Proximal vs. Recipient Site for Vascular Lymph Node Transfers for Breast Cancer-Related Lymphedema: A Meta-Analysis and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection (Screening and Exclusion Process)
2.5. Data Collection and Extraction
2.6. Outcomes Assessed
2.7. Risk of Bias and Quality Assessment
2.8. Data Synthesis and Statistical Analysis
2.9. Use of Generative AI and Software
2.10. Publication Bias
2.11. PRISMA Checklist Compliance
3. Results
3.1. Search Methodology
3.2. Patient Demographics and Clinical Characteristics
3.3. Complications
3.4. Clinical Outcomes by Placement Strategy
3.5. Scar Release + VLNT
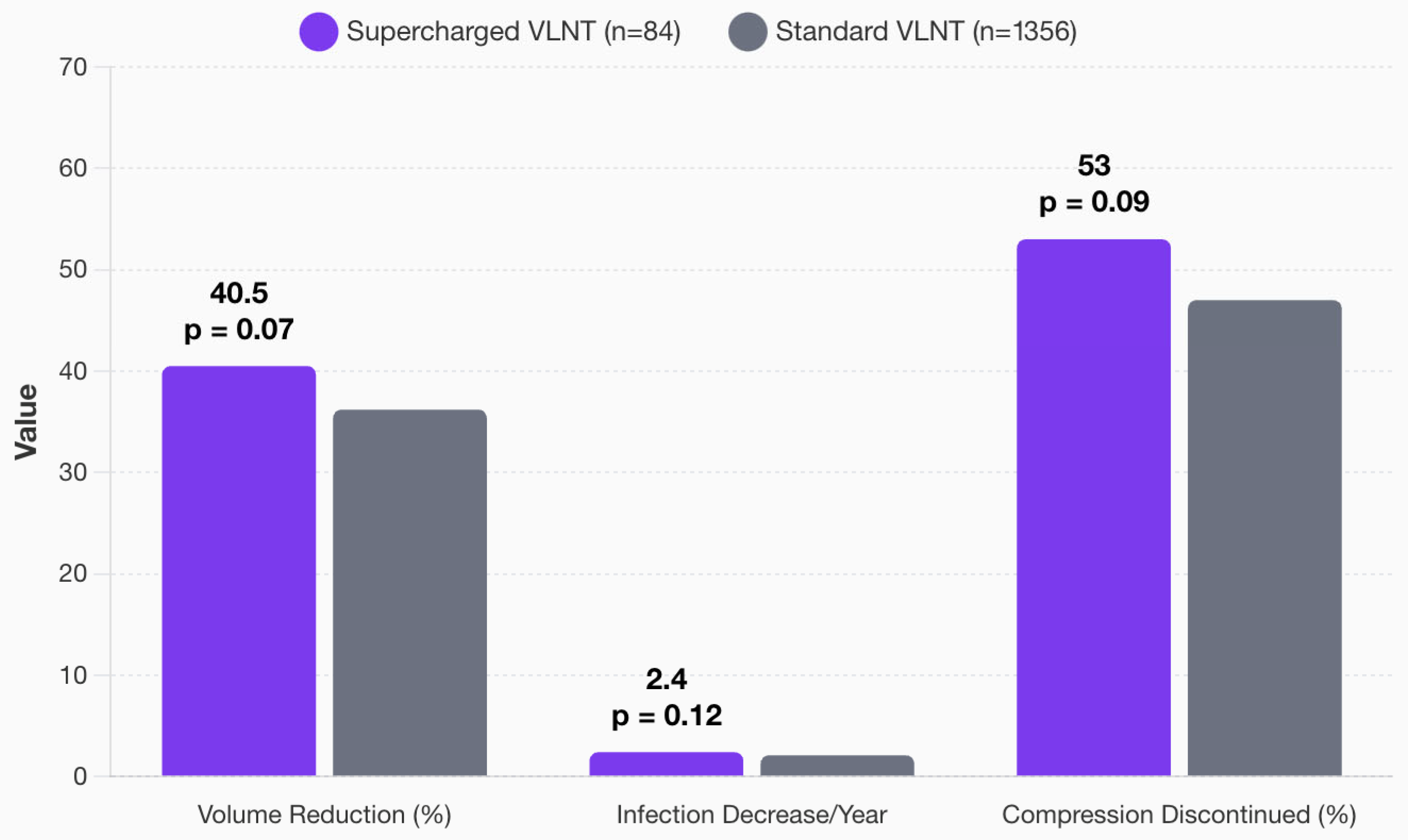
3.6. Supercharging of VLNT
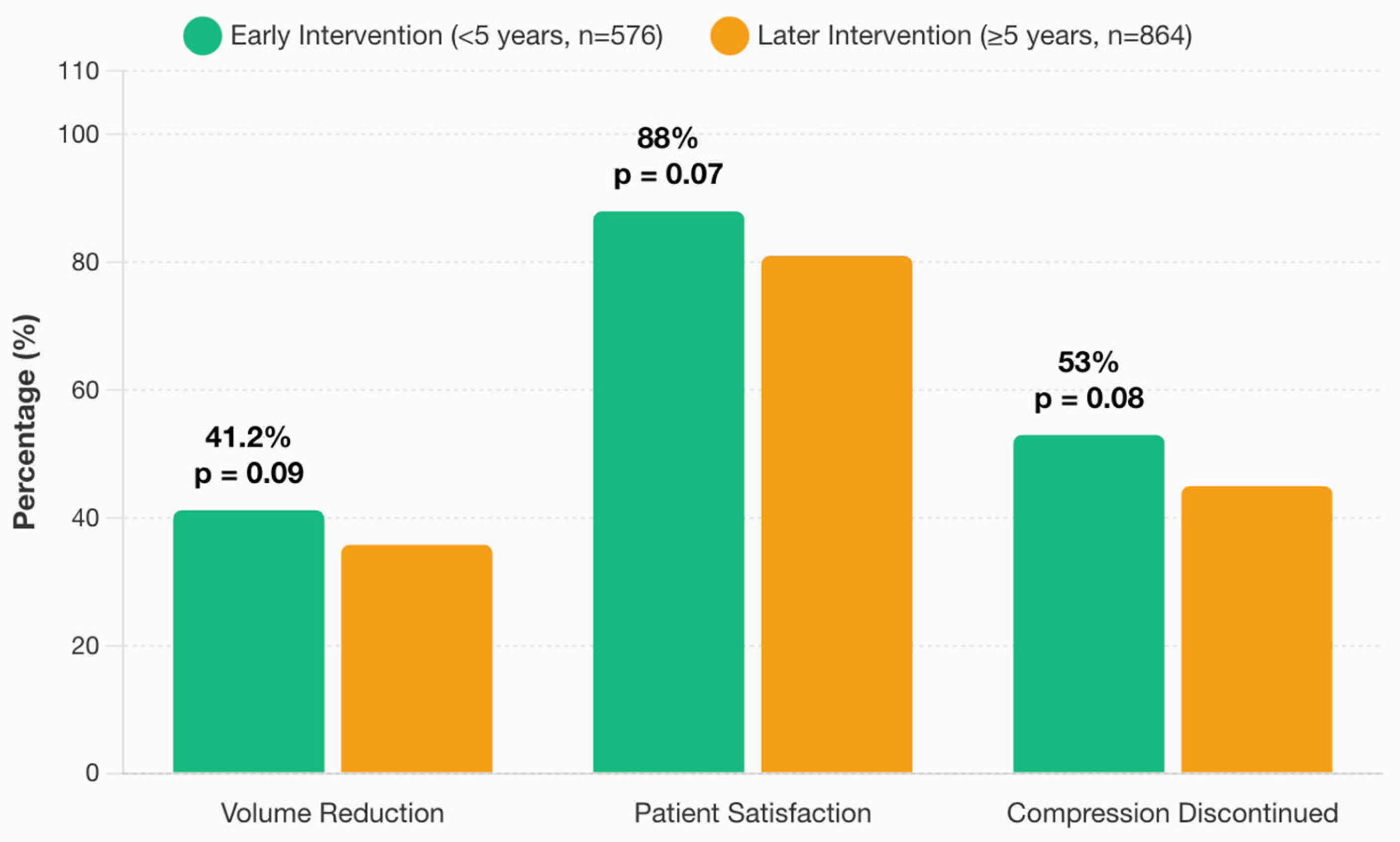
3.7. Timing of VLNT
3.8. Comprehensive Outcome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALND | Axillary lymph node dissection |
| BCRL | Breast cancer-related lymphedema |
| BMI | Body mass index |
| DIEP | Deep inferior epigastric artery perforator |
| LVA | Lymphovenous anastomosis |
| LVB | Lymphovenous bypass |
| MLD | Manual lymphatic drainage |
| pLVB | Prophylactic lymphovenous bypass |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| VLNT | Vascularized lymph node transfer |
References
- Akita, S.; Mitsukawa, N.; Kuriyama, M.; Kubota, Y.; Hasegawa, M.; Tokumoto, H.; Satoh, K.; Ishigaki, T.; Yamaji, Y.; Sugie, T. Comparison of vascularized supraclavicular lymph node transfer and lymphaticovenular anastomosis for advanced stage lower extremity lymphedema. Ann. Plast. Surg. 2015, 74, 573–579. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Cho, M.-J.; Flores Garcia, J.; Myung, Y.; Cha, H.G.; Hayashi, A.; Hong, J.P.; Skoracki, R. Evolving Role of Lymphedema Surgery on Breast Reconstruction: A Systematic Review and Multi-Institutional Algorithmic Approach. J. Clin. Med. 2024, 13, 6518. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-J.; Senger, J.-L.; Park, K.U.; Hansotia, K.; Chratian, S.; Kadle, R.; Skoracki, R.J. Preventing Breast Cancer-Related Lymphedema: A Comprehensive Analysis of a 9-Year Single-Center Experience of Prophylactic Lymphovenous Bypass. Ann. Surg. Oncol. 