Assessment of Serum Endocan Levels and Their Associations with Arterial Stiffness Parameters in Young Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Blood Samples and Standard Laboratory Analyses
2.3. Measurement of Erum Endocan (Endothelial Cell Specific Molecule-1, ESM1)
2.4. Measurement of TNFα
2.5. Measurement of Interleukin-6 (IL-6)
2.6. MPO, MCP-1, MMP-1, MMP-3, MMP-7 and MMP-9 Enzyme-Linked Immunosorbent Assays
2.7. Ultrasound-Based Evaluation of Endothelium-Dependent Vasodilatory Function in the Brachial Artery
2.8. Determination of Carotid Intima-Media Ratio (cIMT)
2.9. Analysis of Arterial Stiffness Parameters
2.10. Statistical Analysis
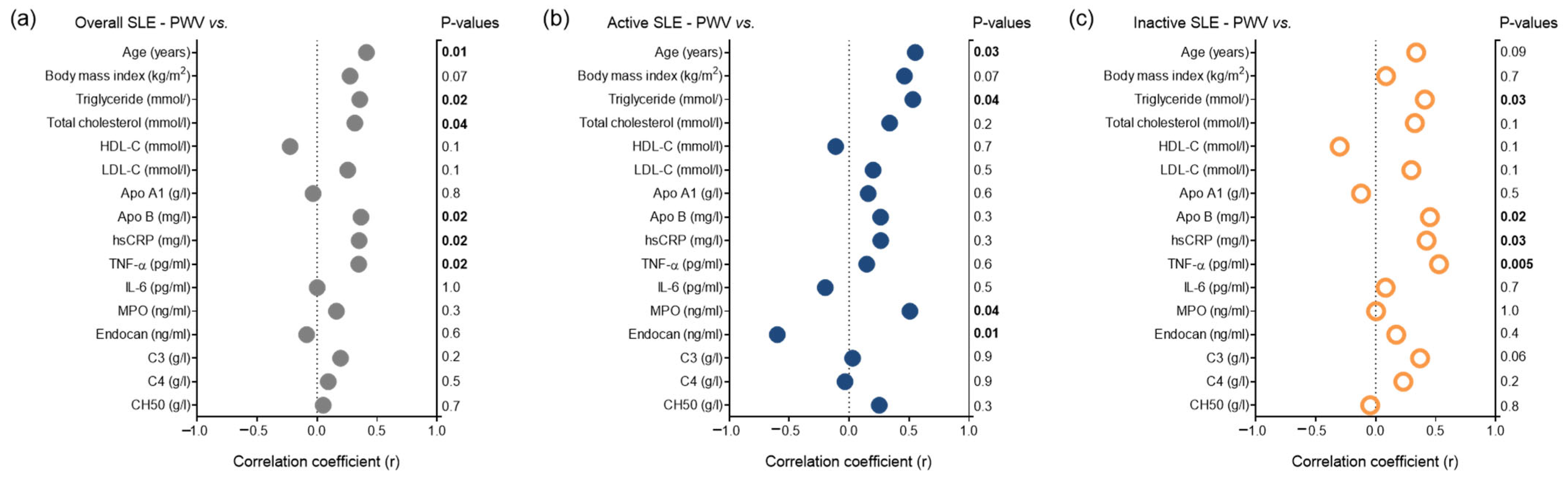
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCL | Anticardiolipin |
| Aix | Augmentation index |
| aPL | Anti-phospholipid antibody |
| APS | Antiphospholipid syndrome |
| B2GPI | Anti-beta-2-glycoprotein I |
| BMI | Body mass index |
| C3 | Complement 3 |
| C4 | Complement 4 |
| CH50 | 50% hemolytic complement |
| cIMT | Carotid intima-media thickness |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DMARDs | Disease-modifying antirheumatic drugs |
| dsDNA | Anti-double-stranded deoxyribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assays |
| FMD | Flow-mediated dilatation |
| HDL-C | High-density lipoprotein-cholesterol |
| hsCRP | High-sensitivity C-reactive protein |
| IL-6 | Interleukin-6 |
| LDL-C | Low-density lipoprotein-cholesterol |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMP-1 | Matrix metalloprotease-1 |
| MMP-3 | Matrix metalloprotease-3 |
| MMP-7 | Matrix metalloprotease-7 |
| MMP-9 | Matrix metalloprotease-9 |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear factor-κB |
| NSAID | Nonsteroidal anti-inflammatory drugs |
| PWV | Pulse wave velocity |
