A Narrative Review on Toxidromes in the Psychiatric Population: Implications for Overdose Prevention
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiology
3.2. Risk Factors and Clinical Relevance in Psychiatric Populations
3.3. Comparison of Major Toxidromes in Psychiatry
- Anticholinergic Toxidrome
- 2.
- Cholinergic Toxidrome
- 3.
- Opioid Toxidrome
- 4.
- Sedative-Hypnotic Toxidrome
- 5.
- Sympathomimetic Toxidrome
- 6.
- Neuroleptic Malignant Syndrome
- 7.
- Serotonergic Toxidrome (Serotonin Toxicity)
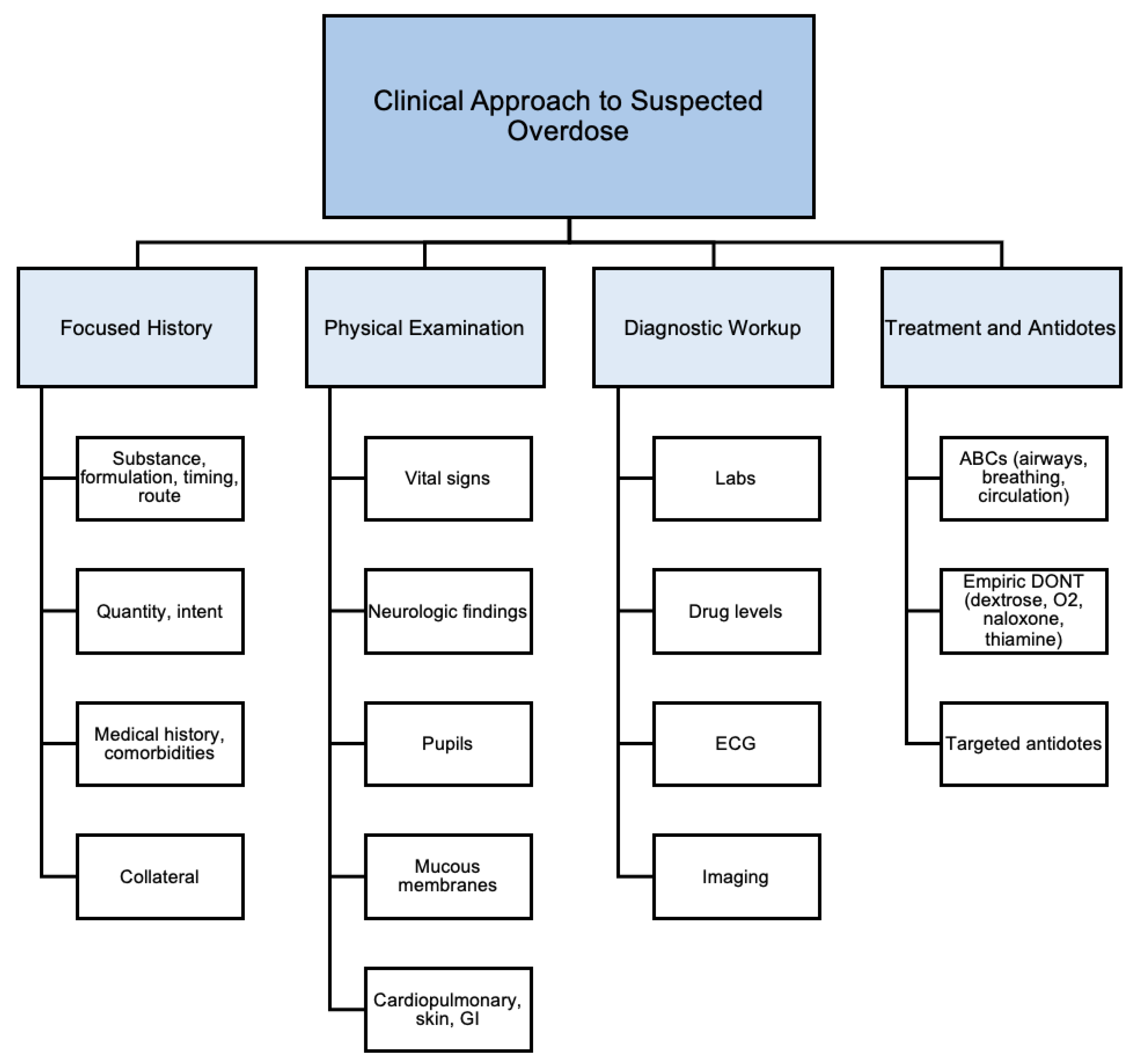
3.4. Clinical Approach to Overdoses
- Focused History
- 2.
- Physical Examination
- 3.
- Diagnostic Workup
- 4.
- Treatment and Antidote Administration
3.5. Distinguishing Toxidromes from Psychiatric Syndromes
3.6. Overdose Prevention and Longitudinal Systems-Level Interventions
- Restricting Access to High-Risk Medications
- 2.
- Enhanced Patient Education and Discharge Planning
- 3.
- Pharmacist Involvement and Collaborative Care Models
- 4.
- Public Health and Policy Interventions
4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Homeland Security. Report on the Toxic Chemical Syndrome; U.S. Department of Homeland Security: Washington, DC, USA, 2012.