2025, 32, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Tokumoto, H.; Yamaji, Y.; Sasahara, Y.; Kubota, Y.; Kubo, M.; Kuriyama, M.; Mitsukawa, N. Contribution of simultaneous breast reconstruction by deep inferior epigastric artery perforator flap to the efficacy of vascularized lymph node transfer in patients with breast cancer-related lymphedema. J. Reconstr. Microsurg. 2017, 33, 571–578. [Google Scholar] [CrossRef]
- Aljaaly, H.A.; Fries, C.A.; Cheng, M.H. Dorsal wrist placement for vascularized submental lymph node transfer significantly improves breast cancer-related lymphedema. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2149. [Google Scholar] [CrossRef]
- Patel, K.M.; Lin, C.Y.; Cheng, M.H. From theory to evidence: Long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J. Reconstr. Microsurg. 2015, 31, 26–30. [Google Scholar] [CrossRef]
- Arrivé, L.; Derhy, S.; El Mouhadi, S.; Monnier-Cholley, L.; Menu, Y.; Becker, C. Noncontrast Magnetic Resonance Lymphography. J. Reconstr. Microsurg. 2016, 32, 80–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciudad, P.; Date, S.; Lee, M.H.; Lo Torto, F.; Nicoli, F.; Sapountzis, S.; Cheng, H.T.; Agko, M.; Chen, H.C. Double gastroepiploic vascularized lymph node transfers to middle and distal limb for the treatment of lymphedema. Microsurgery 2017, 37, 771–779. [Google Scholar] [CrossRef]
- Ciudad, P.; Manrique, O.J.; Bustos, S.S.; Vargas, M.I.; Reynaga, C.; Agko, M.; Huang, T.C.T.; Benites, E.F.; Mayer, H.F.; Forte, A.J. Combined microvascular breast and lymphatic reconstruction with deep inferior epigastric perforator flap and gastroepiploic vascularized lymph node transfer for postmastectomy lymphedema patients. Gland Surg. 2020, 9, 512–520. [Google Scholar] [CrossRef]
- Chocron, Y.; Azzi, A.J.; Bouhadana, G.; Kokosis, G.; Vorstenbosch, J. Axilla versus wrist as the recipient site in vas-cularized lymph node transfer for breast cancer-related lymphedema: A systematic review and meta-analysis. J. Reconstr. Microsurg. 2022, 38, 539–548. [Google Scholar] [CrossRef]
- Brown, S.; Brown, T.; Cederna, P.S.; Rohrich, R.J. A prospective study on the safety and efficacy of vascularized lymph node transplant. Ann. Surg. 2022, 276, 635–643. [Google Scholar] [CrossRef]
