Reducing Peritoneal Cell Dissemination in Laparoscopic Uterine Surgery: A Comparative Pilot Study on Morcellation Techniques and Peritoneal Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Groups and Sample Collection
2.3. Sample Preparation
2.4. Statistical Analysis
2.5. AI Statement
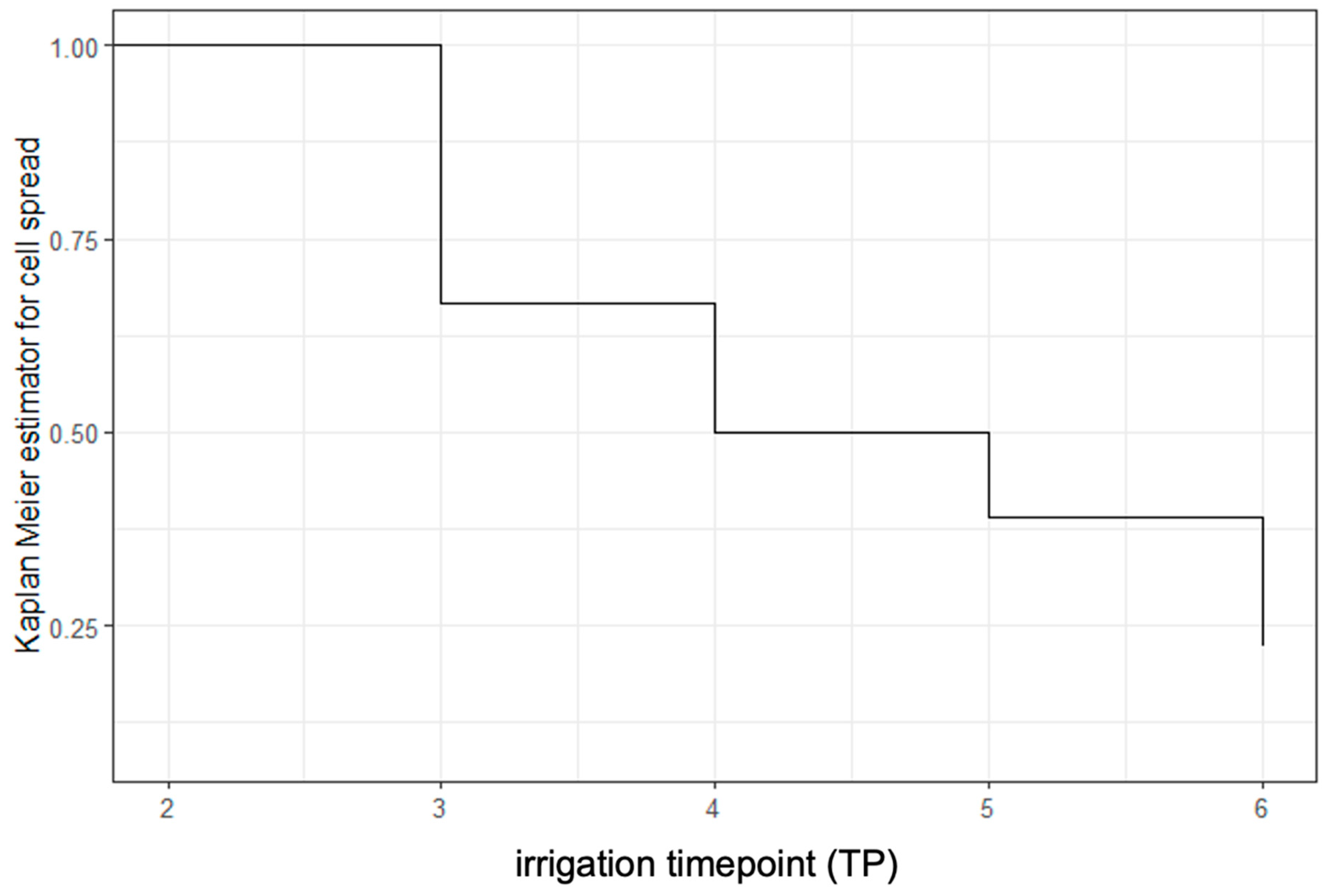
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LM | Laparoscopic myomectomy |
| TLH | Total laparoscopic hysterectomy |
| TP | Timepoint |
| ml | Milliliters |
| FDA | U.S. Food and Drug Administration |
| CRF | Case Report Form |
| cc | Cubic centimeter |
| BMI | Body mass index |
| (u)LMS | (Uterine) leiomyosacroma |
| min | Minimum |
| max | Maximum |
| cm | Centimeter |
| STUMP | Smooth muscle tumor of uncertain malignant potential |
| FIGO | The International Federation of Gynecology and Obstetrics |
References
- Jin, C.; Hu, Y.; Chen, X.; Zheng, F.; Lin, F.; Zhou, K.; Chen, F.; Gu, H. Laparoscopic versus open myomectomy--a meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 14–21. [Google Scholar] [CrossRef]
- Steiner, R.A.; Wight, E.; Tadir, Y.; Haller, U. Electrical cutting device for laparoscopic removal of tissue from the abdominal cavity. Obstet. Gynecol. 1993, 81, 471–474. [Google Scholar] [PubMed]
- FDA. UPDATE: Perform Only Contained Morcellation When Laparoscopic Power Morcellation Is Appropriate: FDA Safety Communication. Published Online 30 December 2020. Available online: https://www.fda.gov/medical-devices/safety-communications/update-perform-only-contained-morcellation-when-laparoscopic-power-morcellation-appropriate-fda (accessed on 14 February 2023).
