Clinical Predictors of Underlying Histologic Activity in Patients with Lupus Nephritis: A Focus on Urinary Soluble CD163
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Kidney Biopsy and Urinary Soluble CD163 Assessment
2.3. Statistical Analysis
3. Results
3.1. Study Population
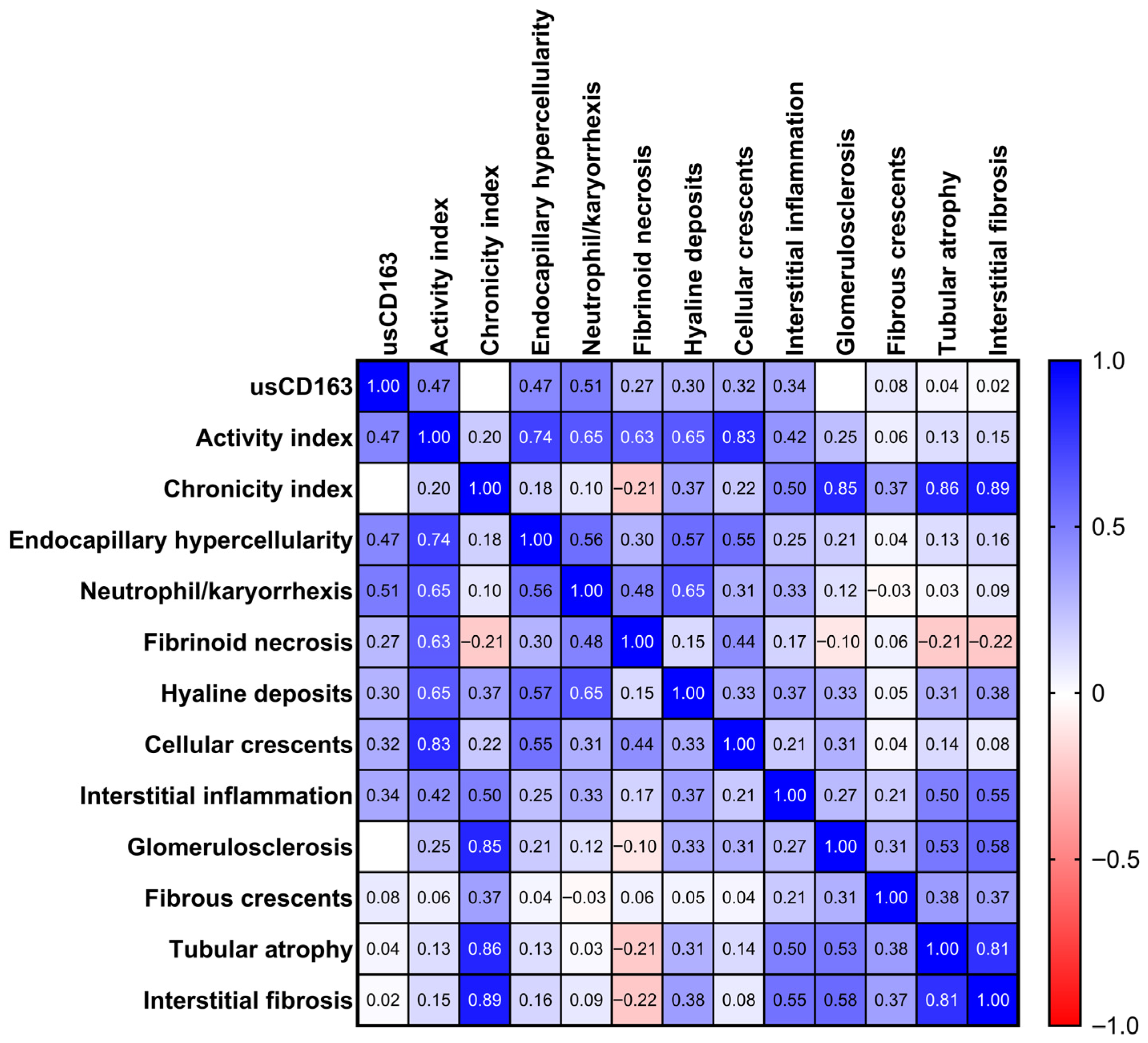
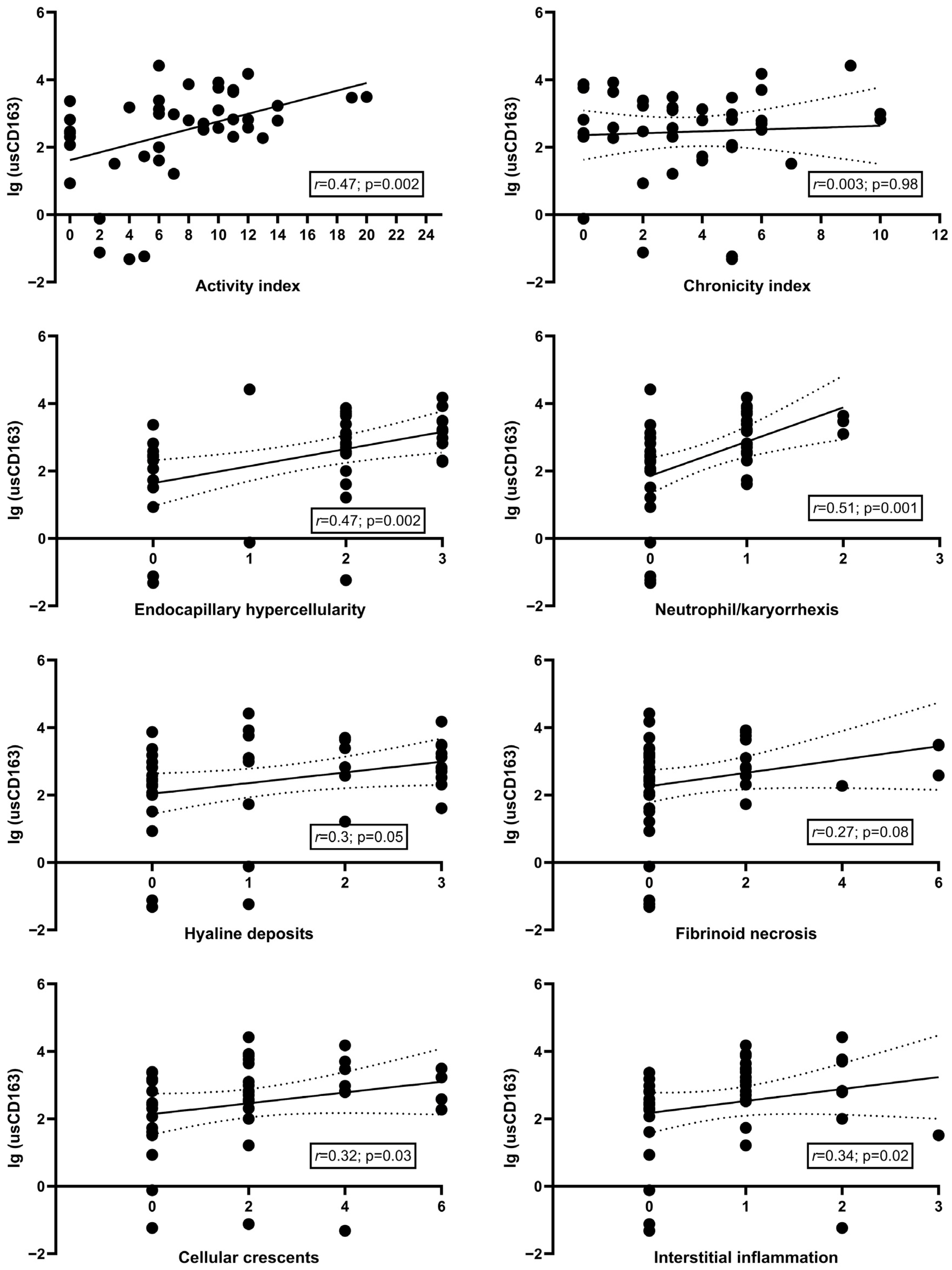
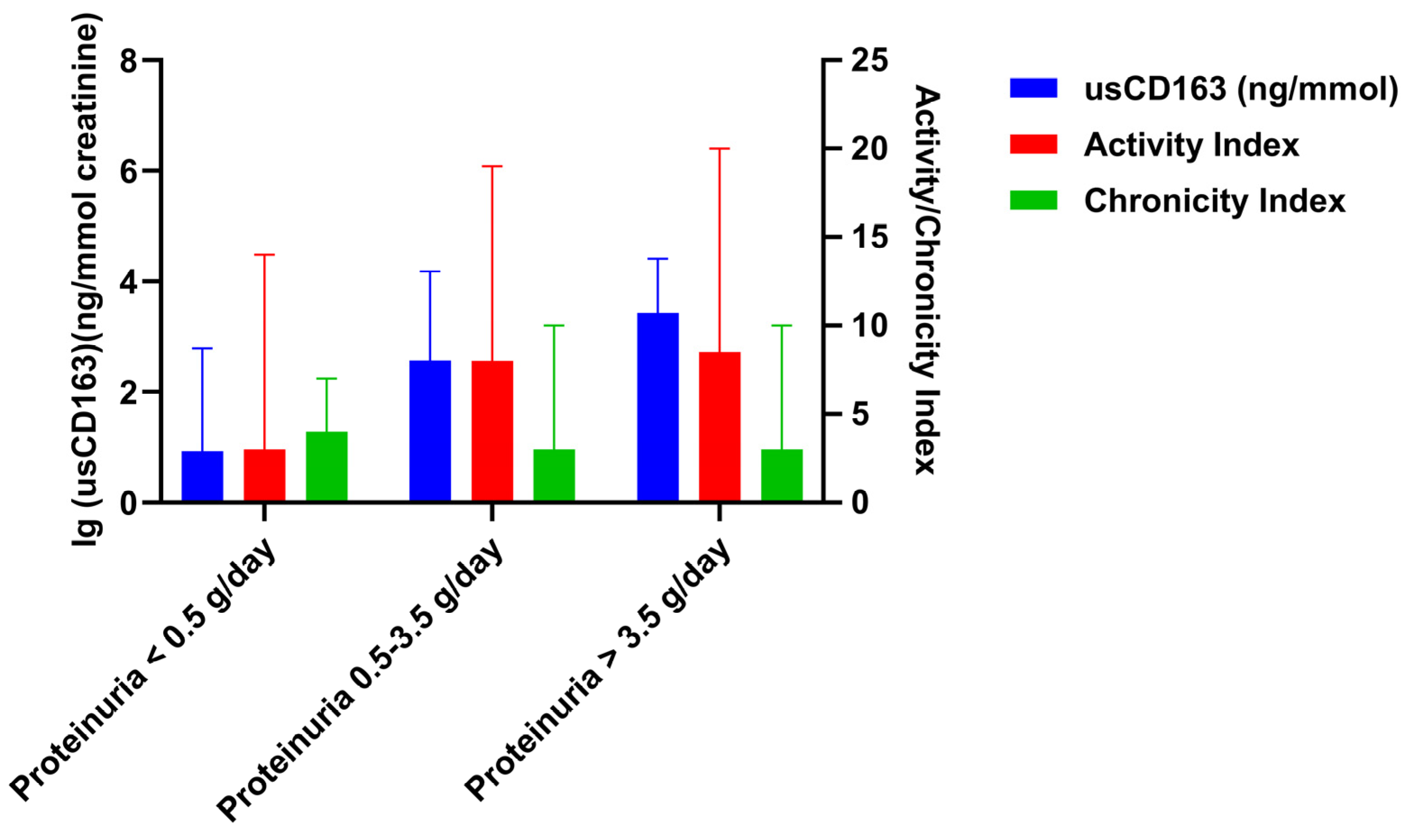
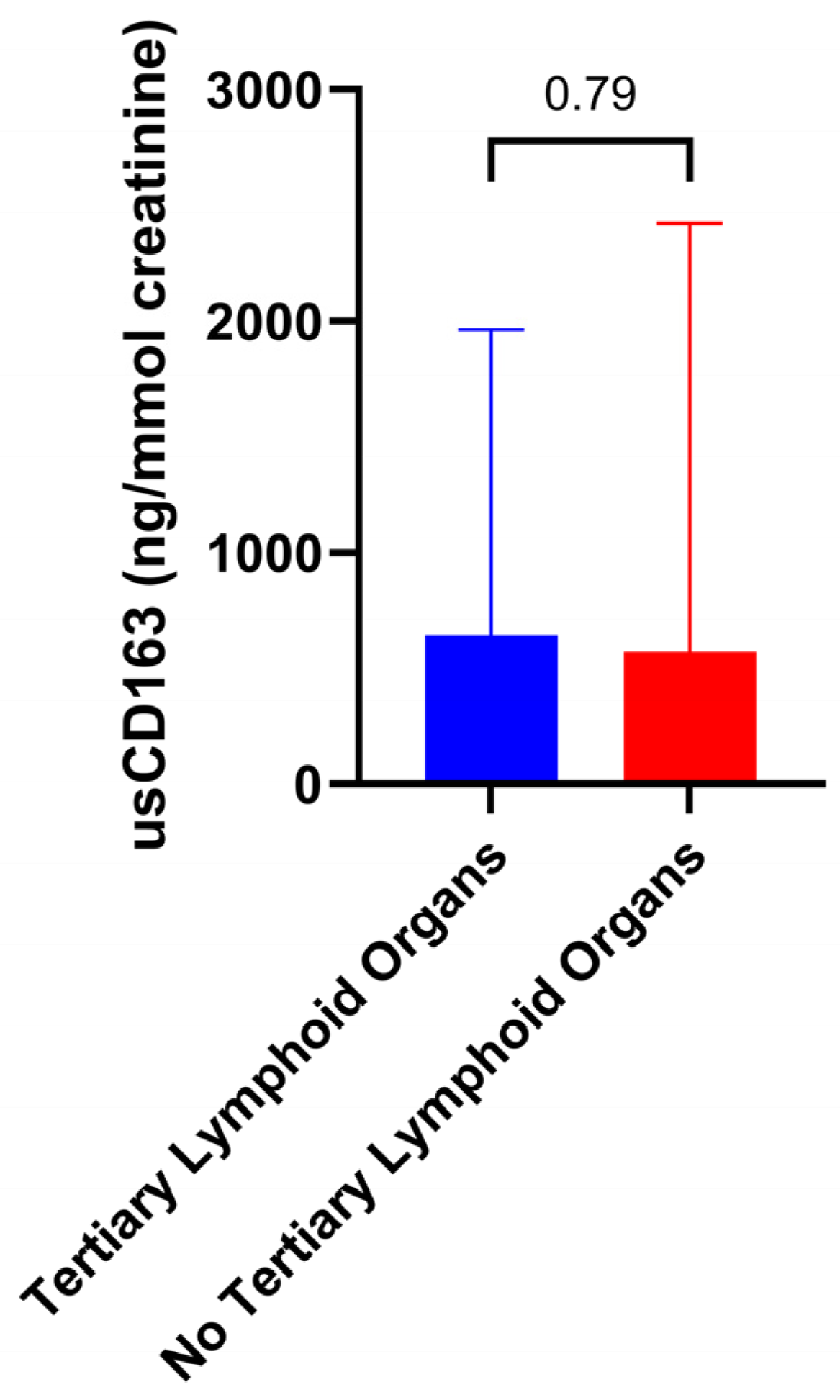
3.2. Cross-Sectional Analysis Evaluating the Relation of Urinary Soluble CD163 with Clinical and Pathologic Parameters
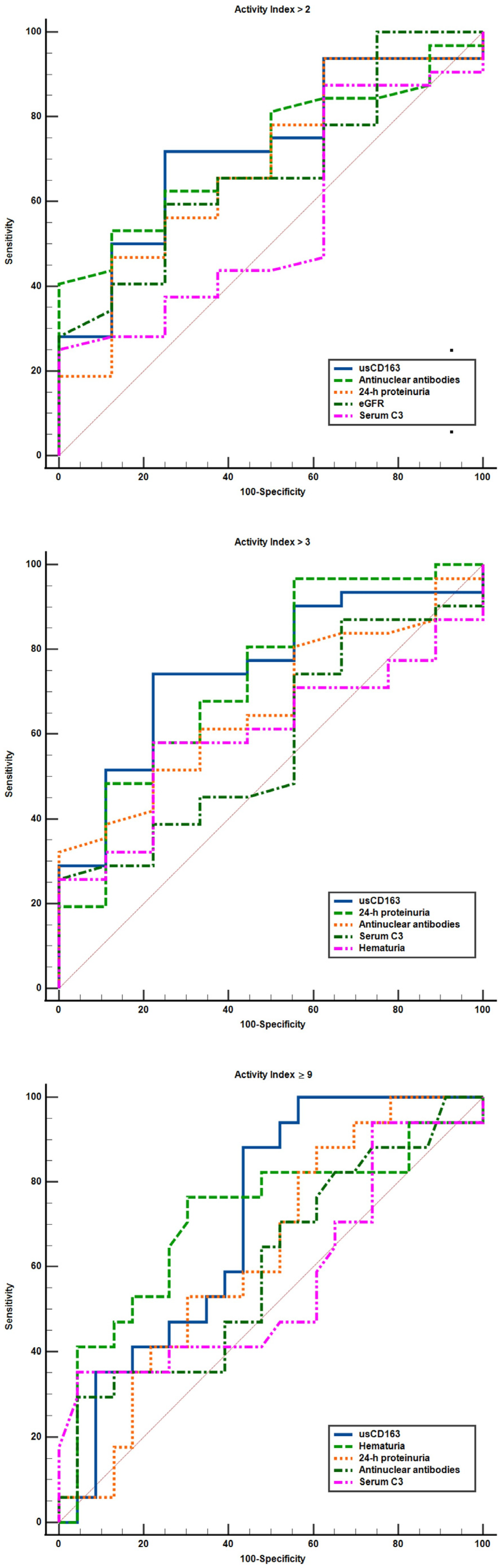
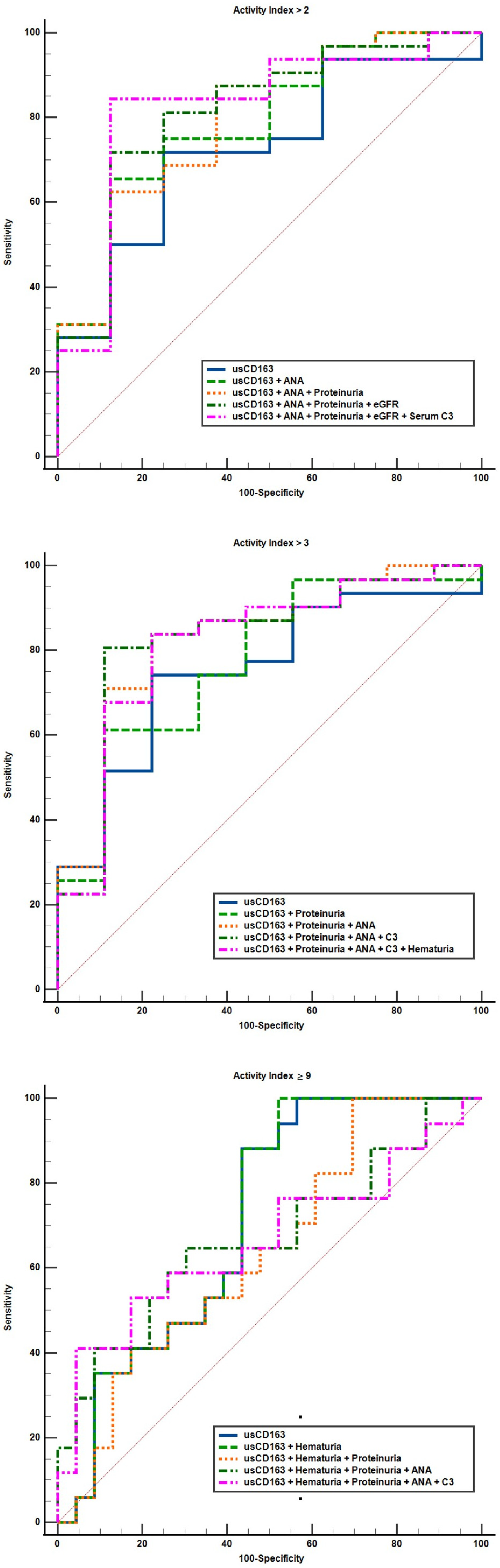
3.3. Cross-Sectional Analysis Evaluating the Performance Characteristics of Clinical Variables to Identify a High Activity Index
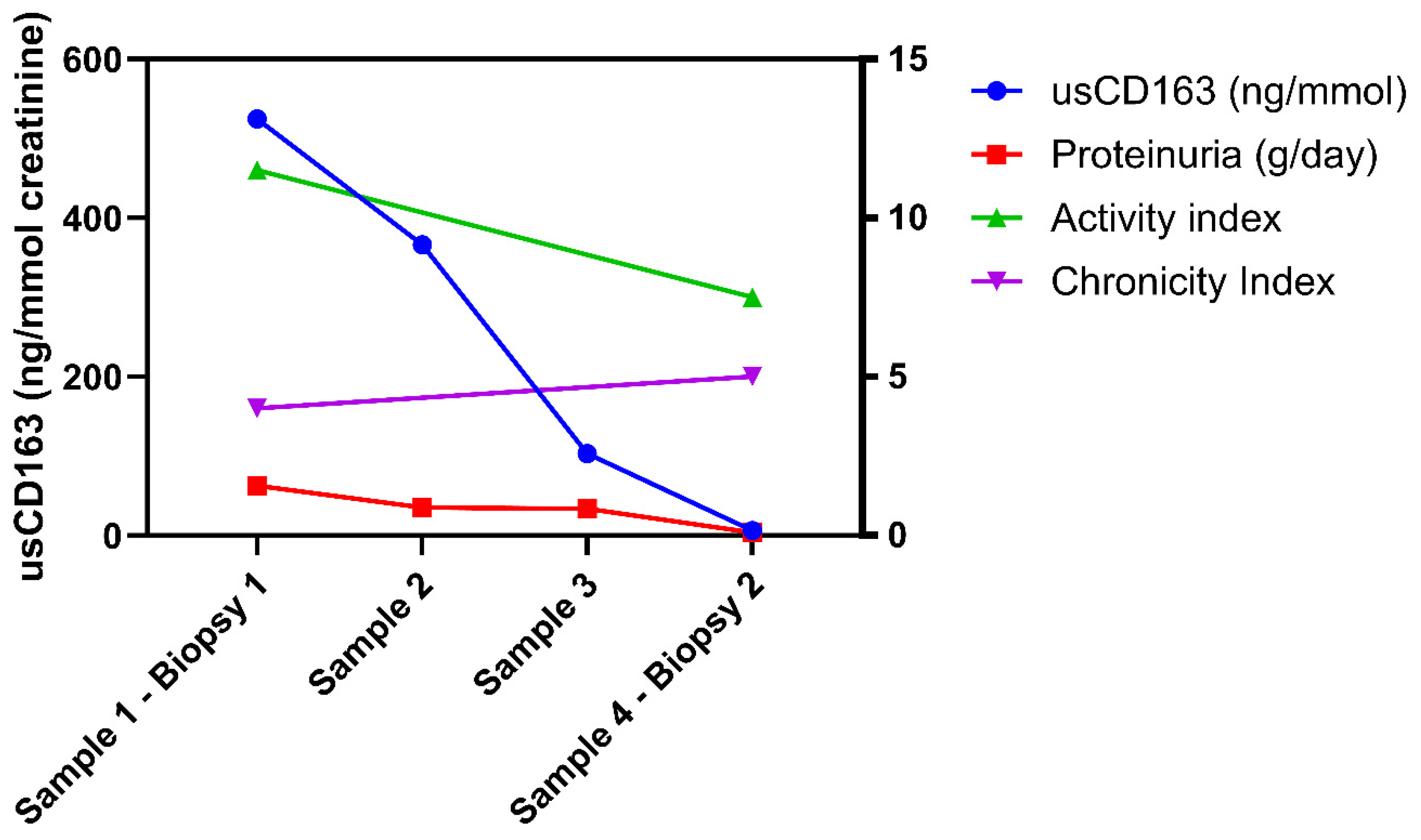
3.4. Longitudinal Analysis of usCD163 During Induction Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moroni, G.; Vercelloni, P.G.; Quaglini, S.; Gatto, M.; Gianfreda, D.; Sacchi, L.; Raffiotta, F.; Zen, M.; Costantini, G.; Urban, M.L.; et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann. Rheum. Dis. 2018, 77, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Obrișcă, B.; Vornicu, A.; Procop, A.; Herlea, V.; Terinte-Balcan, G.; Gherghiceanu, M.; Ismail, G. A Histology-Guided Approach to the Management of Patients with Lupus Nephritis: Are We There Yet? Biomedicines 2022, 10, 1409. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Malvar, A.; Arazi, A.; Rovin, B.H. The lupus nephritis management renaissance. Kidney Int. 2022, 101, 242–255. [Google Scholar] [CrossRef]
- Parodis, I.; Tamirou, F.; Houssiau, F.A. Treat-to-Target in Lupus Nephritis. What is the Role of the Repeat Kidney Biopsy? Arch. Immunol. Ther. Exp. 2022, 70, 8. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Zhang, X.L.; Cruz, C.; Cano-Verduzco, M.L.; Shapiro, J.P.; Nagaraja, H.N.; Morales-Buenrostro, L.E.; Rovin, B.H. Urinary soluble CD163: A novel noninvasive biomarker of activity for lupus nephritis. J. Am. Soc. Nephrol. 2020, 31, 1335–1347. [Google Scholar] [CrossRef]
- Maria, N.I.; Davidson, A. Renal Macrophages and Dendritic Cells in SLE Nephritis. Curr. Rheumatol. Rep. 2017, 19, 81. [Google Scholar] [CrossRef]