- Cheng, M.H.; Chen, S.C.; Henry, S.L.; Tan, B.K.; Lin, M.C.Y.; Huang, J.J. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: Flap anatomy, recipient sites, and outcomes. Plast. Reconstr. Surg. 2013, 131, 1286–1298. [Google Scholar] [CrossRef]
- Ciudad, P.; Escandón, J.M.; Duarte-Bateman, D.; Escandón, L.; Maruccia, M.; Forte, A.J.; Bustos, S.S.; Manrique, O.J.; Langstein, H.N. Surgical management of breast cancer-related lymphedema: A narrative review of contemporary practices. Ann. Transl. Med. 2023, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, P.; Manrique, O.J.; Adabi, K.; Huang, T.C.; Agko, M.; Trignano, E.; Chang, W.; Chen, T.; Salgado, C.J.; Chen, H. Combined double vascularized lymph node transfers and modified radical reduction withpreservation of perforators for advanced stages of lymphedema. J. Surg. Oncol. 2019, 119, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, P.; Manrique, O.J.; Bustos, S.S.; Coca, J.J.P.; Chang, C.; Shih, P.; Nicoli, F.; Lo Torto, F.; Maruccia, M.; Chen, H.C. Comparisons in long-term clinical outcomes among patients with upper or lower extremity lymphedema treated with diverse vascularized lymph node transfer. Microsurgery 2020, 40, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Wee, C.; Meyerson, J.; Eiferman, D.; Skoracki, R. Vascularized jejunal mesenteric lymph node transfer: A novel surgical treatment for extremity lymphedema. J. Am. Coll. Surg. 2017, 225, 650–657. [Google Scholar] [CrossRef]
- Coroneos, C.J.; Wong, F.C.; DeSnyder, S.M.; Shaitelman, S.F.; Schaverien, M.V. Correlation of L-Dex bioimpedance spectroscopy with limb volume and lymphatic function in lymphedema. Lymphat. Res. Biol. 2019, 17, 301–307. [Google Scholar] [CrossRef]
- Dayan, J.H.; Dayan, E.; Smith, M.L. Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast. Reconstr. Surg. 2015, 135, 277–285. [Google Scholar] [CrossRef]
- De Brucker, B.; Zeltzer, A.; Seidenstuecker, K.; Hendrickx, B.; Adriaenssens, N.; Hamdi, M. Breast cancer-related lymphedema: Quality of life after lymph node transfer. Plast. Reconstr. Surg. 2016, 137, 1673–1680. [Google Scholar] [CrossRef]
