Cytoreductive Surgery in Ovarian Cancer: Should the New Optimal Threshold Be 2.5 mm?
Abstract
1. Introduction
2. Material and Methods
2.1. Patient’s Management
2.2. Intraperitoneal Chemotherapy
2.3. Statistical Analysis
3. Results
3.1. Short-Term Outcomes
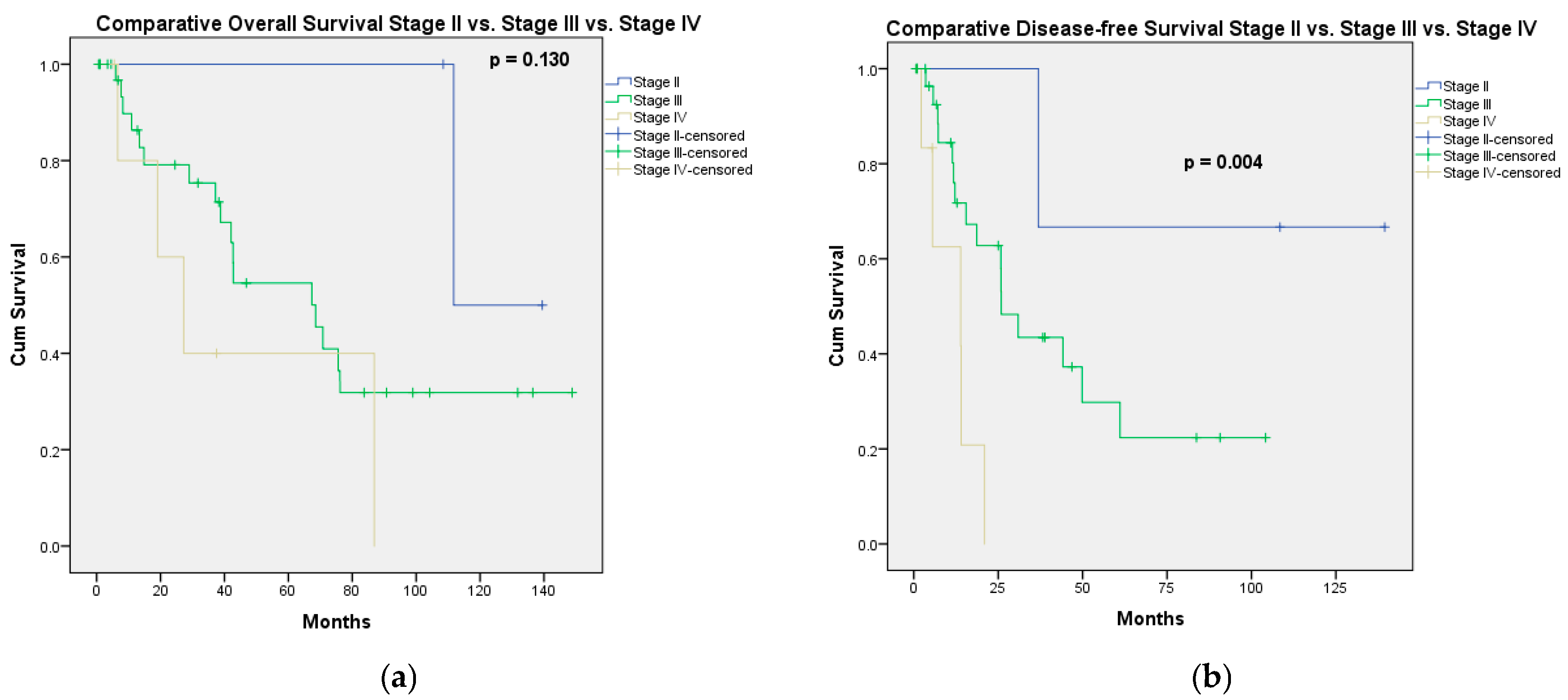
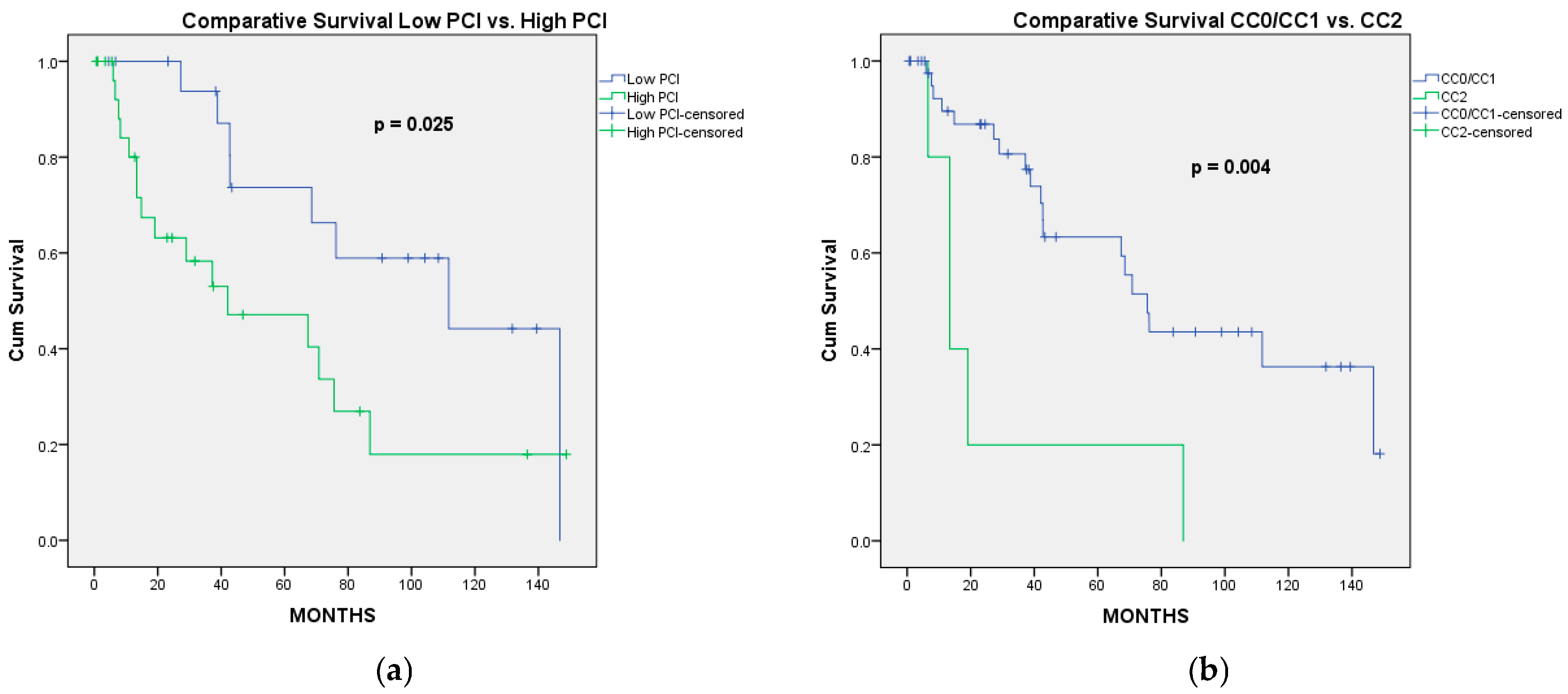
3.2. Long-Term Outcomes
3.2.1. Overall Survival
3.2.2. Progression-Free Survival
4. Discussion
4.1. Limitations
4.2. Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRS | cytoreductive surgery |
| RCT | randomized clinical trial |
| PDS | primary debulking surgery |
| IDS | interval debulking surgery |
| NACT | neoadjuvant chemotherapy |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| CC | completeness of cytoreduction |
| PFS | progression-free survival |
| OS | overall survival |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
References
- Ghirardi, V.; Fagotti, A.; Ansaloni, L.; Valle, M.; Roviello, F.; Sorrentino, L.; Accarpio, F.; Baiocchi, G.; Piccini, L.; De Simone, M.; et al. Diagnostic and Therapeutic Pathway of Advanced Ovarian Cancer with Peritoneal Metastases. Cancers 2023, 15, 407. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.E.; Pothuri, B.; Ray-Coquard, I.; Tan, D.S.P.; Bellet, E.; Oaknin, A.; et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef]
- Bois, A.D.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef]
- Liu, J.; Berchuck, A.; Backes, F.J.; Cohen, J.; Grisham, R.; Leath, C.A.; Martin, L.; Matei, D.; Miller, D.S.; Robertson, S.; et al. NCCN Guidelines® Insights: Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer, Version 3.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 512–519. [Google Scholar] [CrossRef]
- Chang, S.-J.; Bristow, R.E. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: Redefining “optimal” residual disease. Gynecol. Oncol. 2012, 125, 483–492. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Lang, J.; Huh, J.; Haddad, L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Hensley, M.; Barakat, R.R. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol. Oncol. 2006, 103, 559–564. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef]