- Anders, H.J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef]
- Gong, S.; Jin, S.; Li, Y.; Jiang, W.; Zhang, Z.; Shen, Z.; Wang, J.; Zhou, H.; Liu, X.; Xu, X.; et al. Urinary Soluble CD163 Levels Predict IgA Nephropathy Remission Status. Front. Immunol. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.M.; Scott, J.; Clarkson, M.R.; Conlon, N.; Dunne, J.; Griffin, M.D.; Griffin, T.P.; Groarke, E.; Holian, J.; Judge, C.; et al. The Clinical Application of Urine Soluble CD163 in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2021, 32, 2920–2932. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Yadav, A.; Aggarwal, A. Urinary soluble CD163 is a good biomarker for renal disease activity in lupus nephritis. Clin. Rheumatol. 2021, 40, 941–948. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Azzato, F.; Toblli, J.E.; De Rosa, G.; Fuentes, F.; Nagaraja, H.N.; Nash, R.; Rovin, B.H. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int. 2018, 94, 788–794. [Google Scholar] [CrossRef]
- Parodis, I.; Adamichou, C.; Aydin, S.; Gomez, A.; Demoulin, N.; Weinmann-Menke, J.; Houssiau, F.A.; Tamirou, F. Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology 2020, 59, 3424–3434. [Google Scholar] [CrossRef]
- Austin, H.A., III; Klippel, J.H.; Balow, J.E.; le Riche, N.G.; Steinberg, A.D.; Plotz, P.H.; Decker, J.L. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N. Engl. J Med. 1986, 314, 614–619. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Rocha, A.S.; De Rosa, G.; Dubinsky, D.; Almaani, S.J.; Rovin, B.H. Low-Grade Proteinuria Does Not Exclude Significant Kidney Injury in Lupus Nephritis. Kidney Int. Rep. 2020, 5, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024, 105, S1–S69. [Google Scholar] [CrossRef] [PubMed]
- Frangou, E.; Anders, H.J.; Bajema, I.M.; Teng, Y.K.O.; Malvar, A.; Rovin, B.H.; Kronbichler, A. Immunosuppression Withdrawal in Patients with Lupus Nephritis: When, How, and for Whom Will It Be Safe? J. Am. Soc. Nephrol. 2024, 35, 955–958. [Google Scholar] [CrossRef]
- Malvar, A.; Pirruccio, P.; Alberton, V.; Lococo, B.; Recalde, C.; Fazini, B.; Nagaraja, H.; Indrakanti, D.; Rovin, B.H. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol. Dial. Transplant. 2017, 32, 1338–1344. [Google Scholar] [CrossRef]