- Di Taranto, G.; Coleman, G.J.; Hardwicke, J.; Wallis, K.L.; Skillman, J. A comparative study between deep inferior epigastric artery perforator flap breast reconstruction and DIEP flap breast reconstruction coupled with vascularized lymph node transfer: Improving the quality of life of patients with breast cancer related lymphedema without affecting donor site outcomes. Microsurgery 2023, 43, 213–221. [Google Scholar] [CrossRef]
- Dionyssiou, D.; Demiri, E.; Tsimponis, A.; Sarafis, A.; Mpalaris, V.; Tatsidou, G.; Arsos, G. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res. Treat. 2016, 156, 73–79. [Google Scholar] [CrossRef]
- Dionyssiou, D.; Demiri, E.; Sarafis, A.; Goula, C.-O.; Tsimponis, A.; Arsos, G. Functional lymphatic reconstruction with the “Selected Lymph Node” technique guided by a SPECT-CT lymphoscintigraphy. J. Surg. Oncol. 2019, 120, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Engel, H.; Lin, C.Y.; Huang, J.J.; Cheng, M.H. Outcomes of lymphedema microsurgery for breast cancer-related lymphedema with or without microvascular breast reconstruction. Ann. Surg. 2018, 268, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Garcés, M.M.; Mirapeix, R.; Pons, G.; Sadri, A.; Masià, J. A comprehensive review of the natural lymphaticovenous communications and their role in lymphedema surgery. J. Surg. Oncol. 2016, 113, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ghazaleh, A.A.; Handschin, T.M.; Buckowiecki, J.; Chammartin, F.S.; Andree, C.; Schaefer, D.J.; Haug, M.; Kappos, E.A.; Seidenstuecker, K. Combining reconstructive and ablative surgical treatment of chronic breast cancer-relatedlymphedema (BCRL): Safe and effective. Breast Cancer Res. Treat. 2023, 197, 83–92. [Google Scholar] [CrossRef]
- Gratzon, A.; Schultz, J.; Secrest, K.; Lee, K.; Feiner, J.; Klein, R.D. Clinical and psychosocial outcomes of vascularized lymph node transfer for the treatment of upper extremity lymphedema after breast cancer therapy. Ann. Surg. Oncol. 2017, 24, 1475–1481. [Google Scholar] [CrossRef]
- Hamdi, M.; Ramaut, L.; De Baerdemaeker, R.; Zeltzer, A. Decreasing donor site morbidity after groin VLNT: Lessons from a 12-year experience and literature review. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 540–548. [Google Scholar] [CrossRef]
- Ho, O.A.; Lin, C.Y.; Pappalardo, M.; Cheng, M.H. Comparisons of submental and groin vascularized lymph node flaps transfer for breast cancer-related lymphedema. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1923. [Google Scholar] [CrossRef]