| SLE | Systemic lupus erythematosus |
| SLE DAI | SLE Disease activity index |
| SSA | Anti-Sjögren’s-syndrome-related antigen A |
| SSB | Anti-Sjögren’s-syndrome-related antigen B |
| TNFα | Tumor necrosis factor-alpha |
References
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef]
- Drosos, G.C.; Konstantonis, G.; Sfikakis, P.P.; Tektonidou, M.G. Underperformance of clinical risk scores in identifying vascular ultrasound-based high cardiovascular risk in systemic lupus erythematosus. Eur. J. Prev. Cardiol. 2020, 28, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Charras, A.; Smith, E.; Hedrich, C.M. Systemic Lupus Erythematosus in Children and Young People. Curr. Rheumatol. Rep. 2021, 23, 20. [Google Scholar] [CrossRef]
- Sinicato, N.A.; da Silva Cardoso, P.A.; Appenzeller, S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr. Cardiol. Rev. 2013, 9, 15–19. [Google Scholar] [CrossRef][Green Version]
- Diószegi, Á.; Lőrincz, H.; Kaáli, E.; Soltész, P.; Perge, B.; Varga, É.; Harangi, M.; Tarr, T. Role of Altered Metabolism of Triglyceride-Rich Lipoprotein Particles in the Development of Vascular Dysfunction in Systemic Lupus Erythematosus. Biomolecules 2023, 13, 401. [Google Scholar] [CrossRef]
- Nagy, N.; Bói, B.; Papp, G.; Fiák, E.; Gáspár-Kiss, E.; Perge, B.; Farmasi, N.; Tarr, T. Antiphospholipid Antibodies Are Major Risk Factors for Non-Thrombotic Cardiac Complications in Systemic Lupus Erythematosus. Biomedicines 2024, 12, 530. [Google Scholar] [CrossRef]
- Woodridge, L.; Tektonidou, M.G.; Robinson, G.A.; Peng, J.; Coelewij, L.; Martin-Gutierrez, L.; Navarro, E.C.; Griffin, M.; Nicolaides, A.; Ciurtin, C.; et al. Subclinical Atherosclerosis Risk Can Be Predicted in Female Patients With Systemic Lupus Erythematosus Using Metabolomic Signatures: An Observational Study. J. Am. Heart Assoc. 