- Angarita, A.M.; Stone, R.; Temkin, S.M.; Levinson, K.; Fader, A.N.; Tanner, E.J. The use of “Optimal Cytoreduction” Nomenclature in Ovarian Cancer Literature: Can We Move Toward a More Optimal Classification System? Int. J. Gynecol. Cancer 2016, 26, 1421–1427. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Review of a personal experience in the management of carcinomatosis and sarcomatosis. Jpn. J. Clin. Oncol. 2001, 31, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Res. Treat. 1996, 82, 359–374. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef] [PubMed]
- Vachez, E.; Kefleyesus, A.; Bakrin, N.; Ranchon, F.; Rioufol, C.; Vassal, O.; Al-Hadeedi, O.; Kepenekian, V.; Glehen, O. Eliminating the need for preoperative intravenous hyperhydration: Sodium thiosulfate as nephrotoxicity prevention in HIPEC-treated patients—A retrospective analysis. Eur. J. Surg. Oncol. 2024, 50, 107955. [Google Scholar] [CrossRef]
- Meigs, J.V. Tumors of the Female Pelvic Organs; Macmillan: New York, NY, USA, 1934. [Google Scholar]
- Griffiths, C.T. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. J. Natl. Cancer Inst. Monogr. 1975, 42, 101–104. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 1.2025. Mar. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 30 March 2025).
- DiSilvestro, P.A.; O’Malley, D.M.; Eskander, R.N.; Moore, K.N.; Coleman, R.L. Ovarian Cancer Trials; The GOG Foundation, Inc.: Philadelphia, PA, USA, 2023. [Google Scholar]
- Winter, W.E.; Maxwell, G.L.; Tian, C.; Sundborg, M.J.; Rose, G.S.; Rose, P.G.; Rubin, S.C.; Muggia, F.; McGuire, W.P.; Gynecologic Oncology Group. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2008, 26, 83–89. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; van Leeuwen, J.H.S.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.-J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.-Y.; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Torun, B.C.; Glehen, O.; Kepenekian, V.; Sardi, A.; Arjona-Sanchez, A.; Yonemura, Y.; Barat, S.; Morris, D.; Spiliotis, J.; Coccolini, F.; et al. Peritoneal metastasis of advanced epithelial ovarian carcinoma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A retrospective international multicentric data analysis. Eur. J. Surg. Oncol. 2023, 49, 1489–1494. [Google Scholar] [CrossRef]
- Look, M.; Chang, D.; Sugarbaker, P.H. Long-term results of cytoreductive surgery for advanced and recurrent epithelial ovarian cancers and papillary serous carcinoma of the peritoneum. Int. J. Gynecol. Cancer 2003, 13, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.-A.K.; Tripsiannis, G.; Markakidis, S.K.; Karanikiotis, C.N.; Tzegas, G.; Georgiadis, G.; Avgidou, K. Peritoneal cancer index: A prognostic indicator of survival in advanced ovarian cancer. Eur. J. Surg. Oncol. 2003, 29, 69–73. [Google Scholar] [CrossRef]