- Ho, O.A.; Chu, S.-Y.; Huang, Y.-L.; Chen, W.-H.; Lin, C.-Y.; Cheng, M.-H. Effectiveness of Vascularized Lymph Node Transfer for Extremity Lymphedema Using Volumetric and Circumferential Differences. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2003. [Google Scholar] [CrossRef]
- Inbal, A.; Teven, C.M.; Chang, D.W. Latissimus dorsi flap with VLNT for lymphedema treatment: Technique, outcomes, indications and literature review. J. Surg. Oncol. 2017, 115, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Bravo, M.G.; Granoff, M.D.; Kang, C.O.; Critchlow, J.F.; Tsai, L.L.; Lee, B.T.; Singhal, D. Flow-through Omental Flap for Vascularized Lymph Node Transfer: A Novel Surgical Approach for Delayed Lymphatic Reconstruction. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2436. [Google Scholar] [CrossRef]
- Johnson, A.R.; Kimball, S.; Epstein, S.; Recht, A.; Lin, S.J.; Lee, B.T.; Singhal, D. Lymphedema incidence after axillary lymph node dissection: Quantifying the impact of radiation and the lymphatic microsurgical preventive healing approach. Ann. Plast. Surg. 2019, 82 (Suppl. S4), S234–S241. [Google Scholar] [CrossRef] [PubMed]
- Kappos, E.A.; Fabi, A.; Halbeisen, F.S.; Abu-Ghazaleh, A.; Stoffel, J.; Aufmesser-Freyhardt, B.; Simmen, H.P.; Scaglioni, M.F.; Haug, M. VLNT versus LV A for chronic BCRL: A retrospective cohort study of effectiveness over time. Breast Cancer Res. Treat. 2025, 210, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kappos, E.A.; Haas, Y.; Schulz, A.; Peters, F.; Savanthrapadian, S.; Stoffel, J.; Katapodi, M.C.; Mucklow, R.; Kaiser, B.; Haumer, A.; et al. The LYMPH trial: Comparing microsurgical with conservative treatment for chronic breast cancer-associated lymphoedema—Study protocol of a pragmatic randomised international multicentre superiority trial. BMJ Open 2025, 15, e090662. [Google Scholar] [CrossRef]
- Kenworthy, E.O.; Nelson, J.A.; Verma, R.; Mbabuike, J.; Mehrara, B.J.; Dayan, J.H. Double vascularized omentum lymphatic transplant (VOLT) for the treatment of lymphedema. J. Surg. Oncol. 2018, 117, 1413–1419. [Google Scholar] [CrossRef]