- Abesamis, M.G. Toxidromes. In Psychosomatic Medicine; Ackerman, K.D., Dimartini, A.F., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 295–314. ISBN 978-0-19-932931-1. [Google Scholar]
- Rasimas, J.J.; Sinclair, C.M. Assessment and Management of Toxidromes in the Critical Care Unit. Crit. Care Clin. 2017, 33, 521–541. [Google Scholar] [CrossRef]
- Greene, S.; AufderHeide, E.; French-Rosas, L. Toxicologic Emergencies in Patients with Mental Illness. Psychiatr. Clin. N. Am. 2017, 40, 519–532. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Akpunonu, P.D.; Arens, A.M.; Babu, K.M.; Cao, D.; Hoffman, R.S.; Hoyte, C.O.; Mazer-Amirshahi, M.E.; Stolbach, A.; St-Onge, M.; et al. 2023 American Heart Association Focused Update on the Management of Patients with Cardiac Arrest or Life-Threatening Toxicity Due to Poisoning: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2023, 148, e149–e184. [Google Scholar] [CrossRef]
- World Health Organization. Suicide; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Choi, N.G.; Marti, C.N.; Choi, B.Y. Three Leading Suicide Methods in the United States, 2017–2019: Associations with Decedents’ Demographic and Clinical Characteristics. Front. Public Health 2022, 10, 955008. [Google Scholar] [CrossRef]
- Fazel, S.; Runeson, B. Suicide. N. Engl. J. Med. 2020, 382, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S. Epidemiology of Suicide and the Psychiatric Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef] [PubMed]
- Shoib, S.; Kim, Y.-K. The Frontiers of Suicide. In Frontiers in Psychiatry; Kim, Y.-K., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1192, pp. 503–517. ISBN 978-981-329-720-3. [Google Scholar]
- National Center for National Center for Drug Abuse Statistics. Drug Overdose Death Rates; CDC: Atlanta, GA, USA, 2024. [Google Scholar]
- Lim, J.S.; Buckley, N.A.; Cairns, R.; Schumann, J.; Schaffer, A.L.; Chitty, K.M. Substances Detected During Coroner Postmortem Toxicology Analyses in Poisoning- and Nonpoisoning-Related Suicides. JAMA Psychiatry 2023, 80, 1121. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Swedler, D.I.; Lawrence, B.A.; Ali, B.; Rockett, I.R.H.; Carlson, N.N.; Leonardo, J. Incidence and Lethality of Suicidal Overdoses by Drug Class. JAMA Netw. Open 2020, 3, e200607. [Google Scholar] [CrossRef]
- Trott, M.; Suetani, S.; Arnautovska, U.; Kisely, S.; Kar Ray, M.; Theodoros, T.; Le, V.; Leske, S.; Lu, M.; Soole, R.; et al. Suicide Methods and Severe Mental Illness: A Systematic Review and Meta-analysis. Acta Psychiatr. Scand. 2025, 151, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.; Carr, M.J.; Mok, P.L.H.; Antonsen, S.; Pedersen, C.B.; Shaw, J.; Webb, R.T. Suicide Methods and Specific Types of Accidental Death and Fatal Poisoning Among Discharged Psychiatric Patients: A National Cohort Study. J. Clin. Psychiatry 2018, 79, 17m11809. [Google Scholar] [CrossRef]
- Levenson, J.L. (Ed.) The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry, 3rd ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2019; ISBN 978-1-61537-136-5. [Google Scholar]
- Fu, X.-L.; Qian, Y.; Jin, X.-H.; Yu, H.-R.; Wu, H.; Du, L.; Chen, H.-L.; Shi, Y.-Q. Suicide Rates among People with Serious Mental Illness: A Systematic Review and Meta-Analysis. Psychol. Med. 2021, 53, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Arnone, D.; Karmegam, S.R.; Östlundh, L.; Alkhyeli, F.; Alhammadi, L.; Alhammadi, S.; Alkhoori, A.; Selvaraj, S. Risk of Suicidal Behavior in Patients with Major Depression and Bipolar Disorder—A Systematic Review and Meta-Analysis of Registry-Based Studies. Neurosci. Biobehav. Rev. 2024, 159, 105594. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-H.; Westphal, J.; Hu, Y.; Peterson, E.L.; Williams, L.K.; Prabhakar, D.; Frank, C.; Autio, K.; Elsiss, F.; Simon, G.E.; et al. Diagnosed Mental Health Conditions and Risk of Suicide Mortality. Psychiatr. Serv. 2019, 70, 750–757. [Google Scholar] [CrossRef]
- Qin, P. The Impact of Psychiatric Illness on Suicide: Differences by Diagnosis of Disorders and by Sex and Age of Subjects. J. Psychiatr. Res. 2011, 45, 1445–1452. [Google Scholar] [CrossRef]
- Palmer, B.A.; Pankratz, V.S.; Bostwick, J.M. The Lifetime Risk of Suicide in Schizophrenia: A Reexamination. Arch. Gen. Psychiatry 2005, 62, 247. [Google Scholar] [CrossRef]
- Wang, J.; Sumner, S.A.; Simon, T.R.; Crosby, A.E.; Annor, F.B.; Gaylor, E.; Xu, L.; Holland, K.M. Trends in the Incidence and Lethality of Suicidal Acts in the United States, 2006 to 2015. JAMA Psychiatry 2020, 77, 684. [Google Scholar] [CrossRef] [PubMed]