- Birmingham, D.J.; Merchant, M.; Waikar, S.S.; Nagaraja, H.; Klein, J.B.; Rovin, B.H. Biomarkers of lupus nephritis histology and flare: Deciphering the relevant amidst the noise. Nephrol. Dial. Transplant. 2017, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, H.; Vanarsa, K.; Gidley, G.; Mok, C.C.; Petri, M.; Saxena, R.; Mohan, C. Association of Urine sCD163 With Proliferative Lupus Nephritis, Fibrinoid Necrosis, Cellular Crescents and Intrarenal M2 Macrophages. Front. Immunol. 2020, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, J.; Wong, M.G.; Shi, S.; Zan, J.; Monaghan, H.; Perkovic, V.; Zhang, H.; TESTING Study Biomarker Group. Correlation of Urinary Soluble CD163 Levels with Disease Activity and Treatment Response in IgA Nephropathy. Kidney Int. Rep. 2024, 9, 3016–3026. [Google Scholar] [CrossRef]
- Malvar, A.; Alberton, V.; Lococo, B.; Lourenco, M.; Martinez, J.; Burna, L.; Besso, C.; Navarro, J.; Nagaraja, H.N.; Khatiwada, A.; et al. Remission of lupus nephritis: The trajectory of histological response in successfully treated patients. Lupus Sci. Med. 2023, 10, e000932. [Google Scholar] [CrossRef] [PubMed]
- Vodehnal, S.; Mohan, C. Urinary biomarkers for active Lupus Nephritis that have survived independent validation across cohorts. Kidney Int. 2024, 106, 1135–1145. [Google Scholar] [CrossRef]
- Fava, A.; Buyon, J.; Magder, L.; Hodgin, J.; Rosenberg, A.; Demeke, D.S.; Rao, D.A.; Arazi, A.; Celia, A.I.; Putterman, C.; et al. Urine proteomic signatures of histological class, activity, chronicity, and treatment response in lupus nephritis. JCI Insight 2024, 9, e172569. [Google Scholar] [CrossRef]

| Clinical Variable | Value |
|---|---|
| Age (y) | 33.5 (24–42.75) |
| Gender (n, %F/%M) | 34/8 (81%/19%) |
| Time since SLE diagnosis (years) | 4 (1–8) |
| Time from induction therapy initiation to biopsy (months) | 1 (0–11.5) |
| Serum creatinine (mg/dL) | 1.07 (0.8–1.34) |
| eGFR (mL/min/1.73 m2) | 76.6 ± 33.9 |
| 24-h proteinuria (g/day) | 1.98 (0.83–4.52) |
| 24-h proteinuria (n, % of pts) | |
| 7 (16.7%) |
| 21 (50%) |
| 14 (33.3%) |
| Albumin/creatinine ratio (mg/g) | 849 (298–3770) |
| Albuminuria (mg/dL) | 791 (230–3233) |
| Urinary creatinine (mmol/L) | 8.4 (5.4–14.8) |