- Lin, C.Y.; Liu, H.E.; Cheng, M.H. Factors associated with professional healthcare advice seeking in breast cancer-related lymphedema. J. Surg. Oncol. 2020, 121, 67–74. [Google Scholar] [CrossRef]
- Liu, H.L.; Pang, S.Y.; Lee, C.C.; Wong, M.M.K.; Chung, H.P.; Chan, Y.W. Orthotopic transfer of vascularized groin lymph node flap in the treatment of breast cancer-related lymphedema: Clinical results, lymphoscintigraphy findings, and proposed mechanism. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1033–1040. [Google Scholar] [CrossRef]
- Maldonado, A.A.; Chen, R.; Chang, D.W.; Mirza, N.Q. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J. Surg. Oncol. 2017, 115, 68–71. [Google Scholar] [CrossRef]
- Maruccia, M.; Elia, R.; Ciudad, P.; Nacchiero, E.; Nicoli, F.; Vestita, M.; Chen, H.C.; Giudice, G. Postmastectomy upper limb lymphedema: Combined vascularized lymph node transfer and scar release with fat graft expedites surgical and patients’ related outcomes. A retrospective comparative study. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 892–901. [Google Scholar] [CrossRef]
- Maruccia, M.; Pezzolla, A.; Nacchiero, E.; Dicillo, P.; Macchia, L.; Fiore, P.; Giudice, G.; Elia, R. Efficacy and early results after combining laparoscopic harvest of double gastroepiploic lymph node flap and active physiotherapy for lower extremity lymphedema. Microsurgery 2019, 39, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Masia, J.; Pons, G.; Nardulli, M.L. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J. Reconstr. Microsurg. 2016, 32, 16–27. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T.; Giron, G.L.; Sampson, M.R.; Brockway, J.P.; Hurley, K.E.; Beard, J.R.; Portman, S.G.; Wilcox, J.; et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J. Clin. Oncol. 2008, 26, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Montag, E.; Okada, A.Y.; Arruda, E.G.P.; Fonseca, A.S.; Bromley, M.; Munhoz, A.M.; Busnardo, F.F.; Gemperli, R. Influence of vascularized lymph node transfer (VLNT) flap positioning on the response to breast cancer-related lymphedema treatment. Rev. Col. Bras. Cir. 2019, 46, e2156. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Arvanitakis, M.; Chen, Y.C.; Giovanoli, P.; Chia-Shen Yang, J.; Chang, E.I. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery 2018, 38, 222–229. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; DeSnyder, S.M.; Klimberg, S.; Alatriste, M.; Boccardo, F.; Smith, M.L.; Stout, N.L.; Staley, A.C.; Thiruchelvam, P.T.R.; Hutchison, N.A.; et al. Considerations for Clinicians in the Diagnosis, Prevention, and Treatment of Breast Cancer-Related Lymphedema, Recommendations from an Expert Panel: Part 2: Preventive and Therapeutic Options. Ann. Surg. Oncol. 2017, 24, 2827–2835. [Google Scholar] [CrossRef]
- Chang, D.W.; Dayan, J.; Greene, A.K.; MacDonald, J.K.; Masia, J.; Mehrara, B.; Neligan, P.C.; Nguyen, D. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast. Reconstr. Surg. 2021, 147, 975–993. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical Treatment of Lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Patel, K.M.; Lin, C.Y.; Cheng, M.H. A prospective evaluation of lymphedema-specific quality-of-life outcomes fol-lowing vascularized lymph node transfer. Ann. Surg. Oncol. 2015, 22, 2424–2430. [Google Scholar] [CrossRef]
- Seidenstuecker, K.; Fertsch, S.; Ghazaleh, A.A.; Fabi, A.; Stoffel, J.; Bukowiecki, J.; Wolter, A.; Aghlmandi, S.; Nadella, A.; Halbeisen, F.S.; et al. Improving quality of life after breast cancer: A comparison of two microsurgical treatment options for BCRL. Clin. Exp. Med. 2024, 24, 82. [Google Scholar] [CrossRef]
- Visconti, G.; Tartaglione, G.; Bartoletti, R.; Salgarello, M. Compartimental harvesting of dual lymph node flap from the right supraclavicular area for the treatment of lower extremity lymphedema: A case series. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 211–215. [Google Scholar] [CrossRef]
- Shesol, B.F.; Nakashima, R.; Alavi, A.; Hamilton, R.W. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast. Reconstr. Surg. 1979, 63, 817–823. [Google Scholar] [CrossRef]

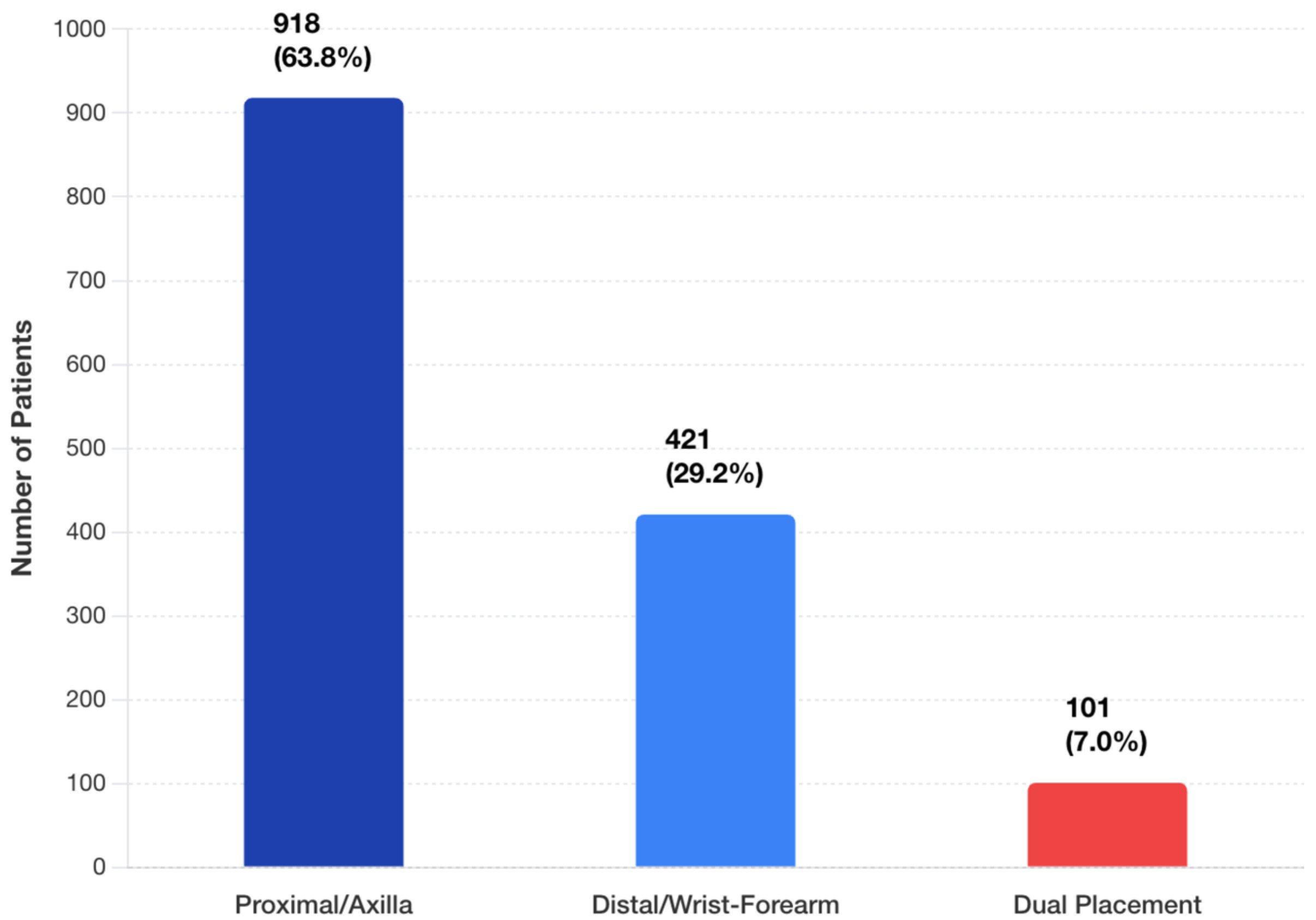



| Characteristic | Total (n = 1440) | Proximal (n = 918) | Distal (n = 421) | Both (n = 101) |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean ± SD | 54.0 ± 8.2 | 55.2 ± 8.5 | 53.8 ± 7.8 | 54.5 ± 8.0 |
| Range | 16–80 | 18–80 | 16–78 | 22–75 |
| BMI, kg/m2, mean ± SD | 27.1 ± 4.2 | 27.5 ± 4.3 | 27.0 ± 4.0 | 27.2 ± 4.1 |
| Range | 22.5–33.0 | 22.8–33.0 | 22.5–32.5 | 23.0–32.0 |