- Kegler, S.R.; Simon, T.R.; Zwald, M.L.; Chen, M.S.; Mercy, J.A.; Jones, C.M.; Mercado-Crespo, M.C.; Blair, J.M.; Stone, D.M.; Ottley, P.G.; et al. Vital Signs: Changes in Firearm Homicide and Suicide Rates—United States, 2019–2020. Mmwr-Morbidity Mortal. Wkly. Rep. 2022, 71, 656–663. [Google Scholar] [CrossRef]
- DeJong, T.M.; Overholser, J.C.; Stockmeier, C.A. Apples to Oranges?: A Direct Comparison between Suicide Attempters and Suicide Completers. J. Affect. Disord. 2010, 124, 90–97. [Google Scholar] [CrossRef]
- McMorrow, C.; Nerney, D.; Cullen, N.; Kielty, J.; VanLaar, A.; Davoren, M.; Conlon, L.; Brodie, C.; McDonald, C.; Hallahan, B. Psychiatric and Psychosocial Characteristics of Suicide Completers: A 13-Year Comprehensive Evaluation of Psychiatric Case Records and Postmortem Findings. Eur. Psychiatr. 2022, 65, e14. [Google Scholar] [CrossRef]
- Martínez-Alés, G.; Jiang, T.; Keyes, K.M.; Gradus, J.L. The Recent Rise of Suicide Mortality in the United States. Annu. Rev. Public Health 2022, 43, 99–116. [Google Scholar] [CrossRef]
- Ivey-Stephenson, A.Z.; Crosby, A.E.; Hoenig, J.M.; Gyawali, S.; Park-Lee, E.; Hedden, S.L. Suicidal Thoughts and Behaviors Among Adults Aged ≥18 Years—United States, 2015–2019. MMWR Surveill. Summ. 2022, 71, 1–19. [Google Scholar] [CrossRef]
- American Psychiatric Association. The American Psychiatric Association Practice Guidelines for the Psychiatric Evaluation of Adults, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 2015; ISBN 978-0-89042-465-0. [Google Scholar]
- Merrill, R.M.; Ashton, M.K. How Do Mental Disorders and Combinations of Disorders Affect the Odds of Injuries and Poisoning? J. Nerv. Ment. Dis. 2024, 212, 303–311. [Google Scholar] [CrossRef]
- Bohnert, A.S.B.; Ilgen, M.A.; Ignacio, R.V.; McCarthy, J.F.; Valenstein, M.; Blow, F.C. Risk of Death From Accidental Overdose Associated with Psychiatric and Substance Use Disorders. Am. J. Psychiatry 2012, 169, 64–70. [Google Scholar] [CrossRef]
- McHugh, C.M.; Chun Lee, R.S.; Hermens, D.F.; Corderoy, A.; Large, M.; Hickie, I.B. Impulsivity in the Self-Harm and Suicidal Behavior of Young People: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2019, 116, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.; Daley, D.; Townsend, E.; Sayal, K. Impulsivity and Self-Harm in Adolescence: A Systematic Review. Eur. Child Adolesc. Psychiatry 2017, 26, 387–402. [Google Scholar] [CrossRef]
- Cáceda, R.; Durand, D.; Cortes, E.; Prendes-Alvarez, S.; Moskovciak, T.; Harvey, P.D.; Nemeroff, C.B. Impulsive Choice and Psychological Pain in Acutely Suicidal Depressed Patients. Psychosom. Med. 2014, 76, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.K.; Lee, J.W.; Lee, S.Y.; Moon, J.; Jeon, D.-W.; Shim, S.-H.; Cho, S.-J.; Kim, S.G.; Lee, J.; Paik, J.-W.; et al. Suicide Risk Factors across Suicidal Ideators, Single Suicide Attempters, and Multiple Suicide Attempters. J. Psychiatr. Res. 2020, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benton, T.D.; Muhrer, E.; Jones, J.D.; Lewis, J. Dysregulation and Suicide in Children and Adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2021, 30, 389–399. [Google Scholar] [CrossRef]
- Rutter, S.B.; Cipriani, N.; Smith, E.C.; Ramjas, E.; Vaccaro, D.H.; Martin Lopez, M.; Calabrese, W.R.; Torres, D.; Campos-Abraham, P.; Llaguno, M.; et al. Neurocognition and the Suicidal Process. In Behavioral Neurobiology of Suicide and Self Harm; Baca-Garcia, E., Ed.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2020; Volume 46, pp. 117–153. ISBN 978-3-030-57573-1. [Google Scholar]
- Miranda, R.; Valderrama, J.; Tsypes, A.; Gadol, E.; Gallagher, M. Cognitive Inflexibility and Suicidal Ideation: Mediating Role of Brooding and Hopelessness. Psychiatry Res. 2013, 210, 174–181. [Google Scholar] [CrossRef]
- Vinckier, F.; Gourion, D.; Mouchabac, S. Anhedonia Predicts Poor Psychosocial Functioning: Results from a Large Cohort of Patients Treated for Major Depressive Disorder by General Practitioners. Eur. Psychiatry 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Wong, S.; Le, G.H.; Phan, L.; Rhee, T.G.; Ho, R.; Meshkat, S.; Teopiz, K.M.; Kwan, A.T.H.; Mansur, R.B.; Rosenblat, J.D.; et al. Effects of Anhedonia on Health-Related Quality of Life and Functional Outcomes in Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2024, 356, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.; Peters, J.R.; Nishar, S.; Grilo, C.M.; Sanislow, C.A.; Shea, M.T.; Zanarini, M.C.; McGlashan, T.H.; Morey, L.C.; Skodol, A.E. Association of Borderline Personality Disorder Criteria with Suicide Attempts: Findings From the Collaborative Longitudinal Study of Personality Disorders Over 10 Years of Follow-Up. JAMA Psychiatry 2021, 78, 187. [Google Scholar] [CrossRef]