- Harris, J.A.; Swenson, C.W.; Uppal, S.; Kamdar, N.; Mahnert, N.; As-Sanie, S.; Morgan, D.M. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am. J. Obstet. Gynecol. 2016, 214, e1–e98. [Google Scholar] [CrossRef]
- Brölmann, H.; Tanos, V.; Grimbizis, G.; Ind, T.; Philips, K.; van den Bosch, T.; Sawalhe, S.; van den Haak, L.; Jansen, F.-W.; Pijnenborg, J.; et al. Options on fibroid morcellation: A literature review. Gynecol. Surg. 2015, 12, 3–15. [Google Scholar] [CrossRef]
- Kho, K.A.; Brown, D.N. Surgical Treatment of Uterine Fibroids Within a Containment System and Without Power Morcellation. Clin. Obstet. Gynecol. 2016, 59, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.V.; Cohen, S.L.; Fuchs-Weizman, N.; Wang, K.C.; Manoucheri, E.; Vitonis, A.F.; Einarsson, J.I. Open power morcellation versus contained power morcellation within an insufflated isolation bag: Comparison of perioperative outcomes. J. Minim. Invasive Gynecol. 2015, 22, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sandrieser, L.; Kuessel, L.; Perricos, A.; Wenzl, R.; Husslein, H. Myomectomy for a large uterine fibroid via mini-laparotomy: A step-by-step video tutorial. Fertil. Steril. 2022, 117, 456–457. [Google Scholar] [CrossRef]
- Sandberg, E.M.; van den Haak, L.; Bosse, T.; Jansen, F.W. Disseminated leiomyoma cells can be identified following conventional myomectomy. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 2183–2187. [Google Scholar] [CrossRef]
- Toubia, T.; Moulder, J.K.; Schiff, L.D.; Clarke-Pearson, D.; O’Connor, S.M.; Siedhoff, M.T. Peritoneal Washings After Power Morcellation in Laparoscopic Myomectomy: A Pilot Study. J. Minim. Invasive Gynecol. 2016, 23, 578–581. [Google Scholar] [CrossRef]
- Yu, S.P.; Lee, B.B.; Han, M.N.; Chan, C.; Rao, J.; Levin, M.; Fung, P.C.; Parker, W. Irrigation after Laparoscopic Power Morcellation and the Dispersal of Leiomyoma Cells: A Pilot Study. J. Minim. Invasive Gynecol. 2018, 25, 632–637. [Google Scholar] [CrossRef]
- Cheung, C.C.; Banerjee, D.; Barnes, P.J.; Berendt, R.C.; Butany, J.; Canil, S.; Clarke, B.A.; El-Zimaity, H.; Garratt, J.; Geldenhuys, L.; et al. Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee for High Complexity Testing/Immunohistochemistry: Guidelines for the preparation, release, and storage of unstained archived diagnostic tissue sections for immunohistochemistry. Am. J. Clin. Pathol. 2014, 142, 629–633. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Published Online 2022. Available online: https://www.R-project.org/ (accessed on 17 January 2024).
- Erguler, K. Barnard: Barnard’s Unconditional Test. Published online 20 October 2016. Available online: https://github.com/kerguler/Barnard (accessed on 17 January 2024).