2025, 14, e036507. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Le Master, E.; Granados, S.T.; Levitan, I. Impairment of endothelial glycocalyx in atherosclerosis and obesity. Curr. Top. Membr. 2023, 91, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gaudette, S.; Hughes, D.; Boller, M. The endothelial glycocalyx: Structure and function in health and critical illness. J. Vet. Emerg. Crit. Care 2020, 30, 117–134. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, L.; Yu, X.H.; Hu, M.; Zhang, Y.K.; Liu, X.; He, P.; Ouyang, X. Endocan: A Key Player of Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 798699. [Google Scholar] [CrossRef]
- Klisic, A.; Patoulias, D. The Role of Endocan in Cardiometabolic Disorders. Metabolites 2023, 13, 640. [Google Scholar] [CrossRef]
- Voiosu, T.; Bălănescu, P.; Benguş, A.; Voiosu, A.; Baicuş, C.R.; Barbu, M.; Ladaru, A.; Nitipir, C.; Mateescu, B.; Diculescu, M.; et al. Serum endocan levels are increased in patients with inflammatory bowel disease. Clin. Lab. 2014, 60, 505–510. [Google Scholar] [CrossRef]
- Jin, H.; Rugira, T.; Ko, Y.S.; Park, S.W.; Yun, S.P.; Kim, H.J. ESM-1 Overexpression is Involved in Increased Tumorigenesis of Radiotherapy-Resistant Breast Cancer Cells. Cancers 2020, 12, 1363. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.A.; Ji, H.I.; Song, R.; Kim, J.Y.; Lee, S.H.; Hong, S.J.; Yoo, M.C.; Yang, H.I. Increased expression of endocan in arthritic synovial tissues: Effects of adiponectin on the expression of endocan in fibroblast-like synoviocytes. Mol. Med. Rep. 2015, 11, 2695–2702. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Endocan--the new endothelial activation marker independently associated with soluble endothelial adhesion molecules in uraemic patients with cardiovascular disease. Clin. Biochem. 2015, 48, 425–430. [Google Scholar] [CrossRef]