- Wong, Z.; Öngür, D.; Cohen, B.; Ravichandran, C.; Noam, G.; Murphy, B. Command Hallucinations and Clinical Characteristics of Suicidality in Patients with Psychotic Spectrum Disorders. Compr. Psychiatry 2013, 54, 611–617. [Google Scholar] [CrossRef]
- Wolff, J.; Hefner, G.; Normann, C.; Kaier, K.; Binder, H.; Hiemke, C.; Toto, S.; Domschke, K.; Marschollek, M.; Klimke, A. Polypharmacy and the Risk of Drug–Drug Interactions and Potentially Inappropriate Medications in Hospital Psychiatry. Pharmacoepidemiol. Drug 2021, 30, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Højlund, M.; Köhler-Forsberg, O.; Gregersen, A.T.; Rohde, C.; Mellentin, A.I.; Anhøj, S.J.; Kemp, A.F.; Fuglsang, N.B.; Wiuff, A.C.; Nissen, L.; et al. Prevalence, Correlates, Tolerability-Related Outcomes, and Efficacy-Related Outcomes of Antipsychotic Polypharmacy: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2024, 11, 975–989. [Google Scholar] [CrossRef]
- Hu, J.; McMillan, S.S.; Theodoros, T.; Collins, J.C.; El-Den, S.; O’Reilly, C.L.; Wheeler, A.J. Psychotropic Medication Use in People Living with Severe and Persistent Mental Illness in the Australian Community: A Cross-Sectional Study. BMC Psychiatry 2022, 22, 705. [Google Scholar] [CrossRef]
- Delara, M.; Murray, L.; Jafari, B.; Bahji, A.; Goodarzi, Z.; Kirkham, J.; Chowdhury, M.; Seitz, D.P. Prevalence and Factors Associated with Polypharmacy: A Systematic Review and Meta-Analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar] [CrossRef]
- Shekho, D.; Mishra, R.; Kamal, R.; Khurana, D.; Chauhan, A.; Awasthi, A. Polypharmacy in Psychiatry: An In-Depth Examination of Drug-drugInteractions and Treatment Challenges. Curr. Pharm. Des. 2024, 30, 1641–1649. [Google Scholar] [CrossRef]
- Crombie, I.K.; McLoone, P. Does the Availability of Prescribed Drugs Affect Rates of Self Poisoning? Br. J. Gen. Pract. 1998, 48, 1505–1506. [Google Scholar] [PubMed]
- Gurewich, D.; Linsky, A.M.; Harvey, K.L.; Li, M.; Griesemer, I.; MacLaren, R.Z.; Ostrow, R.; Mohr, D. Relationship Between Unmet Social Needs and Care Access in a Veteran Cohort. J. Gen. Intern. Med. 2023, 38, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, O.E.; Liaw, W.; Patel, N.C.; Rastegar, J.; Ruble, M.; Franklin, S.; Renda, A.; Obasi, E.; Woodard, L. Assessment of Unmet Health-Related Social Needs Among Patients with Mental Illness Enrolled in Medicare Advantage. JAMA Netw. Open 2022, 5, e2239855. [Google Scholar] [CrossRef]
- Alang, S.M. Sociodemographic Disparities Associated with Perceived Causes of Unmet Need for Mental Health Care. Psychiatr. Rehabil. J. 2015, 38, 293–299. [Google Scholar] [CrossRef]
- Meyer, D.; Lowensen, K.; Perrin, N.; Moore, A.; Mehta, S.H.; Himmelfarb, C.R.; Inglesby, T.V.; Jennings, J.M.; Mueller, A.K.; LaRicci, J.N.; et al. An Evaluation of the Impact of Social and Structural Determinants of Health on Forgone Care during the COVID-19 Pandemic in Baltimore, Maryland. PLoS ONE 2024, 19, e0302064. [Google Scholar] [CrossRef]
- Yang, G.; Chan, J.; Choi, M.; Singh, K.; Slocum, G.W.; Calixte, R.; Gore, R.; Gottlieb, M. The Association of Social Determinants of Health and Medication Adherence: A Cross-Sectional Analysis across Three Urban Emergency Departments. Am. J. Emerg. Med. 2025, 95, 16–27. [Google Scholar] [CrossRef]
- Simpson, S.A.; Shy, B.D.; Loh, R.M. More Than Suicide: Mortality After Emergency Psychiatric Care and Implications for Practice. J. Acad. Consult.-Liaison Psychiatry 2022, 63, 354–362. [Google Scholar] [CrossRef]
- Joo, J.Y. Fragmented Care and Chronic Illness Patient Outcomes: A Systematic Review. Nurs. Open 2023, 10, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; McCance-Katz, E.F. Co-Occurring Substance Use and Mental Disorders among Adults with Opioid Use Disorder. Drug Alcohol Depend. 2019, 197, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.; Lucatch, A.M.; Lowe, D.J.E.; Balodis, I.M.; MacKillop, J.; George, T.P. The Neurobiology of Impulsivity and Substance Use Disorders: Implications for Treatment. Ann. N. Y. Acad. Sci. 2019, 1451, 71–91. [Google Scholar] [CrossRef]
- Wrege, J.; Schmidt, A.; Walter, A.; Smieskova, R.; Bendfeldt, K.; Radue, E.-W.; Lang, U.; Borgwardt, S. Effects of Cannabis on Impulsivity: A Systematic Review of Neuroimaging Findings. Curr. Pharm. Des. 2014, 20, 2126–2137. [Google Scholar] [CrossRef]
- Lees, B.; Meredith, L.R.; Kirkland, A.E.; Bryant, B.E.; Squeglia, L.M. Effect of Alcohol Use on the Adolescent Brain and Behavior. Pharmacol. Biochem. Behav. 2020, 192, 172906. [Google Scholar] [CrossRef] [PubMed]
- Ordak, M.; Zmysłowska, A.; Bielski, M.; Rybak, D.; Tomaszewska, M.; Wyszomierska, K.; Kmiec, A.; Garlicka, N.; Zalewska, M.; Zalewski, M.; et al. Pharmacotherapy of Patients Taking New Psychoactive Substances: A Systematic Review and Analysis of Case Reports. Front. Psychiatry 2021, 12, 669921. [Google Scholar] [CrossRef] [PubMed]