- Hadley, W. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Published online 12 October 2023. Available online: https://ggplot2.tidyverse.org/ (accessed on 17 January 2024).
- Daniel, D.S. ggsurvfit: Flexible Time-to-Event Figures. Published Online 31 October 2023. Available online: https://github.com/pharmaverse/ggsurvfit (accessed on 17 January 2024).
- Paul, E. Power and Sample Size Calculation for the Cochran-Mantel-Haenszel Test. Published online 10 December 2023. Available online: https://github.com/pegeler/samplesizeCMH (accessed on 17 January 2024).
- Therneau, T.M. survival: Survival Analysis. Therneau T (2024). A Package for Survival Analysis in R. R Package Version 3.8-3. Available online: https://CRAN.R-project.org/package=survival (accessed on 12 May 2024).
- Lum, D.A.; Sokol, E.R.; Berek, J.S.; Schulkin, J.; Chen, L.; McElwain, C.-A.; Wright, J.D. Impact of the 2014 Food and Drug Administration Warnings Against Power Morcellation. J. Minim. Invasive Gynecol. 2016, 23, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Raine-Bennett, T.; Tucker, L.-Y.; Zaritsky, E.; Littell, R.D.; Palen, T.; Neugebauer, R.; Axtell, A.; Schultze, P.M.; Kronbach, D.W.; Embry-Schubert, J.; et al. Occult Uterine Sarcoma and Leiomyosarcoma: Incidence of and Survival Associated With Morcellation. Obstet. Gynecol. 2016, 127, 29–39. [Google Scholar] [CrossRef]
- Bretthauer, M.; Goderstad, J.M.; Løberg, M.; Emilsson, L.; Ye, W.; Adami, H.-O.; Kalager, M. Uterine morcellation and survival in uterine sarcomas. Eur. J. Cancer 2018, 101, 62–68. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Maltese, G.; Bogani, G.; Fucà, G.; Lepori, S.; De Iaco, P.; Perrone, M.; Scambia, G.; Cormio, G.; Bogliolo, S.; et al. Morcellation worsens survival outcomes in patients with undiagnosed uterine leiomyosarcomas: A retrospective MITO group study. Gynecol. Oncol. 2017, 144, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H.; Berek, J.S.; Pritts, E.A.; Olive, D.; Chalas, E.; Clarke-Pearson, D. Regarding “Incidence of Occult Uterine Malignancy Following Vaginal Hysterectomy with Morcellation.”. J. Minim. Invasive Gynecol. 2018, 25, 187–188. [Google Scholar] [CrossRef]
- Lin, K.-H.; Torng, P.-L.; Tsai, K.-H.; Shih, H.-J.; Chen, C.-L. Clinical outcome affected by tumor morcellation in unexpected early uterine leiomyosarcoma. Taiwan. J. Obstet. Gynecol. 2015, 54, 172–177. [Google Scholar] [CrossRef]
- Van der Meulen, J.F.; Pijnenborg, J.M.A.; Boomsma, C.M.; Verberg, M.F.G.; Geomini, P.M.a.J.; Bongers, M.Y. Parasitic myoma after laparoscopic morcellation: A systematic review of the literature. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 69–75. [Google Scholar] [CrossRef]
- Kill, L.M.; Kapetanakis, V.; McCullough, A.E.; Magrina, J.F. Progression of pelvic implants to complex atypical endometrial hyperplasia after uterine morcellation. Obstet. Gynecol. 2011, 117, 447–449. [Google Scholar] [CrossRef]
- Zhang, H.M.; Christianson, L.A.; Templeman, C.L.; Lentz, S.E. Non-malignant Sequelae after Unconfined Power Morcellation. J. Minim. Invasive Gynecol. 2019, 26, 434–440. [Google Scholar] [CrossRef]
- Zhang, S.J.; Guo, L.S.; Deng, P.Z.; Dai, S.R.; Ren, Q.Z.; Tao, X.M.; Zhu, W.P. Application of transvaginal morcellation within disposable extraction bag with traction wire in laparoscopic myomectomy. Zhonghua Yi Xue Za Zhi 2022, 102, 2030–2032. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, S.; Schempershofe, M. In-Bag Morcellation as a Routine for Laparoscopic Hysterectomy. BioMed Res. Int. 2017, 2017, 6701916. [Google Scholar] [CrossRef]
