Correlation Between Urinary Osteopontin Concentration and the Mineral Content and Composition of Kidney Stones
Abstract
1. Introduction
2. Materials and Methods
2.1. Urine Analysis
2.2. Stone Specimen Analysis
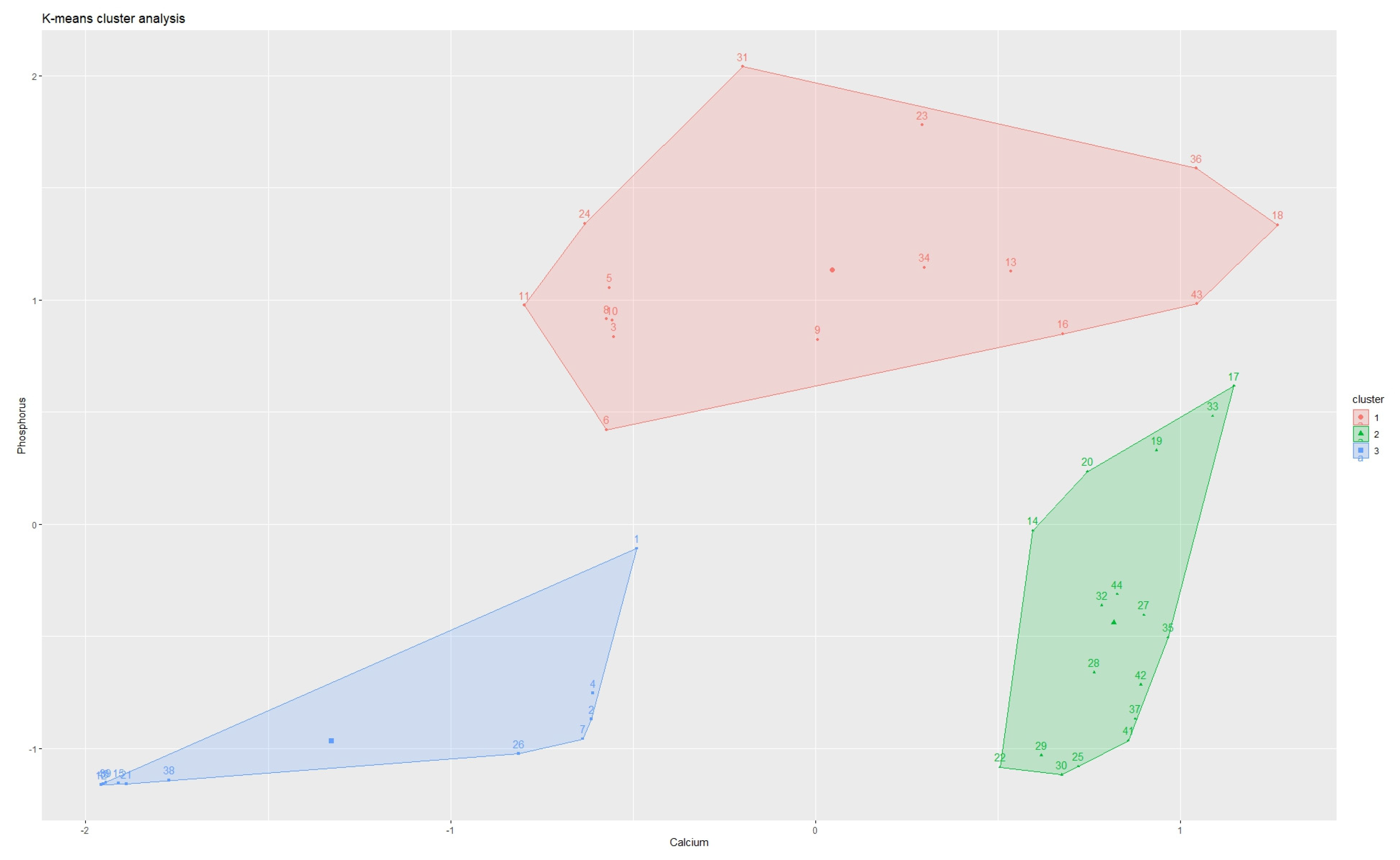
2.3. Statistical Analysis
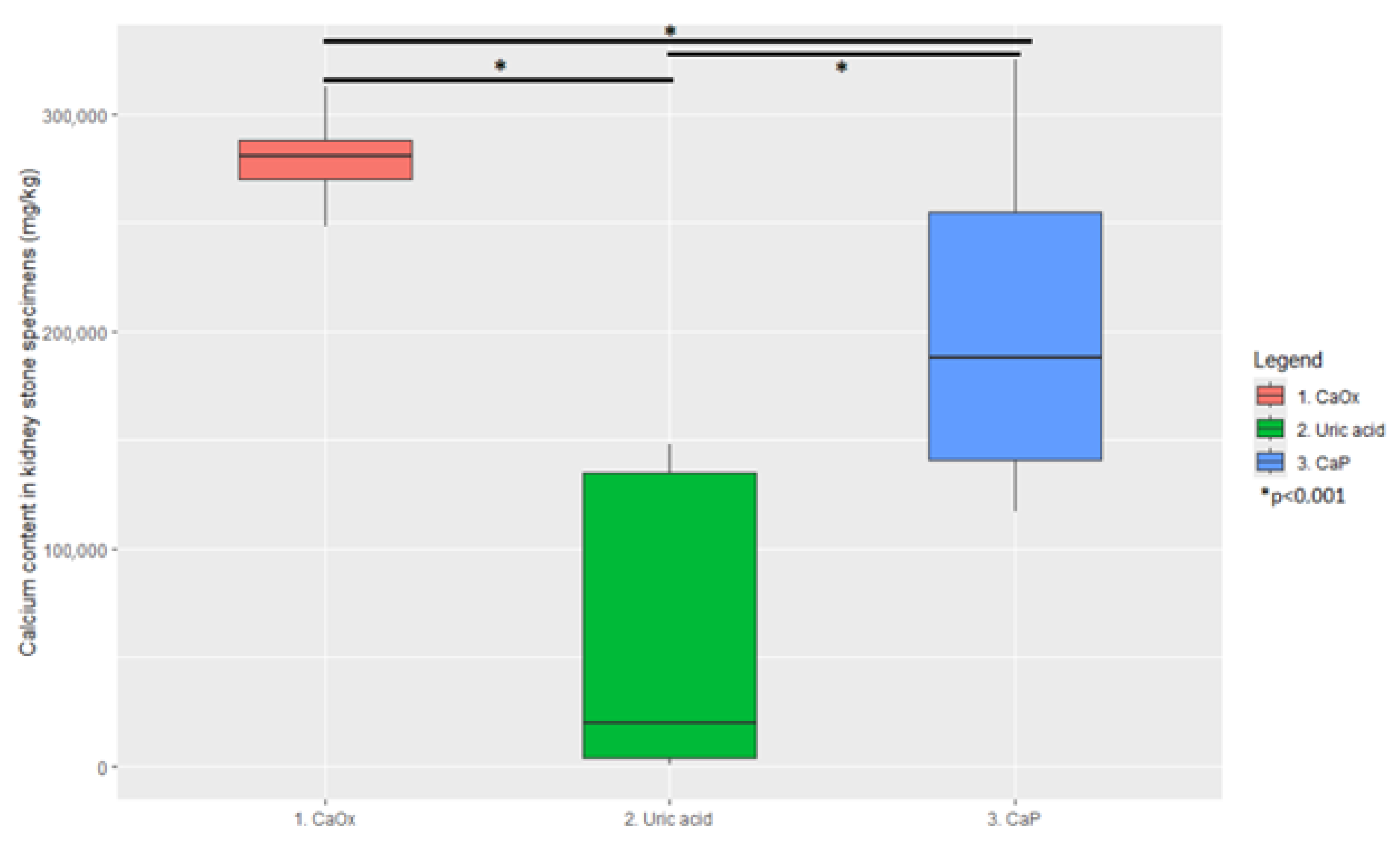
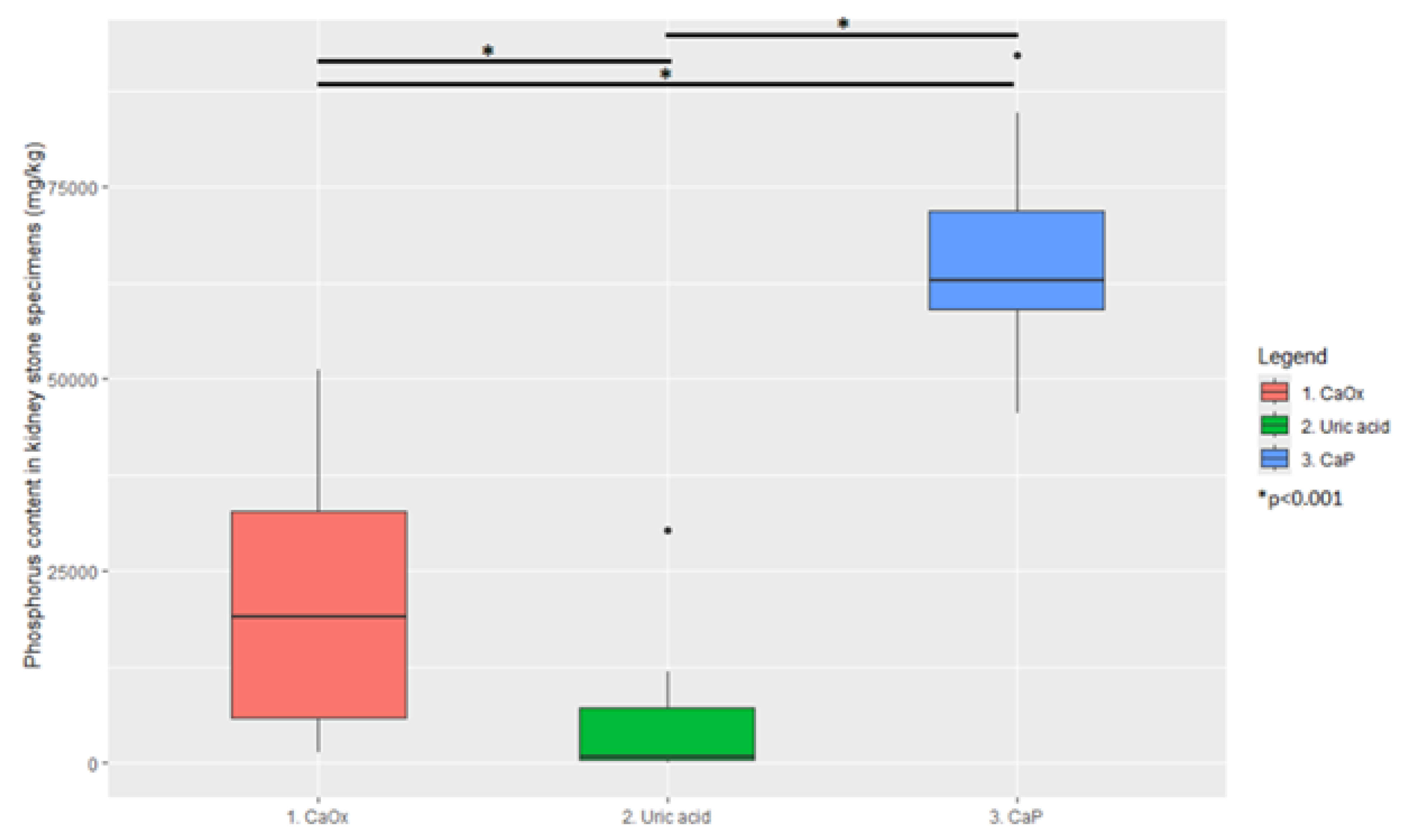
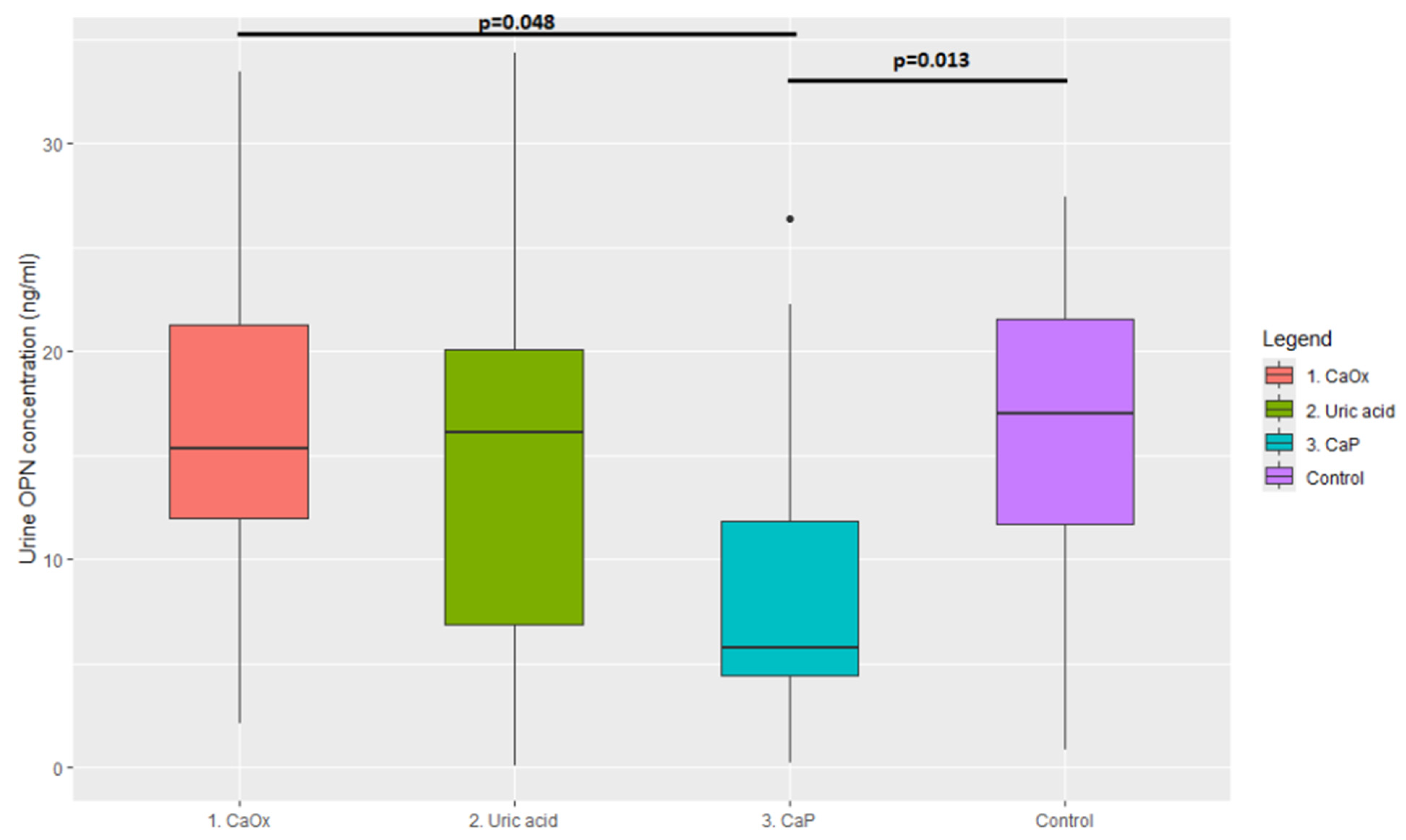
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of Kidney Stones. Healthcare 2023, 11, 424. [Google Scholar] [CrossRef]
- Hesse, A.; Brändle, E.; Wilbert, D.; Köhrmann, K.-U.; Alken, P. Study on the Prevalence and Incidence of Urolithiasis in Germany Comparing the Years 1979 vs. 2000. Eur. Urol. 2003, 44, 709–713. [Google Scholar] [CrossRef]
- Maruyama, M.; Michibata, U.; Tanaka, Y.; Yoshikawa, H.; Takano, K.; Tajiri, R.; Taguchi, K.; Hamamoto, S.; Okada, A.; Yasui, T.; et al. MP05-07 validating the theory of phase transition from calcium oxalate dihydrate to monohydrate in the stone formation: The first artificial in vivo experiment. J. Urol. 2023, 209, e46. [Google Scholar] [CrossRef]
- Pak, C.Y.C.; Poindexter, J.R.; Adams-Huet, B.; Pearle, M.S. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am. J. Med. 2003, 115, 26–32. [Google Scholar] [CrossRef]
- Spivacow, F.R.; Del Valle, E.E.; Lores, E.; Rey, P.G. Kidney stones: Composition, frequency and relation to metabolic diagnosis. Medicina 2016, 76, 343–348. [Google Scholar] [PubMed]
- Kumar, M.; Xie, J.; Chittur, K.; Riley, C. Transformation of modified brushite to hydroxyapatite in aqueous solution: Effects of potassium substitution. Biomaterials 1999, 20, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef]