- Elkamshoushi, A.A.M.; Hassan, E.M.; El Abd, A.M.; Hassan, S.Z.; Maher, A.A. Serum endocan as a predictive biomarker of cardiovascular risk in erectile dysfunction patients. Andrologia 2018, 50, e13113. [Google Scholar] [CrossRef]
- Tokarska, K.; Bogaczewicz, J.; Robak, E.; Woźniacka, A. The role of endocan and selected pro-inflammatory cytokines in systemic lupus erythematosus. Postep. Dermatol. Alergol. 2020, 37, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Icli, A.; Cure, E.; Cure, M.C.; Uslu, A.U.; Balta, S.; Mikhailidis, D.P.; Ozturk, C.; Arslan, S.; Sakız, D.; Sahin, M.; et al. Endocan Levels and Subclinical Atherosclerosis in Patients With Systemic Lupus Erythematosus. Angiology 2016, 67, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Szucs, G.; Tímár, O.; Szekanecz, Z.; Dér, H.; Kerekes, G.; Szamosi, S.; Shoenfeld, Y.; Szegedi, G.; Soltész, P. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis--relevance for prevention of vascular complications. Rheumatology 2007, 46, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Baulmann, J.; Schillings, U.; Rickert, S.; Uen, S.; Düsing, R.; Illyes, M.; Cziraki, A.; Nickering, G.; Mengden, T. A new oscillometric method for assessment of arterial stiffness: Comparison with tonometric and piezo-electronic methods. J. Hypertens. 2008, 26, 523–528. [Google Scholar] [CrossRef]
- Magometschnigg, D. Blood pressure and arterial stiffness. A comparison of two devices for measuring augmentationindex and pulse wave velocity. Wien. Med. Wochenschr. 2005, 155, 404–410. [Google Scholar] [CrossRef]
- Horváth, I.G.; Németh, A.; Lenkey, Z.; Alessandri, N.; Tufano, F.; Kis, P.; Gaszner, B.; Cziráki, A. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J. Hypertens. 2010, 28, 2068–2075. [Google Scholar] [CrossRef]