- Muñoz-Casares, F.C.; Medina-Fernández, F.J.; Arjona-Sánchez, Á.; Casado-Adam, Á.; Sánchez-Hidalgo, J.M.; Rubio, M.J.; Ortega-Salas, R.; Muñoz-Villanueva, M.C.; Rufián-Peña, S.; Briceño, F.J. Peritonectomy procedures and HIPEC in the treatment of peritoneal carcinomatosis from ovarian cancer: Long-term outcomes and perspectives from a high-volume center. Eur. J. Surg. Oncol. 2016, 42, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Naticchioni, E.; Biacchi, D.; Sibio, S.; Accarpio, F.; Rocco, M.; Tarquini, S.; Di Seri, M.; Ciardi, A.; Montruccoli, D.; et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008, 113, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Eisenkop, S.M.; Spirtos, N.M.; Friedman, R.L.; Lin, W.-C.M.; Pisani, A.L.; Perticucci, S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: A prospective study. Gynecol. Oncol. 2003, 90, 390–396. [Google Scholar] [CrossRef]
- Bakrin, N.; Bereder, J.M.; Decullier, E.; Classe, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Yang, S.-L.; Si, L.-H.; Lin, R.-X.; Gu, S.-Y.; Li, J.-H.; Cui, J.-Z.; Yan, C.-H.; Farah, A.M.; Jia, Y. Prognostic role of the peritoneal cancer index in ovarian cancer patients who undergo cytoreductive surgery: A meta-analysis. Curr. Probl. Cancer 2023, 47, 101014. [Google Scholar] [CrossRef]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef]
| Age | 62 [53.25–66.5] Years |
|---|---|
| CA-125 | 400 [30.5–1000] U/mL * |
| PCI | 11.5 [3–17] |
| Stage | |
| IIA | 1 |
| IIB | 2 |
| IIIA | 2 |
| IIIB | 8 |
| IIIC | 27 |
| IVA | 1 |
| IVB | 5 |
| Recurrent peritoneal metastases | 6 |
| Preoperative chemotherapy | |
| Yes | 17 |
| No | 35 |
| Resected organs | |
| THSO | 46 |
| Omentectomy | 49 |
| Peritonectomy | |
| No | 9 |
| Partial | 24 |
| Complete | 19 |
| Small bowel resection | 12 |
| Colorectal resection | 28 |
| Splenectomy | 11 |
| Cholecistectomy | 10 |
| Liver resection | 4 |
| Gastrectomy | 3 |
| Cistectomy | 1 |
| Aletti score | |
| Low (1–3) | 9 |
| Intermediate (4–7) | 24 |
| High (≥8) | 19 |
| HIPEC | |
| No | 32 |
| Yes | 20 |
| Completeness of cytoreduction | |
| CC0 | 35 |
| CC1 | 12 |
| CC2 “optimal” (<10 mm.) | 5 |
| Pathologic type | |
| High-grade serous carcinoma | 39 |
| Low-grade serous carcinoma | 8 |
| Carcino-sarcoma | 2 |
| Borderline serous tumor with peritoneal implants | 2 |
| Mucinous cystic carcinoma | 1 |