- Xie, Y.; Sakatsume, M.; Nishi, S.; Narita, I.; Arakawa, M.; Gejyo, F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001, 60, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular Mechanism of Renal Stone Formation and the Critical Role Played by Modulators. Biomed Res. Int. 2013, 2013, 1–21. [Google Scholar] [CrossRef]
- Kaneko, K.; Kobayashi, R.; Yasuda, M.; Izumi, Y.; Yamanobe, T.; Shimizu, T. Comparison of matrix proteins in different types of urinary stone by proteomic analysis using liquid chromatography–tandem mass spectrometry. Int. J. Urol. 2012, 19, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Tsai, Y.S.; Huang, H.S. Atorvastatin Decreases Renal Calcium Oxalate Stone Deposits by Enhancing Renal Osteopontin Expression in Hyperoxaluric Stone-Forming Rats Fed a High-Fat Diet. Int. J. Mol. Sci. 2022, 23, 3048. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, G.; Yang, B.; Li, P.; Yang, T.; Wu, Y.; Yang, X.; Liu, J.; Li, J. Mechanism of ketotifen fumarate inhibiting renal calcium oxalate stone formation in SD rats. Biomed. Pharmacother. 2022, 151, 113147. [Google Scholar] [CrossRef]
- Hedgepeth, R.C.; Yang, L.; Resnick, M.I.; Marengo, S.R. Expression of proteins that inhibit calcium oxalate crystallization in vitro in the urine of normal and stone-forming individuals. Am. J. Kidney Dis. 2001, 37, 104–112. [Google Scholar] [CrossRef]
- Anan, G.; Yoneyama, T.; Noro, D.; Tobisawa, Y.; Hatakeyama, S.; Yoneyama, M.S.; Yamamoto, H.; Imai, A.; Iwamura, H.; Kohada, Y.; et al. The Impact of Glycosylation of Osteopontin on Urinary Stone Formation. Int. J. Mol. Sci. 2019, 21, 93. [Google Scholar] [CrossRef]