- Nayak, S.S.; Ameen, D.; Nobakht, S.; Nayak, R.; Prabhu, S.V.; Keivanlou, M.H.; Hassanipour, S.; Amini-Salehi, E.; Thakker, N. The predictive value of endocan as a novel biomarker: An umbrella study on meta-analyses. Syst. Rev. 2025, 14, 98. [Google Scholar] [CrossRef]
- Tuzcu, G.; Uslu, A.U.; Tuzcu, A.; Aydoğan Baykara, R.; Omma, A.; Küçük, A. A novel marker relationship between carotid intima media thickness and disease activity score-28 in patients with rheumatoid arthritis: Human endothelial cell-specific molecule-1. Turk. J. Med. Sci. 2019, 49, 1599–1605. [Google Scholar] [CrossRef]
- Nalbantoğlu, A.; Kızılca, Ö.; Güzel, S.; Emeksiz, H.C.; Nalbantoğlu, B. Increased Carotid Intima-Media Thickness and Endothelial Cell-Specific Molecule-1 (Endocan) Levels in Obese Children. Angiology 2021, 72, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Ayano, M.; Horiuchi, T. Complement as a Biomarker for Systemic Lupus Erythematosus. Biomolecules 2023, 13, 367. [Google Scholar] [CrossRef]
- Spronk, P.E.; Limburg, P.C.; Kallenberg, C.G. Serological markers of disease activity in systemic lupus erythematosus. Lupus 1995, 4, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260. [Google Scholar] [CrossRef]
- Kirkgöz, K. C-Reactive Protein in Atherosclerosis-More than a Biomarker, but not Just a Culprit. Rev. Cardiovasc. Med. 2023, 24, 297. [Google Scholar] [CrossRef]
- Sakurai, S.; Kitamura, A.; Cui, R.; Yamagishi, K.; Tanigawa, T.; Iso, H. Relationships of soluble E-selectin and high-sensitivity C-reactive protein with carotid atherosclerosis in Japanese men. J. Atheroscler. Thromb. 2009, 16, 339–345. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 2005, 46, 1118–1122. [Google Scholar] [CrossRef]