| Hematuria (cells/µL) | 25 (3–54) |
| Total cholesterol (mg/dL) | 214 (170–258) |
| Triglycerides (mg/dL) | 139 (91–190) |
| ANA (U/mL) | 5.8 (0.9–7.8) |
| Anti-dsDNA antibody positivity (% of pts) | 77.5% |
| Anti-Sm antibody positivity (% of pts) | 37.5% |
| C3 (mg/dL) | 79.6 ± 32.9 |
| C4 (mg/dL) | 12.7 (7–20) |
| Raw usCD163 (ng/mL) | 4.88 (0.96–12.26) |
| usCD163 normalized (ng/mmol creatinine) | 626.4 (113.4–2384.1) |
| Immunosuppressive treatment characteristics | |
| Use of steroids (% of pts) | 100% |
| Induction treatment (% of pts) | |
| 47.6% |
| 38.1% |
| 4.8% |
| 7.1% |
| Use of rituximab during induction | 33.3% |
| Maintenance treatment (% of pts) | |
| 59.5% |
| 23.8% |
| 7.1% |
| 2.4% |
| 2.4% |
| Histological Variable | Value |
|---|---|
| LN class [n, %] | |
| II | 4 (9.5%) |
| III | 15 (35.7%) |
| IV without V | 15 (35.7%) |
| Pure V | 4 (9.5%) |
| VI | 0% |
| IV with V | 4 (9.5%) |
| Activity Index | 7 (3–11) |
| Endocapillary hypercellularity | 2 (0–2) |
| Neutrophils/karyorrhexis | 1 (0–1) |
| Fibrinoid necrosis | 0 (0–2) |
| Hyaline deposits | 1 (0–3) |
| Cellular/fibrocellular crescents | 2 (0–4) |
| Interstitial inflammation | 1 (0–1) |
| Chronicity Index | 3 (1–5) |
| Total glomerulosclerosis score | 1 (0–2) |
| Fibrous crescents | 0 (0–0) |
| Tubular atrophy | 1 (0–1) |
| Interstitial fibrosis | 1 (0–2) |
| Variable | AUC | p | Cutoff | Youden Index | Differences of AUC (Versus 1) | p |
|---|---|---|---|---|---|---|
| (I) Activity index over 2 | ||||||
| (1) usCD163 (ng/mmol) | 0.74 (0.58–0.86) | 0.007 | >296.2 | 0.51 | - | - |
| (2) Hematuria (cells/µL) | 0.54 (0.38–0.7) | 0.65 | >19.5 | 0.24 | 0.19 (−0.01 to 0.4) | 0.07 |
| (3) Proteinuria (g/d) | 0.69 (0.53–0.83) | 0.04 | > 2.9 | 0.37 | 0.04 (−0.06 to 0.15) | 0.46 |
| (4) eGFR (mL/min) | 0.62 (0.46–0.77) | 0.25 | ≤81 | 0.27 | 0.11 (−0.16 to 0.39) | 0.42 |
| (5) C3 level (mg/dL) | 0.57 (0.41–0.72) | 0.47 | ≤48 | 0.24 | 0.16 (−0.08 to 0.41) | 0.19 |
| (6) C4 level (mg/dL) | 0.52 (0.36–0.67) | 0.82 | ≤8 | 0.25 | 0.22 (−0.02 to 0.46) | 0.07 |
| (7) ANA level (U/mL) | 0.72 (0.55–0.84) | 0.01 | 5.9 | 0.41 | 0.005 (−0.21 to 0.22) | 0.95 |
| 1 + 7 | 0.79 (0.63–0.9) | 0.001 | - | 0.53 | 0.06 (−0.07 to 0.21) | 0.35 |
| 1 + 7 + 3 | 0.80 (0.64–0.91) | 0.001 | - | 0.5 | 0.07 (−0.07 to 0.22) | 0.32 |