- Jaromin, M.; Cichocki, M.; Konecki, T.; Kutwin, P.; Maniukiewicz, W.; Wysocki, P.; Gajek, M.; Szynkowska-Jóźwik, M.I.; Moczulski, D. Elevated Zinc and Potassium Levels in Renal Calculi Indicate Distinct Pathophysiological Mechanisms in Urolithiasis. Pathophysiology 2025, 32, 23. [Google Scholar] [CrossRef]
- Moszczuk, B.; Krata, N.; Rudnicki, W.; Foroncewicz, B.; Cysewski, D.; Pączek, L.; Kaleta, B.; Mucha, K. Osteopontin-A Potential Biomarker for IgA Nephropathy: Machine Learning Application. Biomedicines 2022, 10, 734. [Google Scholar] [CrossRef]
- Wirestam, L.; Enocsson, H.; Skogh, T.; Padyukov, L.; Jönsen, A.; Urowitz, M.B.; Gladman, D.D.; Romero-Diaz, J.; Bae, S.-C.; Fortin, P.R.; et al. Osteopontin and Disease Activity in Patients with Recent-onset Systemic Lupus Erythematosus: Results from the SLICC Inception Cohort. J. Rheumatol. 2019, 46, 492–500. [Google Scholar] [CrossRef]
- Kaleta, B.; Krata, N.; Zagożdżon, R.; Mucha, K. Osteopontin Gene Polymorphism and Urinary OPN Excretion in Patients with Immunoglobulin A Nephropathy. Cells 2019, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Van Nynatten, L.R.; Miller, M.R.; Patel, M.A.; Daley, M.; Filler, G.; Badrnya, S.; Miholits, M.; Webb, B.; McIntyre, C.W.; Fraser, D.D. A novel multiplex biomarker panel for profiling human acute and chronic kidney disease. Sci. Rep. 2023, 13, 21210. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.A.Y.; Shokeir, A.A.; Mortada, W.I.; Awadalla, A.; Barakat, L.A.A. Effect of Copper and Zinc Ions on Biochemical and Molecular Characteristics of Calcium Oxalate Renal Stones: A Controlled Clinical Study. Biol. Trace Elem. Res. 2024, 202, 410–422. [Google Scholar] [CrossRef]