- Cassano, V.; Tripepi, G.; Perticone, M.; Miceli, S.; Scopacasa, I.; Armentaro, G.; Greco, M.; Maio, R.; Hribal, M.L.; Sesti, G.; et al. Endothelial progenitor cells predict vascular damage progression in naive hypertensive patients according to sex. Hypertens. Res. 2021, 44, 1451–1461. [Google Scholar] [CrossRef]
- Suszek, D.; Popławska, M.; Prośniak, J.; Siemieniec, K.; Przeniosło, K.; Wallach, W.; Żybowska-Męczyńska, M.; Ostrowicz, K.; Rzewuska-Fijałkowska, A.; Targońska-Stępniak, B. A novel approach to cardiovascular events in patients with systemic lupus erythematosus: Risk factor assessment and treatment analysis. Rheumatol. Int. 2025, 45, 139. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.A.; Kiani, A.N.; Post, W.; Christopher-Stine, L.; Magder, L.S. Lupus Atherosclerosis Prevention Study (LAPS). Ann. Rheum. Dis. 2011, 70, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, K.; Tsuchida, Y.; Komai, T.; Tsuchiya, H.; Fujio, K. Advances in Targeted Therapy for Systemic Lupus Erythematosus: Current Treatments and Novel Approaches. Int. J. Mol. Sci. 2025, 26, 929. [Google Scholar] [CrossRef]
| All Patients | SLE DAI ≥ 6 | SLE DAI < 6 | p-Value | |
|---|---|---|---|---|
| Number of patients (n) | 47 | 19 | 28 | |
| Female (n; %) | 40 (85.1) | 16 (84.2) | 24 (85.7) | 0.845 |
| Age (years) | 31.9 ± 6.0 | 30.0 ± 7.2 | 33.3 ± 4.8 | 0.070 |
| BMI (kg/m2) | 24.4 ± 4.3 | 24.6 ± 5.0 | 24.3 ± 3.8 | 0.818 |
| Smokers (n, %) | 18 (38.3) | 7 (36.8) | 11 (39.3) | 0.771 |
| aPL antibodies (n, %) | 7 (14.9) | 5 (26.3) | 2 (7.1) | <0.001 |
| Prednisolone (n, %) | 47 (100.0) | 19 (100.0) | 28 (100.0) | 1.000 |
| Chloroquine (n, %) | 22 (46.8) | 8 (42.1) | 14 (50.0) | 0.256 |
| NSAIDs (n, %) | 17 (36.2) | 7 (36.8) | 10 (35.7) | 0.883 |