| 1 + 7 + 3 + 4 | 0.81 (0.66–0.92) | <0.001 | - | 0.59 | 0.09 (−0.04 to 0.23) | 0.17 |
| 1 + 7 + 3 + 4 + 5 | 0.82 (0.67–0.93) | <0.001 | - | 0.72 | 0.1 (−0.04 to 0.25) | 0.17 |
| (II) Activity index over 3 | ||||||
| (1) usCD163 (ng/mmol) | 0.77 (0.61–0.88) | 0.001 | >296.2 | 0.55 | - | - |
| (2) Hematuria (cells/µL) | 0.57 (0.41–0.72) | 0.45 | >19.5 | 0.29 | 0.19 (−0.003 to 0.39) | 0.05 |
| (3) Proteinuria (g/d) | 0.75 (0.58–0.86) | 0.008 | >2.9 | 0.4 | 0.02 (−0.08 to 0.12) | 0.73 |
| (4) eGFR (mL/min) | 0.56 (0.4–0.71) | 0.56 | ≤130 | 0.2 | 0.2 (−0.08 to 0.48) | 0.16 |
| (5) C3 level (mg/dL) | 0.6 (0.44–0.75) | 0.31 | ≤48 | 0.25 | 0.16 (−0.05 to 0.39) | 0.15 |
| (6) C4 level (mg/dL) | 0.51 (0.35–0.66) | 0.9 | ≤8 | 0.27 | 0.25 (0.01 to 0.49) | 0.03 |
| (7) ANA level (U/mL) | 0.66 (0.5–0.81) | 0.07 | >7.6 | 0.32 | 0.09 (−0.15 to 0.32) | 0.48 |
| 1 + 3 | 0.79 (0.63–0.9) | <0.001 | - | 0.52 | 0.02 (−0.04 to 0.09) | 0.49 |
| 1 + 3 + 7 | 0.83 (0.67–0.93) | <0.001 | - | 0.62 | 0.07 (−0.05 to 0.2) | 0.24 |
| 1 + 3 + 7 + 5 | 0.83 (0.67–0.93) | <0.001 | - | 0.69 | 0.07 (−0.06 to 0.21) | 0.29 |
| 1 + 3 + 7 + 5 + 2 | 0.82 (0.66–0.92) | <0.001 | - | 0.62 | 0.06 (−0.07 to 0.2) | 0.35 |
| (III) Activity index of at least 9 | ||||||
| (1) usCD163 (ng/mmol) | 0.74 (0.57–0.86) | 0.002 | >296.2 | 0.47 | - | - |
| (2) Hematuria (cells/µL) | 0.7 (0.54–0.83) | 0.02 | > 19.5 | 0.44 | 0.03 (−0.14 to 0.21) | 0.71 |
| (3) Proteinuria (g/d) | 0.64 (0.48–0.78) | 0.1 | > 1.4 | 0.29 | 0.09 (−0.02 to 0.22) | 0.13 |
| (4) eGFR (mL/min) | 0.51 (0.34–0.66) | 0.95 | ≤100 | 0.22 | 0.23 (−0.03 to 0.49) | 0.08 |
| (5) C3 level (mg/dL) | 0.58 (0.42–0.73) | 0.37 | ≤41.2 | 0.29 | 0.15 (−0.03 to 0.34) | 0.11 |
| (6) C4 level (mg/dL) | 0.51 (0.35–0.67) | 0.87 | ≤4.6 | 0.11 | 0.22 (0.02 to 0.42) | 0.02 |
| (7) ANA level (U/mL) | 0.6 (0.43–0.75) | 0.28 | >9.6 | 0.25 | 0.11 (−0.14 to 0.36) | 0.39 |
| 1 + 2 | 0.74 (0.58–0.86) | 0.002 | - | 0.5 | 0.002 (−0.006 to 0.01) | 0.62 |
| 1 + 2 + 3 | 0.65 (0.49–0.79) | 0.06 | - | 0.29 | 0.07 (−0.05 to 0.21) | 0.24 |
| 1 + 2 + 3 + 7 | 0.67 (0.5 to 0.81) | 0.06 | - | 0.34 | 0.04 (−0.18 to 0.26) | 0.72 |
| 1 + 2 + 3 + 7 + 5 | 0.66 (0.49–0.81) | 0.07 | - | 0.36 | 0.04 (−0.17 to 0.27) | 0.68 |