| Radiation therapy, n (%) | 1080 (75.0) | 780 (85.0) | 294 (69.8) | 76 (75.2) |
| Time to VLNT, y, mean ± SD | 6.0 ± 3.5 | 6.2 ± 3.6 | 5.8 ± 3.4 | 5.9 ± 3.5 |
| VLNT Donor Site, n (%) | ||||
| Inguinal | 576 (40.0) | 414 (45.1) | 147 (34.9) | 15 (14.9) |
| Submental | 245 (17.0) | 138 (15.0) | 84 (20.0) | 23 (22.8) |
| Supraclavicular | 173 (12.0) | 101 (11.0) | 59 (14.0) | 13 (12.9) |
| Other a | 446 (31.0) | 265 (28.9) | 131 (31.1) | 50 (49.5) |
| Surgical Technique | ||||
| Recipient vessels, n (%) | ||||
| Thoracodorsal | — | 643 (70.0) | — | — b |
| Radial | — | — | 211 (50.1) | — b |
| Ulnar/other | — | 275 (30.0) | 210 (49.9) | — b |
| Anastomosis type, n (%) | ||||
| End-to-end | 1022 (71.0) | 689 (75.1) | 274 (65.1) | 60 (59.4) |
| End-to-side | 274 (19.0) | 138 (15.0) | 105 (24.9) | 31 (30.7) |
| Flow-through | 144 (10.0) | 91 (9.9) | 42 (10.0) | 10 (9.9) |
| Adjunct procedures, n (%) | ||||
| Scar release | 936 (65.0) | 734 (80.0) | 126 (29.9) | 76 (75.2) |
| Supercharging | 84 (5.8) | 46 (5.0) | 29 (6.9) | 9 (8.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senger, J.-L.B.; Rajaii, R.; Slater, C.; Cho, M.-J. Proximal vs. Recipient Site for Vascular Lymph Node Transfers for Breast Cancer-Related Lymphedema: A Meta-Analysis and Systematic Review. J. Clin. Med. 2025, 14, 7281. https://doi.org/10.3390/jcm14207281
Senger J-LB, Rajaii R, Slater C, Cho M-J. Proximal vs. Recipient Site for Vascular Lymph Node Transfers for Breast Cancer-Related Lymphedema: A Meta-Analysis and Systematic Review. Journal of Clinical Medicine. 2025; 14(20):7281. https://doi.org/10.3390/jcm14207281
Chicago/Turabian StyleSenger, Jenna-Lynn B., Ramin Rajaii, Christopher Slater, and Min-Jeong Cho. 2025. "Proximal vs. Recipient Site for Vascular Lymph Node Transfers for Breast Cancer-Related Lymphedema: A Meta-Analysis and Systematic Review" Journal of Clinical Medicine 14, no. 20: 7281. https://doi.org/10.3390/jcm14207281
APA StyleSenger, J.-L. B., Rajaii, R., Slater, C., & Cho, M.-J. (2025). Proximal vs. Recipient Site for Vascular Lymph Node Transfers for Breast Cancer-Related Lymphedema: A Meta-Analysis and Systematic Review. Journal of Clinical Medicine, 14(20), 7281. https://doi.org/10.3390/jcm14207281