| DMARDs (n, %) | 32 (68.1) | 14 (73.7) | 18 (64.3) | 0.126 |
| SLE DAI | 4.0 (2.0–10.0) | 10.0 (8.0–12.0) | 4.0 (2.0–4.0) | <0.001 |
| Vascular diagnostic tests | ||||
| cIMT (cm) | 0.049 ± 0.010 | 0.052 ± 0.012 | 0.047 ± 0.008 | 0.156 |
| FMD (%) | 7.8 (5.8–12.0) | 7.4 (5.6–11.1) | 8.1 (6.6–12.3) | 0.502 |
| Aix (%) | −15.4 (−36.8–11.9) | −23.2 (−37.6–8.0) | −10.8 (−35.9–15.2) | 0.386 |
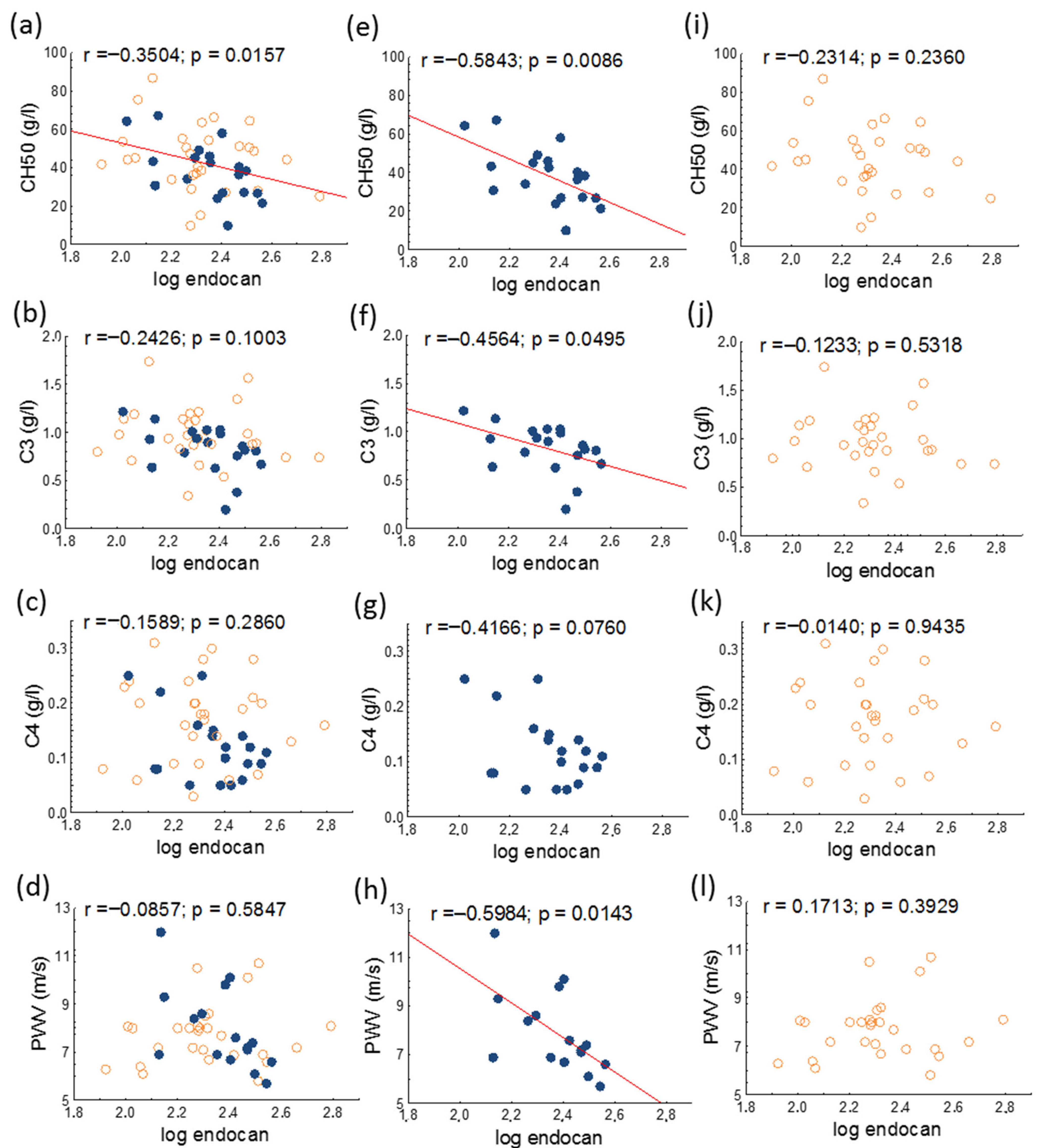
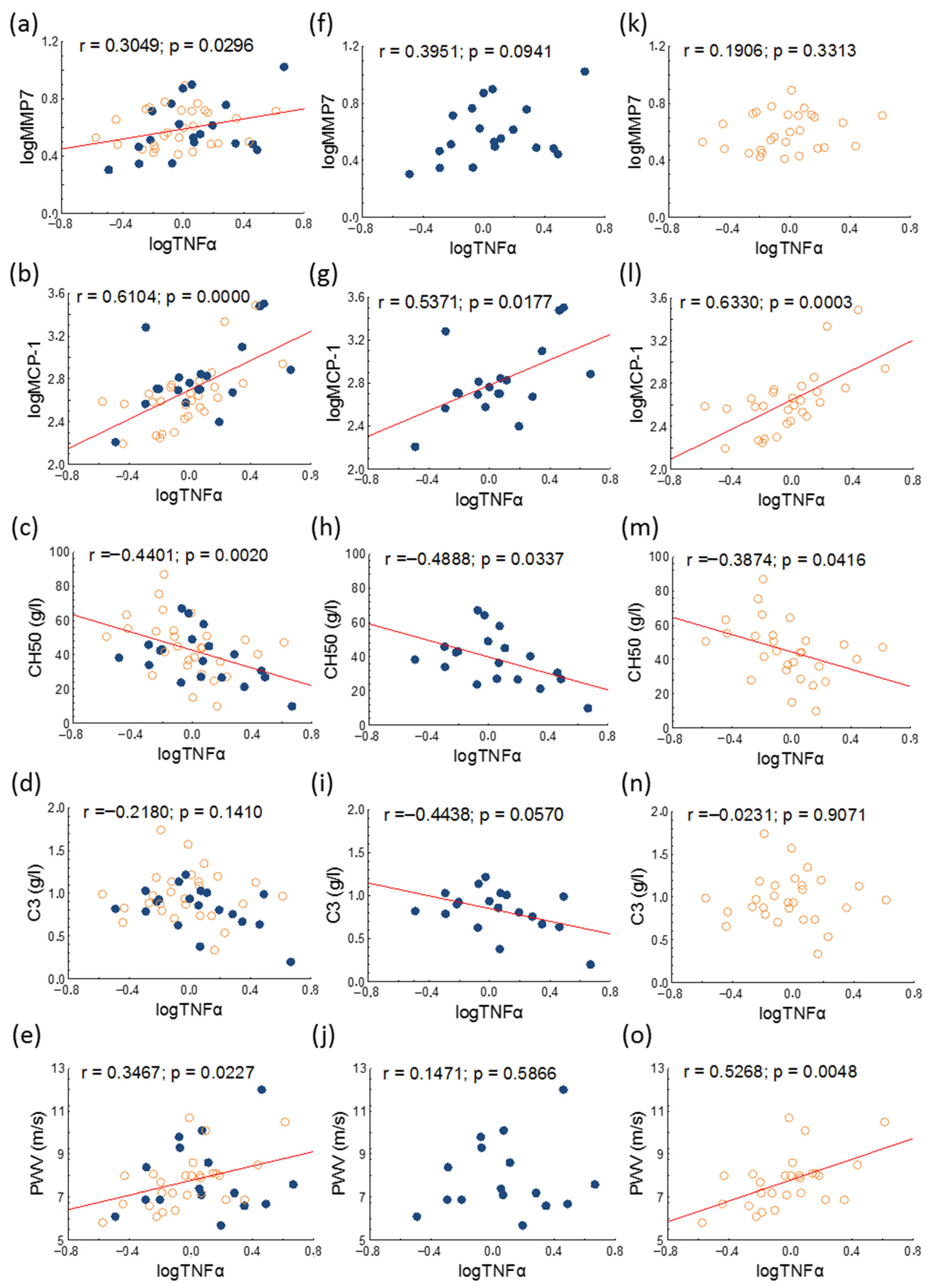
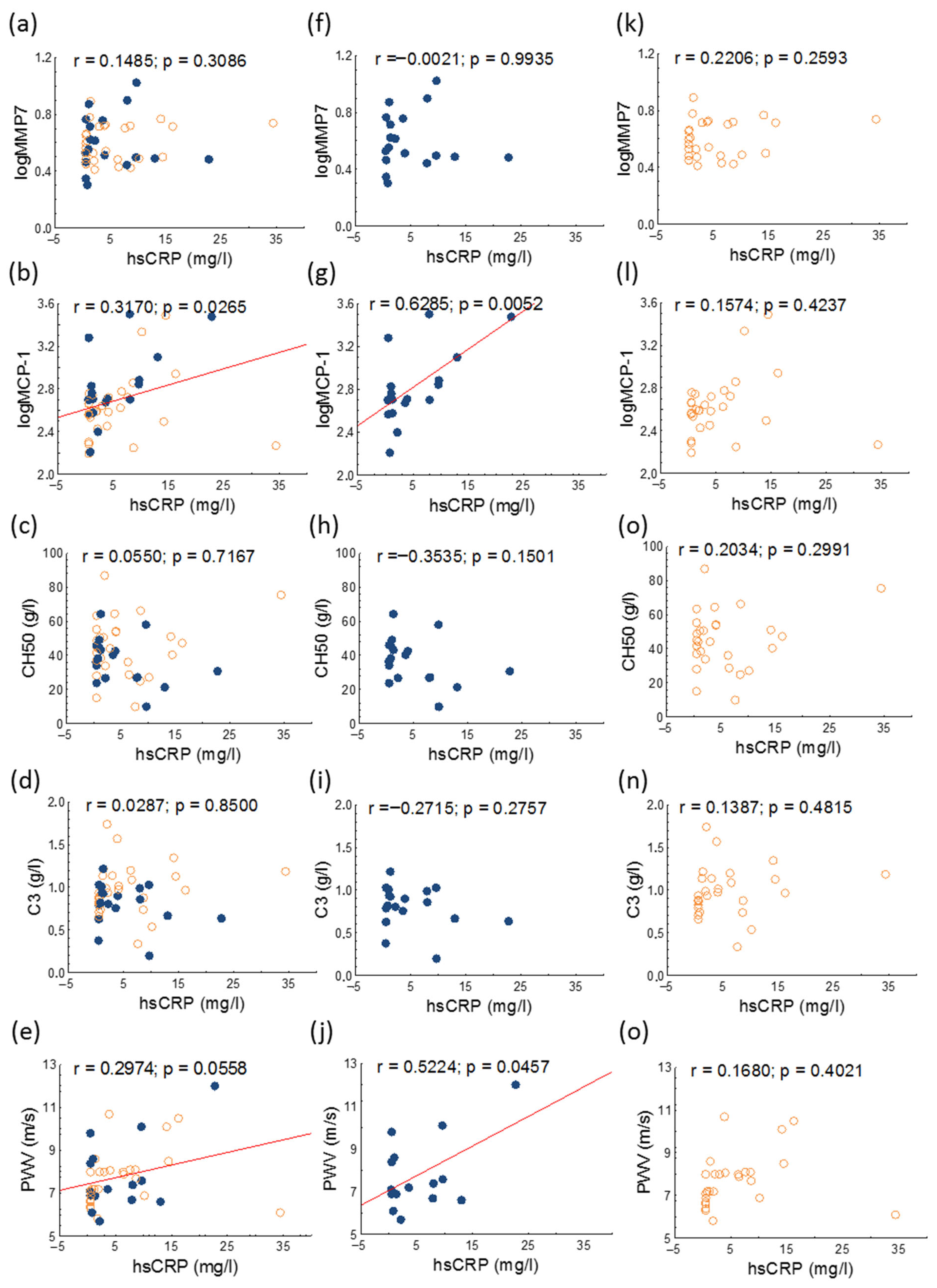
| PWV (m/s) | 7.8 ± 1.4 | 7.9 ± 1.7 | 7.7 ± 1.2 | 0.704 |
| Laboratory parameters | ||||
| Complement 3 (g/L) | 0.92 ± 0.28 | 0.83 ± 0.25 | 0.98 ± 0.29 | 0.068 |
| Complement 4 (g/L) | 0.15 ± 0.08 | 0.12 ± 0.06 | 0.17 ± 0.08 | 0.021 |
| CH50 (g/L) | 42.49 ± 16.39 | 38.54 ± 14.74 | 45.2 ± 17.2 | 0.176 |
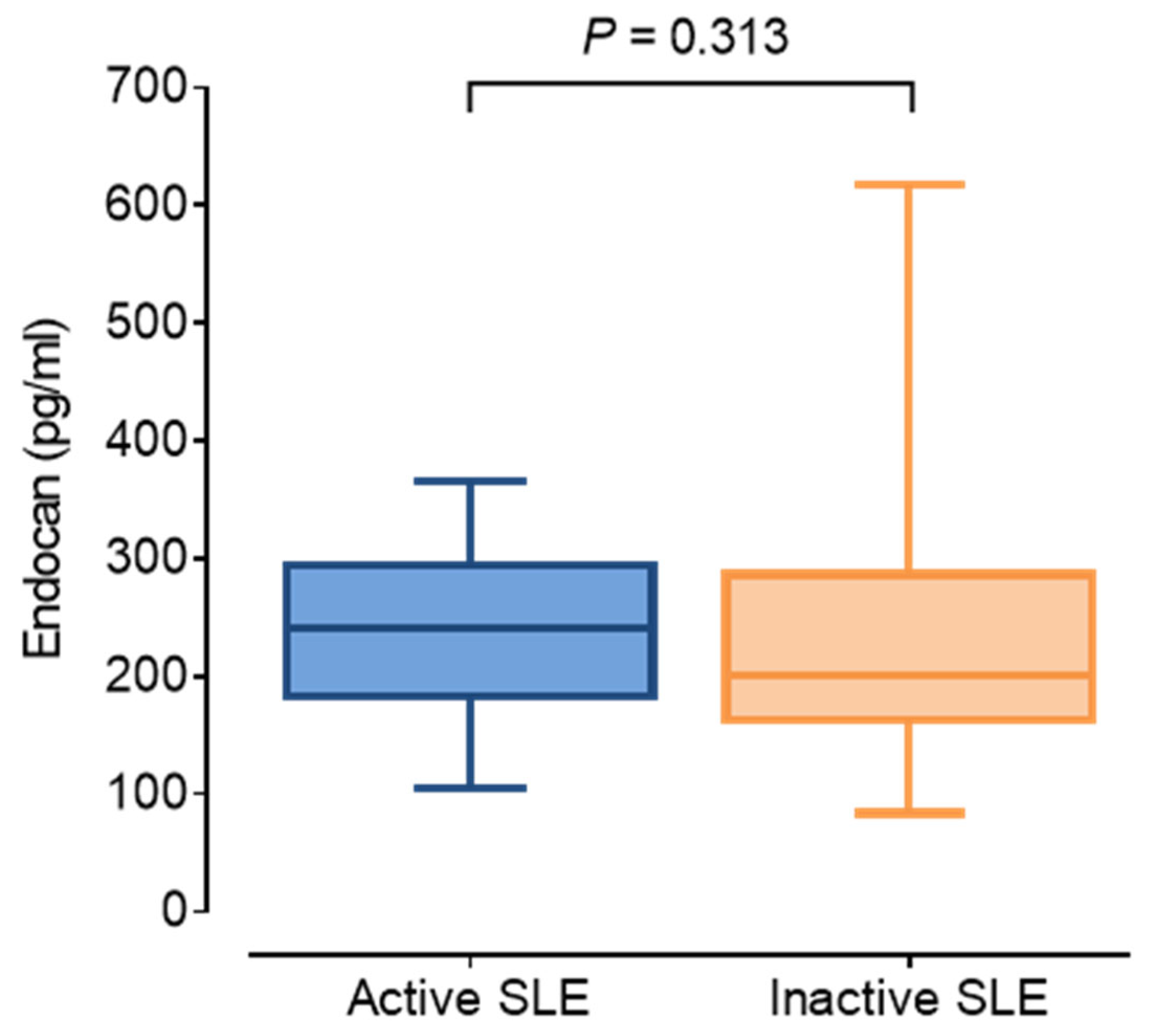
| Endocan (pg/mL) | 208.6 (175.5–294.5) | 241.4 (183.2–294.5) | 200.3 (167.0–277.9) | 0.313 |
| hsCRP (mg/L) | 2.1 (0.57–8.00) | 1.7 (0.76–8.00) | 2.5 (0.54–8.04) | 0.822 |
| Interleukin-6 (pg/mL) | 0.54 (0.0–3.25) | 0.85 (0.0–2.51) | 0.50 (0.07–3.44) | 0.845 |
| TNFα (pg/mL) | 0.99 (0.63–1.46) | 1.13 (0.62–1.91) | 0.96 (0.64–1.32) | 0.398 |
| Myeloperoxidase (ng/mL) | 465.8 (301.9–835.0) | 385.8 (276.8–537.2) | 648.3 (351.9–957.0) | 0.046 |
| Serum amyloid A (μg/mL) | 4.08 (1.49–14.07) | 3.76 (2.53–14.07) | 7.49 (1.40–14.33) | 0.756 |
| MCP-1 (pg/mL) | 473.2 (362.4–649.9) | 512.8 (473.2–772.3) | 395.5 (299.0–545.2) | 0.028 |
| MMP3 (ng/mL) | 31.5 (15.0–50.8) | 29.2 (16.2–45.9) | 32.1 (13.5–53.1) | 0.905 |
| MMP7 (ng/mL) | 3.58 (3.04–5.26) | 3.39 (2.93–5.75) | 3.82 (3.05–5.23) | 0.770 |
| MMP9 (ng/mL) | 298.4 (176.3–479.9) | 215.0 (98.9–324.8) | 339.6 (246.8–687.5) | 0.011 |
| Triglyceride (mmol/L) | 1.30 (1.00–1.90) | 1.25 (1.00–1.90) | 1.35 (1.90–1.15) | 0.893 |