| Variable | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | Accuracy |
|---|---|---|---|---|---|---|---|
| (I) AI over 2 | |||||||
| (1) usCD163 > 296.2 ng/mmol | 72.7% (54.4–86.7) | 77.7% (39.9–97.1) | 92.3% (77.6–97.6) | 43.7% (28.7–86.1) | 3.27 (0.94–11.3) | 0.35 (0.18–0.67) | 73.8% (57.9–86.1) |
| (2) Hematuria > 19.5 cells/µL | 57.5% (39.2–74.5) | 66.6% (29.9–92.5) | 86.3% (70.6–94.3) | 30% (18.9–44) | 1.72 (0.65–4.55) | 0.63 (0.34–1.17) | 59.5% (43.2–74.3) |
| (3) Proteinuria > 2.9 g/day | 48.5% (30.8–66.4) | 88.8% (51.7–99.7) | 94.1% (70.9–99.1) | 32% (23.9–41.3) | 4.36 (0.66–29.6) | 0.58 (0.38–0.86) | 57.1% (40.9–72.2) |
| (4) eGFR ≤ 81 mL/min | 60.6% (42.1–77.1) | 66.6% (29.9–92.5) | 86.9% (71.7–94.5) | 31.5% (19.7–46.3) | 1.81 (0.69–4.76) | 0.59 (0.31–1.1) | 61.9% (45.6–76.4) |
| (5) C3 level ≤ 48 mg/dL | 24.2% (11.1–42.2) | 100% (66.3–100) | 100% (63.1–100) | 26.4% (22.8–30.3) | - | 0.75 (0.62–0.92) | 40.4% (25.6–56.7) |
| (6) C4 level ≤ 8 mg/dL | 36.3% (20.4–54.8) | 88.8% (51.7–99.7) | 92.3% (64.1–98.7) | 27.5% (21.2–35) | 3.27 (0.48–21.9) | 0.71 (0.51–1.01) | 47.6% (32–63.5) |
| (7) ANA level > 5.9 U/mL | 53.1% (34.7–70.9) | 87.5% (47.3–99.6) | 94.4% (72.5–99.1) | 31.8% (22.8–42.3) | 4.25 (0.66–27.3) | 0.53 (0.34–0.84) | 60% (43.3–75.1) |
| 1 + 7 | 34.3% (18.5–53.2) | 100% (63.1–100) | 100% (71.5–100) | 27.5% (22.8–32.8) | - | 0.65 (0.51–0.84) | 47.5% (31.5–63.8) |
| 1 + 7 + 3 | 18.7% (7.2–36.4) | 100% (63.1–100) | 100% (54.1–100) | 23.5% (20.6–51.6) | - | 0.81 (0.68–0.96) | 35% (20.6–51.6) |
| 1 + 7 + 3 + 4 | 12.5% (3.51–28.9) | 100% (63.1–100) | 100% (39.7–100) | 22.2% (20.04–24.5) | - | 0.87 (0.76–0.99) | 30% (16.5–46.5) |
| 1 + 7 + 3 + 4 + 5 | 9.37% (1.97–25.02) | 100% (63.1–100) | 100% (29.2–100) | 21.6% (19.7–23.5) | - | 0.91 (0.81–1.01) | 27.5% (14.6–43.8) |
| (II) AI over 3 | |||||||
| (1) usCD163 > 296.2 ng/mmol | 75% (56.5–88.5) | 80% (44.4–97.4) | 92.3% (77.3–97.6) | 50% (33.7–66.2) | 3.75 (1.06–13.1) | 0.31 (0.16–0.61) | 76.2% (60.5–87.9) |
| (2) Hematuria > 19.5 cells/µL | 59.3% (40.6–76.3) | 70% (34.7–93.3) | 86.3% (70.2–94.4) | 35% (23.1–49.1) | 1.98 (0.73–5.32) | 0.58 (0.32–1.04) | 61.9% (45.6–76.4) |
| (3) Proteinuria > 2.9 g/day | 50% (31.8–68.1) | 90% (55.5–99.7) | 94.1% (70.7–99.1) | 36% (27.3–45.7) | 5 (0.75–33.1) | 0.55 (0.37–0.83) | 59.5% (43.2–74.3) |