| Stage | PCI | CC Score | |||
|---|---|---|---|---|---|
| ≤10 | >10 | CC0 | CC1 | CC2 | |
| Stage 2 | 3 | 0 | 3 | 0 | 0 |
| Stage 3 | 13 | 24 | 24 | 11 | 2 |
| Stage 4 | 1 | 5 | 3 | 1 | 2 |
| Recurrence | 4 | 2 | 5 | 0 | 1 |
| Type of Complication | Number of Complications | Number of Death |
|---|---|---|
| Pleural effusion | 2 | |
| Intestinal leak | 3 | |
| Intraabdominal abscess | 3 | 1 |
| Wound infection | 1 | |
| Bile leak | 1 | |
| Wound hematoma | 1 | |
| Clostridium difficile colitis | 3 | |
| Parastomal abscess | 1 | |
| Pneumonia | 1 | |
| Pancreatic fistula | 1 | |
| MSOF | 1 | 1 |
| Stroke | 1 | |
| Urinary tract infection | 1 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | |
| PCI actual | 0.025 * | 0.121 | ||
| ≤10 | 0.482 | 0.192–1.212 | ||
| >10 | 1 | – | ||
| Preoperative chemo No Yes | 0.366 | |||
| Timing of resection Primary debulking surgery Interval debulking surgery Peritoneal recurrence | 0.766 | |||
| Intraperitoneal chemotherapy No Normothermic HIPEC | 0.254 | |||
| Completeness of cytoreduction | 0.004 * | 0.008 * | ||
| CC0/CC1 | 0.253 | 0.092–0.696 | ||
| CC2 | 1 | – | ||
| FIGO Stage Stage II Stage III Stage IV Recurrence | 0.205 | |||
| Major complications No Yes | 0.345 | |||
| Aletti score Low (1–3) Medium/high (≥4) | 0.090 | 0.490 | ||
| 1 | – | |||
| 1.261 | 0.653–2.437 | |||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p Value | HR | 95% CI | p-Value | |
| PCI actual ≤10 >10 | 0.069 | 0.767 | ||
| 0.819 | 0.220–3.054 | |||
| 1 | – | |||
| Preoperative chemo No Yes | 0.587 | |||
| Timing of resection Primary debulking surgery Interval debulking surgery Peritoneal recurrence | 0.771 | |||
| Intraperitoneal chemotherapy No HIPEC Normothermic | 0.128 | 0.371 | ||
| 0.766 | 0.270–2.175 | |||
| 0.411 | 0.119–1.418 | |||
| 1 | – | |||
| Completeness of cytoreduction CC0/CC1 CC2 | 0.012 * | 0.018 * | ||
| 0.295 | 0.107–0.811 | |||
| 1 | – | |||
| FIGO Stage Stage III Stage IV Recurrence | 0.333 | |||
| Major complications No Yes | 0.454 | |||
| Aletti score Low (1–3) Medium/high (≥4) | 0.103 | 0.195 | ||
| 0.480 | 0.158–1.458 | |||
| 1 | – | |||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | |
| PCI actual ≤10 >10 | 0.028 * | 0.075 | ||
| 0.422 | 0.163–1.091 | |||
| 1 | – | |||
| Preoperative chemo No Yes | 0.110 | 0.041 * | ||
| 0.387 | 0.155–0.963 | |||
| 1 | 1 | |||
| Timing of resection Primary debulking surgery Interval debulking surgery Peritoneal recurrence | 0.479 | |||