- Karnick, A.T.; Caulfield, N.M.; Bauer, B.W.; Martin, R.L.; Kaufman, E.J.; Winchell, R.; Capron, D.W. Substance Use and Suicide Outcomes among Self-Injured Trauma Patients. Drug Alcohol Depend. 2021, 226, 108906. [Google Scholar] [CrossRef]
- Arias, S.A.; Dumas, O.; Sullivan, A.F.; Boudreaux, E.D.; Miller, I.; Camargo, C.A., Jr. Substance Use as a Mediator of the Association Between Demographics, Suicide Attempt History, and Future Suicide Attempts in Emergency Department Patients. Crisis 2016, 37, 385–391. [Google Scholar] [CrossRef][Green Version]
- Nelson, L.S.; Goldfrank, L.R. (Eds.) Goldfrank’s Toxicologic Emergencies, 11th ed.; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 978-1-259-85961-8. [Google Scholar]
- Arens, A.M.; Kearney, T. Adverse Effects of Physostigmine. J. Med. Toxicol. 2019, 15, 184–191. [Google Scholar] [CrossRef]
- Watkins, J.W.; Schwarz, E.S.; Arroyo-Plasencia, A.M.; Mullins, M.E.; Toxicology Investigators Consortium investigators. The Use of Physostigmine by Toxicologists in Anticholinergic Toxicity. J. Med. Toxicol. 2015, 11, 179–184. [Google Scholar] [CrossRef]
- McCoy, C.E.; Honda, R. Anticholinergic Toxicity in the Emergency Department. J. Educ. Teach. Emerg. Med. 2023, 8, S25–S47. [Google Scholar] [CrossRef]
- Calcaterra, S.L.; Bottner, R.; Martin, M.; Englander, H.; Weinstein, Z.M.; Weimer, M.B.; Lambert, E.; Ronan, M.V.; Huerta, S.; Zaman, T.; et al. Management of Opioid Use Disorder, Opioid Withdrawal, and Opioid Overdose Prevention in Hospitalized Adults: A Systematic Review of Existing Guidelines. J. Hosp. Med. 2022, 17, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of Acute Organophosphorus Pesticide Poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.L.; Jones, E.B. Cholinergic Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- King, A.M.; Aaron, C.K. Organophosphate and Carbamate Poisoning. Emerg. Med. Clin. N. Am. 2015, 33, 133–151. [Google Scholar] [CrossRef]
- Hulse, E.J.; Haslam, J.D.; Emmett, S.R.; Woolley, T. Organophosphorus Nerve Agent Poisoning: Managing the Poisoned Patient. Br. J. Anaesth. 2019, 123, 457–463. [Google Scholar] [CrossRef]
- Azevedo, K.; Johnson, M.; Wassermann, M.; Evans-Wall, J. Drugs of Abuse-Opioids, Sedatives, Hypnotics. Crit. Care Clin. 2021, 37, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Morford, K.L.; Levander, X.A. Benzodiazepines and Related Sedatives. Med. Clin. N. Am. 2022, 106, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Sharbaf Shoar, N.; Bistas, K.G.; Patel, P.; Saadabadi, A. Flumazenil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Brown, H.; Pollard, K.A. Drugs of Abuse: Sympathomimetics. Crit. Care Clin. 2021, 37, 487–499. [Google Scholar] [CrossRef]
- Radaelli, D.; Manfredi, A.; Zanon, M.; Fattorini, P.; Scopetti, M.; Neri, M.; Frisoni, P.; D’Errico, S. Synthetic Cannabinoids and Cathinones Cardiotoxicity: Facts and Perspectives. Curr. Neuropharmacol. 2021, 19, 2038–2048. [Google Scholar] [CrossRef]
- Ware, M.R.; Feller, D.B.; Hall, K.L. Neuroleptic Malignant Syndrome: Diagnosis and Management. Prim. Care Companion J. Clin. Psychiatry 2018, 20, 17r02185. [Google Scholar] [CrossRef]
- Pileggi, D.J.; Cook, A.M. Neuroleptic Malignant Syndrome: Focus on Treatment and Rechallenge. Ann. Pharmacother. 2016, 50, 973–981. [Google Scholar] [CrossRef]
- Perry, P.J.; Wilborn, C.A. Serotonin Syndrome vs Neuroleptic Malignant Syndrome: A Contrast of Causes, Diagnoses, and Management. Ann. Clin. Psychiatry 2012, 24, 155–162. [Google Scholar] [PubMed]
- Boyer, E.W.; Shannon, M. The Serotonin Syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef]
- Morarasu, B.C.; Coman, A.E.; Bologa, C.; Lionte, C.; Petris, O.R.; Ceasovschih, A.; Sorodoc, V.; Haliga, R.E.; Puha, G.; Stoica, A.; et al. Recognition and Management of Serotonin Toxidrome in the Emergency Department—Case Based Review. J. Pers. Med. 2022, 12, 2069. [Google Scholar] [CrossRef]
- Mikkelsen, N.; Damkier, P.; Pedersen, S.A. Serotonin Syndrome—A Focused Review. Basic Clin. Pharmacol. Toxicol. 2023, 133, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Maitland, S.; Baker, M. Serotonin Syndrome. Drug Ther. Bull. 2022, 60, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, K.; Doering And, M.; Mullins, M.E.; on behalf of the Toxicology Investigators Consortium. Dexmedetomidine in the Treatment of Toxicologic Conditions: A Systematic Review and Review of the Toxicology Investigators Consortium Database. Clin. Toxicol. 2022, 60, 1356–1375. [Google Scholar] [CrossRef]