- Bensouda-Miguet, C.; Nohuz, E.; Cerruto, E.; Buenerd, A.; Nadaud, B.; Moret, S.; Chene, G. Inbag Morcellation Applied to the Laparoscopic Surgery of Leiomyoma: A Randomized Controlled Trial. BioMed Res. Int. 2021, 2021, 6611448. [Google Scholar] [CrossRef] [PubMed]
- Asgari, Z.; Hashemi, M.; Hosseini, R.; Sepidarkish, M.; Seifollahi, A. Comparison of the Number of Spindle Cells in Peritoneal Washings between Laparoscopic Myomectomy with Morcellation and Open Myomectomy without Morcellation. J. Minim. Invasive Gynecol. 2021, 28, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Valletta, R.; Corato, V.; Lombardo, F.; Avesani, G.; Negri, G.; Steinkasserer, M.; Tagliaferri, T.; Bonatti, M. Leiomyoma or sarcoma? MRI performance in the differential diagnosis of sonographically suspicious uterine masses. Eur. J. Radiol. 2024, 170, 111217. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Casali, P.G.; Croce, S.; Fennessy, F.M.; Fischerova, D.; Jones, R.; Sanfilippo, R.; Zapardiel, I.; Amant, F.; Blay, J.-Y.; et al. ESGO/EURACAN/GCIG guidelines for the management of patients with uterine sarcomas. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2024, 34, 1499–1521. [Google Scholar] [CrossRef]

| Variable | All | Group A | Group B | Group C | Group D | Group B + C + D | p-Value A vs. B + C + D * |
|---|---|---|---|---|---|---|---|
| n | 72 | 21 | 17 | 19 | 15 | 51 | |
| Age (years) | 43 (38–45) | 37 (33–40) | 43 (41–45) | 44 (41–46) | 47 (43–51) | 44 (41–47) | p < 0.001 |
| BMI (kg/m2) | 25 (23–31) | 27 (22–26) | 24 (23–31) | 26 (25–30) | 23.5 (22–25) | 25 (23–29) | p = 0.451 |
| Gravidity | 2 (1–3) | 1 (0–1) | 2 (0–4) | 2 (2–3) | 2 (1–2) | 2 (1–3) | p < 0.001 |
| Parity | 1 (0–2) | 0 (0–1) | 2 (0–2) | 2 (1–2) | 2 (1–2) | 2 (1–2) | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuessel, L.; Sandrieser, L.; Hofstetter, G.; Heinzl, F.; Mara, M.; Richtárová, A.; Montanari, E.; Wenzl, R.; Perricos-Hess, A.; Husslein, H. Reducing Peritoneal Cell Dissemination in Laparoscopic Uterine Surgery: A Comparative Pilot Study on Morcellation Techniques and Peritoneal Irrigation. J. Clin. Med. 2025, 14, 3383. https://doi.org/10.3390/jcm14103383
Kuessel L, Sandrieser L, Hofstetter G, Heinzl F, Mara M, Richtárová A, Montanari E, Wenzl R, Perricos-Hess A, Husslein H. Reducing Peritoneal Cell Dissemination in Laparoscopic Uterine Surgery: A Comparative Pilot Study on Morcellation Techniques and Peritoneal Irrigation. Journal of Clinical Medicine. 2025; 14(10):3383. https://doi.org/10.3390/jcm14103383
Chicago/Turabian StyleKuessel, Lorenz, Lejla Sandrieser, Gerda Hofstetter, Florian Heinzl, Michal Mara, Adéla Richtárová, Eliana Montanari, René Wenzl, Alexandra Perricos-Hess, and Heinrich Husslein. 2025. "Reducing Peritoneal Cell Dissemination in Laparoscopic Uterine Surgery: A Comparative Pilot Study on Morcellation Techniques and Peritoneal Irrigation" Journal of Clinical Medicine 14, no. 10: 3383. https://doi.org/10.3390/jcm14103383
APA StyleKuessel, L., Sandrieser, L., Hofstetter, G., Heinzl, F., Mara, M., Richtárová, A., Montanari, E., Wenzl, R., Perricos-Hess, A., & Husslein, H. (2025). Reducing Peritoneal Cell Dissemination in Laparoscopic Uterine Surgery: A Comparative Pilot Study on Morcellation Techniques and Peritoneal Irrigation. Journal of Clinical Medicine, 14(10), 3383. https://doi.org/10.3390/jcm14103383