| (4) eGFR ≤ 130 mL/min | 100% (89.1–100) | 20% (2.52–55.6) | 80% (74.5–84.5) | 100% (15.8–100) | 1.25 (0.91–1.7) | - | 80.9% (65.8–91.4) |
| (5) C3 level ≤ 48 mg/dL | 25% (11.4–43.4) | 100% (69.1–100) | 100% (63.1–100) | 29.4% (25.4–33.7) | - | 0.75 (0.61–0.91) | 42.8% (27.7–59.04) |
| (6) C4 level ≤ 8 mg/dL | 37.5% (21.1–56.3) | 90% (55.5–99.7) | 92.3% (63.9–98.8) | 31.03% (24.3–38.7) | 3.75 (0.55–25.4) | 0.69 (0.49–0.97) | 50% (34.2–65.8) |
| (7) ANA level > 7.6 U/mL | 32.2% (16.6–51.3) | 100% (66.3–100) | 100% (69.1–100) | 30% (25.1–35.3) | - | 0.67 (0.53–0.86) | 47.5% (31.5–63.8) |
| 1 + 3 | 46.8% (29.1–65.2) | 90% (55.5–99.7) | 93.7% (69.2–99) | 34.6% (26.4–43.7) | 4.68 (0.7–31.2) | 0.59 (0.4–0.86) | 57.1% (40.9–72.2) |
| 1 + 3 + 7 | 6.45% (0.79–21.4) | 100% (66.3–100) | 100% (15.8–100) | 23.6% (22.1–25.4) | - | 0.93 (0.85–1.02) | 27.5% (14.6–43.8) |
| 1 + 3 + 7 + 5 | 3.22% (0.08–16.7) | 100% (66.3–100) | 100% (2.5–100) | 23.1% (21.9–24.2) | - | 0.96 (0.91–1.03) | 25% (12.6–41.2) |
| 1 + 3 + 7 + 5 + 2 | 3.22% (0.08–16.7) | 100% (66.3–100) | 100% (2.5–100) | 23.1% (21.9–24.2) | - | 0.96 (0.91–1.03) | 25% (12.6–41.2) |
| (III) AI of at least 9 | |||||||
| (1) usCD163 > 296.2 ng/mmol | 88.8% (65.2–98.6) | 58.3% (36.6–77.9) | 61.5% (49.2–72.5) | 87.5% (64.4–96.4) | 2.13 (1.29–3.52) | 0.19 (0.05–0.73) | 71.4% (55.4–84.2) |
| (2) Hematuria > 19.5 cells/µL | 77.7% (52.3–93.6) | 66.6% (44.6–84.3) | 63.6% (48.5–76.4) | 80% (61.7–90.8) | 2.33 (1.26–4.32) | 0.33 (0.13–0.82) | 71.4% (55.4–84.2) |
| (3) Proteinuria > 1.4 g/day | 83.3% (58.6–96.4) | 45.8% (25.5–67.2) | 53.6% (43.1–63.7) | 78.5% (54.4–91.8) | 1.54 (1.009–2.34) | 0.36 (0.12–1.11) | 61.9% (45.6–76.4) |
| (4) eGFR ≤ 100 mL/min | 88.8% (65.2–98.6) | 33.3% (15.6–55.3) | 50% (41.9–58.1) | 80% (49.1–94.3) | 1.33 (0.96–1.84) | 0.33 (0.08–1.38) | 57.1% (40.9–72.2) |
| (5) C3 level ≤ 41.2 mg/dL | 33.3% (13.3–59) | 95.8% (78.8–99.9) | 85.7% (44.1–97.8) | 65.7% (57.7–72.8) | 8 (1.05–60.7) | 0.69 (0.49–0.97) | 69.05% (52.9–82.3) |
| (6) C4 level ≤ 4.6 mg/dL | 11.1% (1.37–34.7) | 100% (85.7–100) | 100% (15.8–100) | 60% (56.02–63.8) | - | 0.88 (0.75–1.04) | 61.9% (45.6–76.4) |