- Ohbe, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Cholinergic Crisis Caused by Cholinesterase Inhibitors: A Retrospective Nationwide Database Study. J. Med. Toxicol. 2018, 14, 237–241. [Google Scholar] [CrossRef]
- Tuovinen, K. Organophosphate-Induced Convulsions and Prevention of Neuropathological Damages. Toxicology 2004, 196, 31–39. [Google Scholar] [CrossRef]
- Berde, C.; Nurko, S. Opioid Side Effects--Mechanism-Based Therapy. N. Engl. J. Med. 2008, 358, 2400–2402. [Google Scholar] [CrossRef]
- Baldo, B.A.; Rose, M.A. Mechanisms of Opioid-Induced Respiratory Depression. Arch. Toxicol. 2022, 96, 2247–2260. [Google Scholar] [CrossRef]
- Bateman, J.T.; Saunders, S.E.; Levitt, E.S. Understanding and Countering Opioid-Induced Respiratory Depression. Br. J. Pharmacol. 2023, 180, 813–828. [Google Scholar] [CrossRef]
- Han, Y.; Yan, W.; Zheng, Y.; Khan, M.Z.; Yuan, K.; Lu, L. The Rising Crisis of Illicit Fentanyl Use, Overdose, and Potential Therapeutic Strategies. Transl. Psychiatry 2019, 9, 282. [Google Scholar] [CrossRef]
- Kummer, R.L.; Kempainen, R.R.; Olives, T.D.; Leatherman, J.W.; Prekker, M.E. Naloxone-Associated Pulmonary Edema Following Recreational Opioid Overdose. Am. J. Emerg. Med. 2022, 53, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.R.; Patel, P.; Morrisonponce, D. Naloxone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Abdelal, R.; Banerjee, A.R.; Carlberg-Racich, S.; Darwaza, N.; Ito, D.; Epstein, J. The Need for Multiple Naloxone Administrations for Opioid Overdose Reversals: A Review of the Literature. Subst. Abus. 2022, 43, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Hocker, S. Toxin-Induced Coma and Central Nervous System Depression. Neurol. Clin. 2020, 38, 825–841. [Google Scholar] [CrossRef]
- Stewart, P.T.; Rahman, M.B.; Chiew, A.L.; Fitzpatrick, M.; Osborne, N.J.; Chan, B.S. Cognitive Impairment Following Sedative Overdose. Clin. Toxicol. 2024, 62, 152–163. [Google Scholar] [CrossRef]
- Penninga, E.I.; Graudal, N.; Ladekarl, M.B.; Jürgens, G. Adverse Events Associated with Flumazenil Treatment for the Management of Suspected Benzodiazepine Intoxication—A Systematic Review with Meta-Analyses of Randomised Trials. Basic Clin. Pharmacol. Toxicol. 2016, 118, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B.; Walker, D.J. The Potential for Misuse and Abuse of Medications in ADHD: A Review. Postgrad. Med. 2014, 126, 64–81. [Google Scholar] [CrossRef]
- Dominic, P.; Ahmad, J.; Awwab, H.; Bhuiyan, M.S.; Kevil, C.G.; Goeders, N.E.; Murnane, K.S.; Patterson, J.C.; Sandau, K.E.; Gopinathannair, R.; et al. Stimulant Drugs of Abuse and Cardiac Arrhythmias. Circ. Arrhythmia Electrophysiol. 2022, 15, e010273. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological Mechanisms of Catecholamine and Cocaine-Mediated Cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.; Li, L.; Du Rietz, E.; Andell, P.; Garcia-Argibay, M.; D’Onofrio, B.M.; Cortese, S.; Larsson, H.; Chang, Z. Risk of Cardiovascular Diseases Associated with Medications Used in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2243597. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Compton, W.M.; Jones, C.M.; Einstein, E.B.; Volkow, N.D. Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry 2021, 78, 1329. [Google Scholar] [CrossRef]
- Dunkley, E.J.C.; Isbister, G.K.; Sibbritt, D.; Dawson, A.H.; Whyte, I.M. The Hunter Serotonin Toxicity Criteria: Simple and Accurate Diagnostic Decision Rules for Serotonin Toxicity. Qjm Int. J. Med. 2003, 96, 635–642. [Google Scholar] [CrossRef]
- Prakash, S.; Patel, H.; Kumar, S.; Shah, C.S. Cyproheptadine in Serotonin Syndrome: A Retrospective Study. J. Fam. Med. Prim. Care 2024, 13, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Francescangeli, J.; Karamchandani, K.; Powell, M.; Bonavia, A. The Serotonin Syndrome: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2019, 20, 2288. [Google Scholar] [CrossRef]
- Horseman, M.; Panahi, L.; Udeani, G.; Tenpas, A.S.; Verduzco, R., Jr.; Patel, P.H.; Bazan, D.Z.; Mora, A.; Samuel, N.; Mingle, A.-C.; et al. Drug-Induced Hyperthermia Review. Cureus 2022, 14, e27278. [Google Scholar] [CrossRef]
- Dao, C.K.; Nowinski, S.M.; Mills, E.M. The Heat Is on: Molecular Mechanisms of Drug-Induced Hyperthermia. Temperature 2014, 1, 183–191. [Google Scholar] [CrossRef]
- Erickson, T.B.; Thompson, T.M.; Lu, J.J. The Approach to the Patient with an Unknown Overdose. Emerg. Med. Clin. N. Am. 2007, 25, 249–281. [Google Scholar] [CrossRef]
- Holstege, C.P.; Borek, H.A. Toxidromes. Crit. Care Clin. 2012, 28, 479–498. [Google Scholar] [CrossRef]