- Wu, F.; Cheng, Y.; Zhou, J.; Liu, X.; Lin, R.; Xiang, S.; Liu, Z.; Wang, C. Zn2+ regulates human oxalate metabolism by manipulating oxalate decarboxylase to treat calcium oxalate stones. Int. J. Biol. Macromol. 2023, 234, 123320. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Ashayeri, N.Y.; Moore, F.; Irani, D.; Asadi, S.; Zarasvandi, A.; Salari, M. Mineralogical Composition of Urinary Stones and Their Frequency in Patients: Relationship to Gender and Age. Minerals 2016, 6, 131. [Google Scholar] [CrossRef]
- González-Enguita, C.; García-Giménez, R. Kidney Stones: Crystal Characterization. Crystals 2024, 14, 238. [Google Scholar] [CrossRef]
- Williams, J.C.; Saw, K.C.; Paterson, R.F.; Hatt, E.K.; McAteer, J.A.; Lingeman, J.E. Variability of renal stone fragility in shock wave lithotripsy. Urology 2003, 61, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Huang, Z.; Wang, G.; Sun, X.; Wu, Y.; Yang, B.; Yang, T.; Liu, J.; Li, P.; Li, J. Osteopontin: An important protein in the formation of kidney stones. Front. Pharmacol. 2022, 13, 1036423. [Google Scholar] [CrossRef]
- Chang, L.; Feng, T.; Li, J.; Dou, C.; Wei, J.; Guo, Y. Regulation of osteopontin expression in a rat model of urolithiasis. Chin. Med. J. 2001, 114, 829–832. [Google Scholar] [PubMed]
- Wesson, J.A.; Johnson, R.J.; Mazzali, M.; Beshensky, A.M.; Stietz, S.; Giachelli, C.; Liaw, L.; Alpers, C.E.; Couser, W.G.; Kleinman, J.G.; et al. Osteopontin Is a Critical Inhibitor of Calcium Oxalate Crystal Formation and Retention in Renal Tubules. J. Am. Soc. Nephrol. 2003, 14, 139–147. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Putnis, C.V. Energetic Basis for Inhibition of Calcium Phosphate Biomineralization by Osteopontin. J. Phys. Chem. B 2017, 121, 5968–5976. [Google Scholar] [CrossRef]