| Intraperitoneal chemotherapy No Normothermic HIPEC | 0.552 | |||
| Completeness of cytoreduction CC0/CC1 CC2 | 0.001 * | 0.003 * | ||
| 0.155 | 0.046–0.527 | |||
| 1 | – | |||
| FIGO Stage Stage II Stage III Stage IV Recurrence | 0.004 * | 0.313 | ||
| 0.422 | 0.039–4.565 | |||
| 1.023 | 0.259–4.039 | |||
| 2.969 | 0.629–14.008 | |||
| 1 | – | |||
| Major complications No Yes | 0.806 | |||
| Aletti score Low (1–3) Medium/high (≥4) | 0.180 | |||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | |
| PCI actual ≤10 >10 | 0.092 | 0.429 | ||
| 0.672 | 0.251—1.797 | |||
| 1 | – | |||
| Preoperative chemo No Yes | 0.198 | |||
| Timing of resection Primary debulking surgery Interval debulking surgery Peritoneal recurrence | 0.554 | |||
| Intraperitoneal chemotherapy No Normothermic HIPEC | 0.403 | |||
| Completeness of cytoreduction CC0/CC1 CC2 | 0.001 * | 0.001 * | ||
| 0.127 | 0.040—0.407 | |||
| 1 | – | |||
| FIGO Stage Stage III Stage IV Recurrence | 0.008 * | 0.354 | ||
| 1.449 | 0.408–5.148 | |||
| 3.242 | 0.626–16.782 | |||
| 1 | – | |||
| Major complications No Yes | 0.916 | |||
| Aletti score Low (1–3) Medium/high (≥4) | 0.194 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorie, T.R.; Potlog, G.; Verdea, C.; Chiriac, T.D.; Popescu, G.A.; Minca, D.G.; Costea, R.V.; Brebu, D.; Alexandrescu, S.T. Cytoreductive Surgery in Ovarian Cancer: Should the New Optimal Threshold Be 2.5 mm? J. Clin. Med. 2025, 14, 6094. https://doi.org/10.3390/jcm14176094
Grigorie TR, Potlog G, Verdea C, Chiriac TD, Popescu GA, Minca DG, Costea RV, Brebu D, Alexandrescu ST. Cytoreductive Surgery in Ovarian Cancer: Should the New Optimal Threshold Be 2.5 mm? Journal of Clinical Medicine. 2025; 14(17):6094. https://doi.org/10.3390/jcm14176094
Chicago/Turabian StyleGrigorie, Tudor Razvan, Gheorghe Potlog, Cosmin Verdea, Teodora Delia Chiriac, George Andrei Popescu, Dana Galieta Minca, Radu Virgil Costea, Dan Brebu, and Sorin Tiberiu Alexandrescu. 2025. "Cytoreductive Surgery in Ovarian Cancer: Should the New Optimal Threshold Be 2.5 mm?" Journal of Clinical Medicine 14, no. 17: 6094. https://doi.org/10.3390/jcm14176094
APA StyleGrigorie, T. R., Potlog, G., Verdea, C., Chiriac, T. D., Popescu, G. A., Minca, D. G., Costea, R. V., Brebu, D., & Alexandrescu, S. T. (2025). Cytoreductive Surgery in Ovarian Cancer: Should the New Optimal Threshold Be 2.5 mm? Journal of Clinical Medicine, 14(17), 6094. https://doi.org/10.3390/jcm14176094