- Campbell, J.; Matoff-Stepp, S.; Velez, M.L.; Cox, H.H.; Laughon, K. Pregnancy-Associated Deaths from Homicide, Suicide, and Drug Overdose: Review of Research and the Intersection with Intimate Partner Violence. J. Womens Health 2021, 30, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chyka, P.A.; Seger, D.; Krenzelok, E.P.; Vale, J.A.; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. Position Paper: Single-Dose Activated Charcoal. Clin. Toxicol. 2005, 43, 61–87. [Google Scholar] [CrossRef]
- Birtcher, K.K.; Allen, L.A.; Anderson, J.L.; Bonaca, M.P.; Gluckman, T.J.; Hussain, A.; Kosiborod, M.; Mehta, L.S.; Virani, S.S. 2022 ACC Expert Consensus Decision Pathway for Integrating Atherosclerotic Cardiovascular Disease and Multimorbidity Treatment: A Framework for Pragmatic, Patient-Centered Care. J. Am. Coll. Cardiol. 2023, 81, 292–317. [Google Scholar] [CrossRef]
- Babu, K.M.; Brent, J.; Juurlink, D.N. Prevention of Opioid Overdose. N. Engl. J. Med. 2019, 380, 2246–2255. [Google Scholar] [CrossRef]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain-United States, 2022. MMWR. Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, A.; Gonzalez, S.J.; Zoorob, R.J. Substance Misuse in Adults: A Primary Care Approach. Am. Fam. Physician 2024, 109, 430–440. [Google Scholar]
- Beadles, C.A.; Ellis, A.R.; Lichstein, J.C.; Farley, J.F.; Jackson, C.T.; Morrissey, J.P.; Domino, M.E. First Outpatient Follow-up after Psychiatric Hospitalization: Does One Size Fit All? Psychiatr. Serv. 2015, 66, 364–372. [Google Scholar] [CrossRef]
- Mann, J.J.; Michel, C.A.; Auerbach, R.P. Improving Suicide Prevention Through Evidence-Based Strategies: A Systematic Review. Am. J. Psychiatry 2021, 178, 611–624. [Google Scholar] [CrossRef]
- Gallant, K.C.; Harris, B.R. Community Collaboration for Suicide and Overdose Prevention: Attitudes, Perceptions, and Practices of Community-Based Professionals and County Leadership in New York State. Community Ment. Health J. 2024, 60, 859–868. [Google Scholar] [CrossRef]
- Kishi, T.; Matsunaga, S.; Iwata, N. Mortality Risk Associated with Long-Acting Injectable Antipsychotics: A Systematic Review and Meta-Analyses of Randomized Controlled Trials. Schizophr. Bull. 2016, 42, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Vallersnes, O.M.; Jacobsen, D.; Ekeberg, Ø.; Brekke, M. Mortality, Morbidity and Follow-up after Acute Poisoning by Substances of Abuse: A Prospective Observational Cohort Study. Scand. J. Public Health 2019, 47, 452–461. [Google Scholar] [CrossRef]
- Simon, G.E.; Shortreed, S.M.; Rossom, R.C.; Beck, A.; Clarke, G.N.; Whiteside, U.; Richards, J.E.; Penfold, R.B.; Boggs, J.M.; Smith, J. Effect of Offering Care Management or Online Dialectical Behavior Therapy Skills Training vs Usual Care on Self-Harm Among Adult Outpatients with Suicidal Ideation: A Randomized Clinical Trial. JAMA 2022, 327, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Krishna, G.S.; Alla, S.; Kurian, T.D.; Kurian, J.; Ramesh, M.; Kishor, M. Impact of Pharmacist–Psychiatrist Collaborative Patient Education on Medication Adherence and Quality of Life (QOL) of Bipolar Affective Disorder (BPAD) Patients. Front. Pharmacol. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Syrnyk, M.; Glass, B. Pharmacist Interventions in Medication Adherence in Patients with Mental Health Disorders: A Scoping Review. Int. J. Pharm. Pract. 2023, 31, 449–458. [Google Scholar] [CrossRef]
- Wien, K.; Reißner, P.; Hefner, G.; Thern, J.; Borgwardt, S. Prevalence and Solving Strategies of Drug-Related Problems in Adult Psychiatric Inpatients—A Systematic Review. Front. Psychiatry 2024, 15, 1460098. [Google Scholar] [CrossRef]
- Baker, E.; Gwernan-Jones, R.; Britten, N.; Cox, M.; McCabe, C.; Retzer, A.; Gill, L.; Plappert, H.; Reilly, S.; Pinfold, V.; et al. Refining a Model of Collaborative Care for People with a Diagnosis of Bipolar, Schizophrenia or Other Psychoses in England: A Qualitative Formative Evaluation. BMC Psychiatry 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Reist, C.; Petiwala, I.; Latimer, J.; Raffaelli, S.B.; Chiang, M.; Eisenberg, D.; Campbell, S. Collaborative Mental Health Care: A Narrative Review. Medicine 2022, 101, e32554. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A.; Courtwright, A.; Wu, K.C.-C. The Role of Stigma and Denormalization in Suicide-Prevention Laws in East Asia: A Sociocultural, Historical, and Ethical Perspective. Harv. Rev. Psychiatry 2017, 25, 229–240. [Google Scholar] [CrossRef]
- Dreier, M.; Ludwig, J.; Härter, M.; von dem Knesebeck, O.; Rezvani, F.; Baumgardt, J.; Pohontsch, N.J.; Bock, T.; Liebherz, S. Evaluation of an Online Suicide Prevention Program to Improve Suicide Literacy and to Reduce Suicide Stigma: A Mixed Methods Study. PLoS ONE 2023, 18, e0284944. [Google Scholar] [CrossRef]