- Wallace, B.; Chmiel, J.A.; Al, K.F.; Bjazevic, J.; Burton, J.P.; Goldberg, H.A.; Razvi, H. The Role of Urinary Modulators in the Development of Infectious Kidney Stones. J. Endourol. 2023, 37, 358–366. [Google Scholar] [CrossRef]
- Hoyer, J.R.; Asplin, J.R.; Otvos, L. Phosphorylated osteopontin peptides suppress crystallization by inhibiting the growth of calcium oxalate crystals. Kidney Int. 2001, 60, 77–82. [Google Scholar] [CrossRef]
- Li, S.; Wang, L. Phosphorylated osteopontin peptides inhibit crystallization by resisting the aggregation of calcium phosphate nanoparticles. CrystEngComm 2012, 14, 8037. [Google Scholar] [CrossRef]
- Iline-Vul, T.; Nanda, R.; Mateos, B.; Hazan, S.; Matlahov, I.; Perelshtein, I.; Keinan-Adamsky, K.; Althoff-Ospelt, G.; Konrat, R.; Goobes, G. Osteopontin regulates biomimetic calcium phosphate crystallization from disordered mineral layers covering apatite crystallites. Sci. Rep. 2020, 10, 15722. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; Zhang, S.; Jansson, K.P.; Fields, T.A.; Boulanger, J.; Liu, S.; Rowe, P.S. Critical Role of Osteopontin in Maintaining Urinary Phosphate Solubility in CKD. Kidney360 2022, 3, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Nomura, S.; Saeki, Y.; Higashibata, Y.; Hamamoto, S.; Hirose, M.; Itoh, Y.; Yasui, T.; Tozawa, K.; Kohri, K. Morphological Conversion of Calcium Oxalate Crystals Into Stones Is Regulated by Osteopontin in Mouse Kidney. J. Bone Miner. Res. 2008, 23, 1629–1637. [Google Scholar] [CrossRef]
- Safran, J.B.; Butler, W.T.; Farach-Carson, M.C. Modulation of Osteopontin Post-translational State by 1,25-(OH)2-Vitamin D3. J. Biol. Chem. 1998, 273, 29935–29941. [Google Scholar] [CrossRef][Green Version]
