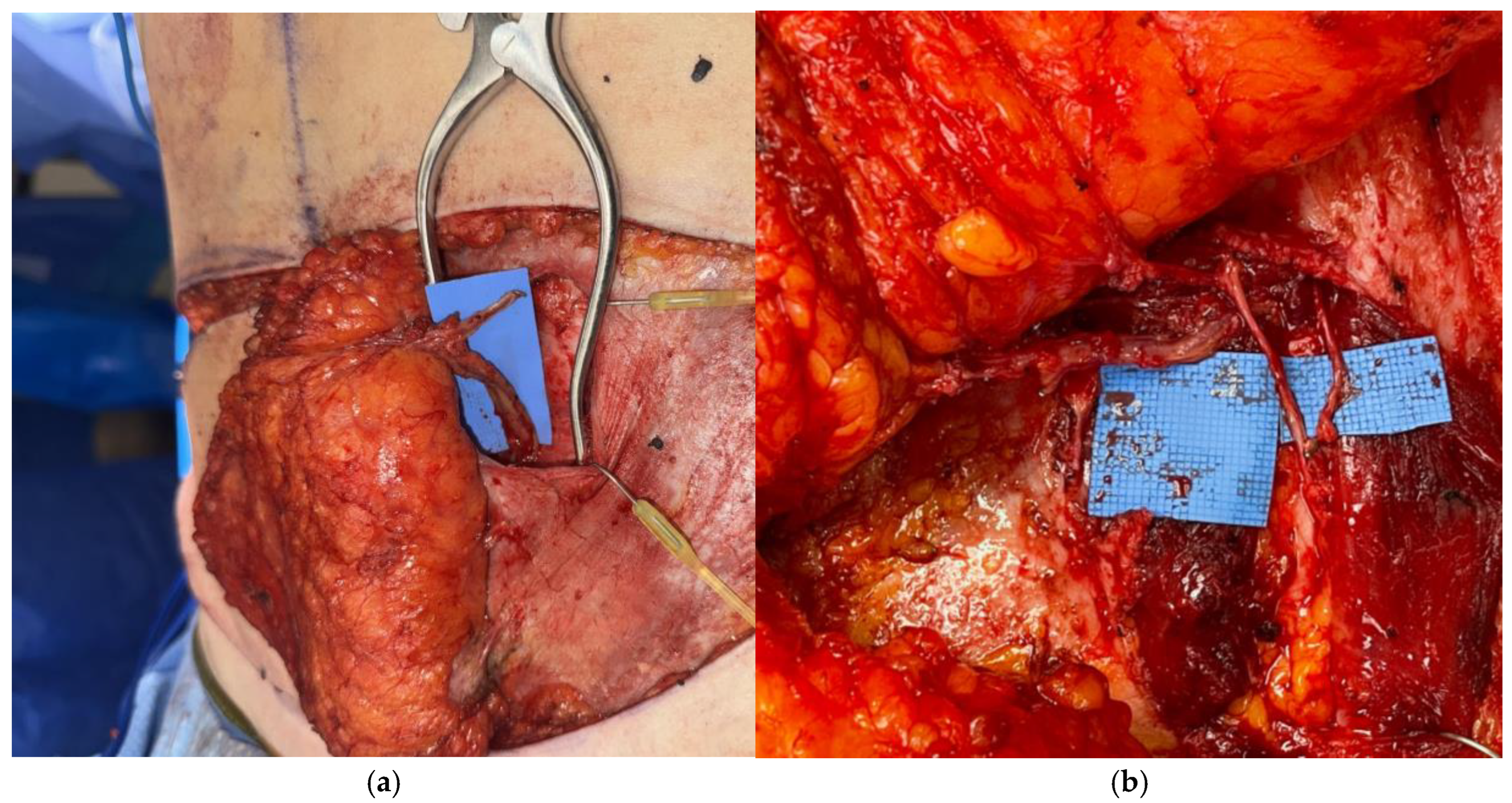
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, C.; Daar, D.A.; Salibian, A.A. Pushing the DIEP Envelope: Where Are We Now? J. Clin. Med. 2025, 14, 6248. https://doi.org/10.3390/jcm14176248
Clark C, Daar DA, Salibian AA. Pushing the DIEP Envelope: Where Are We Now? Journal of Clinical Medicine. 2025; 14(17):6248. https://doi.org/10.3390/jcm14176248
Chicago/Turabian StyleClark, Chase, David A. Daar, and Ara A. Salibian. 2025. "Pushing the DIEP Envelope: Where Are We Now?" Journal of Clinical Medicine 14, no. 17: 6248. https://doi.org/10.3390/jcm14176248
APA StyleClark, C., Daar, D. A., & Salibian, A. A. (2025). Pushing the DIEP Envelope: Where Are We Now? Journal of Clinical Medicine, 14(17), 6248. https://doi.org/10.3390/jcm14176248