- Purtle, J.; Mauri, A.I.; Lindsey, M.A.; Keyes, K.M. Evidence for Public Policies to Prevent Suicide Death in the United States. Annu. Rev. Public Health 2025, 46, 349–367. [Google Scholar] [CrossRef]
- Lynch, F.L. Population Health Outcome Models in Suicide Prevention Policy. Am. J. Prev. Med. 2014, 47, S137–S143. [Google Scholar] [CrossRef] [PubMed]
| Toxidrome | Pathogenesis | Common Agents | Clinical Features | Diagnosis | Management |
|---|---|---|---|---|---|
| Anticholinergic | Muscarinic acetylcholine receptor blockade causes central and peripheral Ach inhibition | Atropine, diphenhydramine, hyoscyamine, TCAs, phenothiazines, benztropine, trihexyphenidyl, scopolamine | Dry skin, mydriasis, urinary retention, ileus, delirium, hyperthermia, tachycardia, flushed skin, and hallucinations. Severe: delirium, seizures, coma | Clinical: dry skin, mydriasis, altered mental status; absent diaphoresis (differentiates from sympathomimetic) | Supportive care, benzodiazepines; physostigmine in severe cases [2,3,62,63,64,65] |
| Opioid | Mu-opioid receptor agonism | Heroin, morphine, oxycodone, fentanyl, carfentanil, methadone | Miosis, respiratory depression, bradycardia, hypotension, coma | Clinical: classic triad—miosis, respiratory depression, loss of consciousness | Naloxone, airway support [2,3,62,66] |
| Cholinergic | Excess acetylcholine due to acetylcholinesterase inhibition | Organophosphates, carbamates, nerve agents, physostigmine | Diarrhea, Urination, Miosis, Bradycardia, Bronchorrhea, Emesis, Lacrimation, Salivation, Sweating | Clinical: muscarinic + nicotinic symptoms, bradycardia, wheezing, secretions | Atropine, pralidoxime, benzodiazepines [2,3,62,67,68,69,70] |
| Sedative-Hypnotic | Potentiation of GABA-A receptor activity | Benzodiazepines, barbiturates, zolpidem, ethanol | CNS depression, slurred speech, ataxia, hypotension, respiratory depression | Clinical: history + CNS depression without other findings (e.g., no miosis or clonus) | Supportive care; flumazenil is rarely used due to risk of seizures or arrhythmia [2,3,62,71,72,73] |
| Sympathomimetic | Excessive stimulation of adrenergic receptors via increased catecholamine release or reuptake inhibition | Amphetamines, methamphetamine, cocaine, methylphenidate, synthetic cathinones, pseudoephedrine, phenylephrine, ephedrine | Hypertension, tachycardia, hyperthermia, agitation, paranoia, hallucinations, mydriasis, tremor, diaphoresis, seizures, rhabdomyolysis | Clinical: agitation, mydriasis, hyperthermia, +diaphoresis (distinguishes from anticholinergic) | Supportive care, benzodiazepines, cooling; avoid beta-blockers alone! [2,3,5,62,74,75] |
| Neuroleptic | Dopamine D2 receptor blockade, primarily in the basal ganglia and hypothalamus | Haloperidol, fluphenazine, risperidone, olanzapine, prochlorperazine, promethazine | Mild (EPS): dystonia, tremor, bradykinesia, akathisia; Severe (NMS): hyperthermia, lead-pipe rigidity, altered mental status, autonomic instability | Clinical: rigidity, altered mental status, fever, increased creatine kinase | Stop the offending agent! EPS: benztropine or diphenhydramine. NMS: bromocriptine, dantrolene, ICU support [2,3,62,76,77,78] |
| Serotonergic | Excess serotonergic activity, particularly at 5-HT2A receptors | SSRIs, SNRIs, MAOIs, TCAs, trazodone, mirtazapine, tramadol, fentanyl, dextromethorphan, buspirone | Agitation, clonus, hyperreflexia, mydriasis, hyperthermia, diarrhea, tremor, altered mental status | Hunter Criteria: clonus + serotonergic agent; hyperreflexia and clonus are key signs | Stop all serotonergic drugs; benzodiazepines, cyproheptadine, cooling; ICU monitoring if severe [2,3,62,79,80,81,82,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Buciuc, A.G.; Barry, P.; Padilla, V. A Narrative Review on Toxidromes in the Psychiatric Population: Implications for Overdose Prevention. J. Clin. Med. 2025, 14, 6160. https://doi.org/10.3390/jcm14176160
Dutta S, Buciuc AG, Barry P, Padilla V. A Narrative Review on Toxidromes in the Psychiatric Population: Implications for Overdose Prevention. Journal of Clinical Medicine. 2025; 14(17):6160. https://doi.org/10.3390/jcm14176160
Chicago/Turabian StyleDutta, Sanjukta, Adela Georgiana Buciuc, Patrick Barry, and Vanessa Padilla. 2025. "A Narrative Review on Toxidromes in the Psychiatric Population: Implications for Overdose Prevention" Journal of Clinical Medicine 14, no. 17: 6160. https://doi.org/10.3390/jcm14176160
APA StyleDutta, S., Buciuc, A. G., Barry, P., & Padilla, V. (2025). A Narrative Review on Toxidromes in the Psychiatric Population: Implications for Overdose Prevention. Journal of Clinical Medicine, 14(17), 6160. https://doi.org/10.3390/jcm14176160