| (7) ANA level > 9.6 U/mL | 29.4% (10.3–55.9) | 95.6% (78.05–99.9) | 83.3% (39.1–97.5) | 64.7% (57.1–71.6) | 6.76 (0.86–52.7) | 0.74 (0.53–1.01) | 67.5% (50.8–81.4) |
| 1 + 2 | 66.6% (40.9–86.6) | 83.3% (62.6–95.2) | 75% (53.6–88.6) | 76.9% (62.8–86.7) | 4 (1.54–10.3) | 0.4 (0.2–0.78) | 76.2% (60.5–87.9) |
| 1 + 2 + 3 | 61.1% (35.7–82.7) | 83.3% (62.6–95.2) | 73.3% (51.1–87.8) | 74.1% (60.9–83.9) | 3.66 (1.39–9.64) | 0.46 (0.25–0.85) | 73.8% (57.9–86.1) |
| 1 + 2 + 3 + 7 | 17.6% (3.8–43.4) | 100% (85.1–100) | 100% (29.2–100) | 62.2% (56.8–67.2) | - | 0.82 (0.66–1.02) | 65% (48.3–79.3) |
| 1 + 2 + 3 + 7 + 5 | 11.7% (1.45–36.4) | 100% (85.2–100) | 100% (15.8–100) | 60.5% (56.3–64.5) | - | 0.88 (0.74–1.05) | 62.5% (45.8–77.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrișcă, B.; Vrabie, A.; Lujinschi, Ș.; Jurubiță, R.; Mocanu, V.; Berechet, A.; Sorohan, B.; Andronesi, A.; Lupușoru, G.; Achim, C.; et al. Clinical Predictors of Underlying Histologic Activity in Patients with Lupus Nephritis: A Focus on Urinary Soluble CD163. J. Clin. Med. 2025, 14, 6162. https://doi.org/10.3390/jcm14176162
Obrișcă B, Vrabie A, Lujinschi Ș, Jurubiță R, Mocanu V, Berechet A, Sorohan B, Andronesi A, Lupușoru G, Achim C, et al. Clinical Predictors of Underlying Histologic Activity in Patients with Lupus Nephritis: A Focus on Urinary Soluble CD163. Journal of Clinical Medicine. 2025; 14(17):6162. https://doi.org/10.3390/jcm14176162
Chicago/Turabian StyleObrișcă, Bogdan, Alexandra Vrabie, Ștefan Lujinschi, Roxana Jurubiță, Valentin Mocanu, Andreea Berechet, Bogdan Sorohan, Andreea Andronesi, Gabriela Lupușoru, Camelia Achim, and et al. 2025. "Clinical Predictors of Underlying Histologic Activity in Patients with Lupus Nephritis: A Focus on Urinary Soluble CD163" Journal of Clinical Medicine 14, no. 17: 6162. https://doi.org/10.3390/jcm14176162
APA StyleObrișcă, B., Vrabie, A., Lujinschi, Ș., Jurubiță, R., Mocanu, V., Berechet, A., Sorohan, B., Andronesi, A., Lupușoru, G., Achim, C., Micu, G., Manda, D., Poalelungi, C., Caceaune, N., Dima, S., & Ismail, G. (2025). Clinical Predictors of Underlying Histologic Activity in Patients with Lupus Nephritis: A Focus on Urinary Soluble CD163. Journal of Clinical Medicine, 14(17), 6162. https://doi.org/10.3390/jcm14176162