- Chang, P.L.; Ridall, A.L.; Prince, C.W. Calcitriol regulation of osteopontin expression in mouse epidermal cells. Endocrinology 1994, 135, 863–869. [Google Scholar] [CrossRef]
- Fukumoto, S. Phosphate metabolism and vitamin D. BoneKEy Rep. 2014, 3, 497. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Mellody, M.; Carpio, M.B.; Damoiseaux, R.; Nicholas, S.B. Osteopontin as a Biomarker in Chronic Kidney Disease. Biomedicines 2023, 11, 1356. [Google Scholar] [CrossRef]
| Study Group n = 44 | Control Group n = 22 | p-Value | |
|---|---|---|---|
| Sex (female; male) | n = 24 (55%); n = 20 (45%) | n = 11 (50%); n = 11 (50%) | |
| Median age (years) | 63.1 | 67.8 | p = 0.1 |
| BMI in male group (kg/m2) | 28.7 ± 4.9 | 27.8 ± 2.76 | p = 0.57 |
| BMI in female group (kg/m2) | 26 ± 4.6 | 24.7 ± 2.66 | p = 0.31 |
| Blood creatinine in male group (μmol/L) | 99.4 ± 31.7 | 87.8 ± 20.1 | p = 0.22 |
| Average eGFR in male group (mL/min/1.73 m2) | 74 | 83 | |
| Blood creatinine concentration in female group (μmol/L) | 75.3 ± 14.8 | 77.9 ± 28.3 | p = 0.77 |
| Average eGFR in female group (mL/min/1.73 m2) | 76 | 71 | |
| Urinary OPN concentration for both sexes (ng/mL) | 13.43 ± 9.78 | 16.17 ± 5.87 | p = 0.17 |
| Urinary OPN concentration in men (ng/mL) | 15.97 ± 9.2 | 17.51 ± 8.5 | p = 0.72 |
| Urinary OPN concentration in women (ng/mL) | 11.23 ± 9.88 | 14.82 ± 7.23 | p = 0.23 |
| Longest stone dimension in CT (mean) | 22.4 mm | - | |
| Longest stone dimension in CT (median) | 21 mm | - |
| Element | Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|---|
| Calcium (mg/kg) | 280,600 (251,940–310,440 95% CI) | 19,420 (868–145,275 95% CI) | 187,800 (123,562–316,537 95% CI) |
| Phosphorus (mg/kg) | 19,000 (1780–49,654 95% CI) | 690 (137–25,732 95% CI) | 62,725 (49,928–89,295 95% CI) |