| Total cholesterol (mmol/L) | 4.5 ± 1.1 | 4.3 ± 1.1 | 4.6 ± 1.1 | 0.440 |
| HDL-C (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.3 ± 0.5 | 0.441 |
| LDL-C (mmol/L) | 2.6 ± 0.8 | 2.6 ± 0.9 | 2.5 ± 0.8 | 0.554 |
| Apolipoprotein B100 (g/L) | 0.85 ± 0.27 | 0.88 ± 0.28 | 0.84 ± 0.26 | 0.618 |
| Apolipoprotein A1 (g/L) | 1.38 ± 0.33 | 1.29 ± 0.26 | 1.44 ± 0.37 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diószegi, Á.; Lőrincz, H.; Kaáli, E.; Csiha, S.; Kaluha, J.; Varga, É.; Páll, D.; Tarr, T.; Harangi, M. Assessment of Serum Endocan Levels and Their Associations with Arterial Stiffness Parameters in Young Patients with Systemic Lupus Erythematosus. J. Clin. Med. 2025, 14, 5955. https://doi.org/10.3390/jcm14175955
Diószegi Á, Lőrincz H, Kaáli E, Csiha S, Kaluha J, Varga É, Páll D, Tarr T, Harangi M. Assessment of Serum Endocan Levels and Their Associations with Arterial Stiffness Parameters in Young Patients with Systemic Lupus Erythematosus. Journal of Clinical Medicine. 2025; 14(17):5955. https://doi.org/10.3390/jcm14175955
Chicago/Turabian StyleDiószegi, Ágnes, Hajnalka Lőrincz, Eszter Kaáli, Sára Csiha, Judit Kaluha, Éva Varga, Dénes Páll, Tünde Tarr, and Mariann Harangi. 2025. "Assessment of Serum Endocan Levels and Their Associations with Arterial Stiffness Parameters in Young Patients with Systemic Lupus Erythematosus" Journal of Clinical Medicine 14, no. 17: 5955. https://doi.org/10.3390/jcm14175955
APA StyleDiószegi, Á., Lőrincz, H., Kaáli, E., Csiha, S., Kaluha, J., Varga, É., Páll, D., Tarr, T., & Harangi, M. (2025). Assessment of Serum Endocan Levels and Their Associations with Arterial Stiffness Parameters in Young Patients with Systemic Lupus Erythematosus. Journal of Clinical Medicine, 14(17), 5955. https://doi.org/10.3390/jcm14175955