| Cluster 1, n = 17 | Median = 15.31 ng/mL | 95% CI = 2.18–32.39 |
| Cluster 2, n = 11 | Median = 16.12 ng/mL | 95% CI = 0.52–32.23 |
| Cluster 3, n = 16 | Median = 5.77 ng/mL | 95% CI = 0.62–24.92 |
| Control group, n = 22 | Median = 17.05 ng/mL | 95% CI = 2.18–32.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaromin, M.; Kutwin, P.; Konecki, T.; Jerczyńska, H.; Wysocki, P.; Gajek, M.; Maniukiewicz, W.; Szynkowska-Józwik, M.I.; Moczulski, D. Correlation Between Urinary Osteopontin Concentration and the Mineral Content and Composition of Kidney Stones. J. Clin. Med. 2025, 14, 6247. https://doi.org/10.3390/jcm14176247
Jaromin M, Kutwin P, Konecki T, Jerczyńska H, Wysocki P, Gajek M, Maniukiewicz W, Szynkowska-Józwik MI, Moczulski D. Correlation Between Urinary Osteopontin Concentration and the Mineral Content and Composition of Kidney Stones. Journal of Clinical Medicine. 2025; 14(17):6247. https://doi.org/10.3390/jcm14176247
Chicago/Turabian StyleJaromin, Maciej, Piotr Kutwin, Tomasz Konecki, Hanna Jerczyńska, Piotr Wysocki, Magdalena Gajek, Waldemar Maniukiewicz, Małgorzata Iwona Szynkowska-Józwik, and Dariusz Moczulski. 2025. "Correlation Between Urinary Osteopontin Concentration and the Mineral Content and Composition of Kidney Stones" Journal of Clinical Medicine 14, no. 17: 6247. https://doi.org/10.3390/jcm14176247
APA StyleJaromin, M., Kutwin, P., Konecki, T., Jerczyńska, H., Wysocki, P., Gajek, M., Maniukiewicz, W., Szynkowska-Józwik, M. I., & Moczulski, D. (2025). Correlation Between Urinary Osteopontin Concentration and the Mineral Content and Composition of Kidney Stones. Journal of Clinical Medicine, 14(17), 6247. https://doi.org/10.3390/jcm14176247








