The Role of Galectin-3 as a Biomarker in the Cardio–Renal–Metabolic Pathology Axis
Abstract
1. Introduction
Gal-3
2. Gal-3 as a Biomarker in Various Diseases
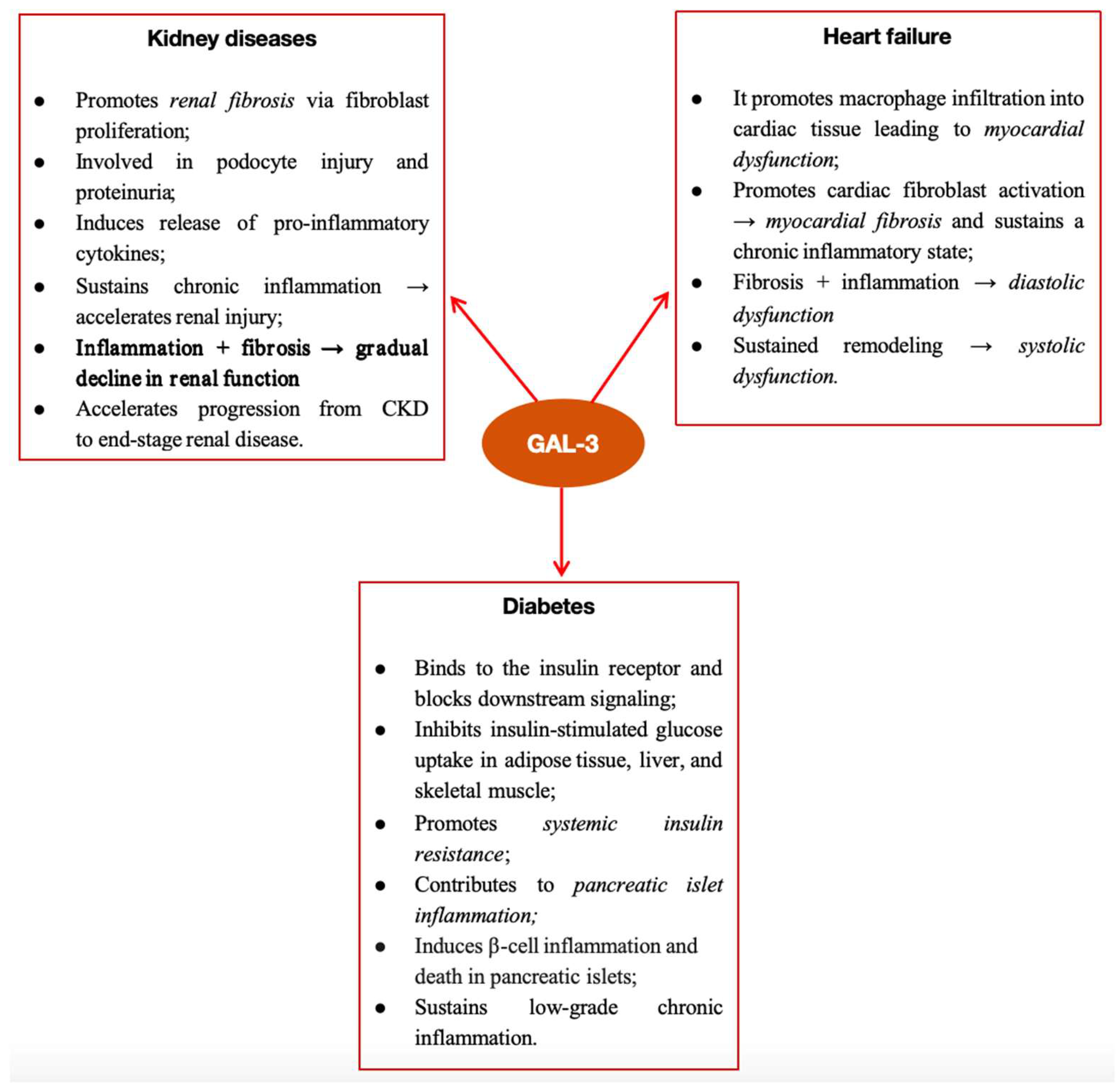
2.1. The Role of Gal-3 in Kidney Diseases
2.2. The Role of Gal-3 in Heart Failure
3. Gal-3 in Cardio–Renal–Metabolic Syndrome
3.1. Gal-3 Implications in Diabetes
3.2. Interferences of Galectin 3 in Cardio–Renal Syndrome
- -
- CRS type 1: A sudden decline in heart function that results in kidney impairment;
- -
- CRS type 2: Chronic cardiac failure that results in renal impairment;
- -
- CRS type 3: Heart dysfunction brought on by an abrupt deterioration in renal function;
- -
- CRS type 4: Chronic renal dysfunction that results in heart disease;
- -
- CRS type 5: Systemic disorders resulting in concurrent renal and cardiac dysfunction [75].
4. Future Perspectives—Anti-Gal-3 Therapy
5. Conclusions
Funding
Conflicts of Interest
References
- Verkerke, H.; Dias-Baruffi, M.; Cummings, R.D.; Arthur, C.M.; Stowell, S.R. Galectins: An Ancient Family of Carbohydrate Binding Proteins with Modern Functions. Methods Mol. Biol. 2022, 2442, 1–40. [Google Scholar]
- Lima, T.; Perpétuo, L.; Henrique, R.; Fardilha, M.; Leite-Moreira, A.; Bastos, J.; Vitorino, R. Gal-3 in prostate cancer and heart diseases: A biomarker for these two frightening pathologies? Mol. Biol. Rep. 2023, 50, 2763–2778. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Gal-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zheng, Y.; Xu, D.; Sun, Z.; Yang, H.; Yin, Q. Gal-3: A key player in microglia-mediated neuroinflammation and Alzheimer’s disease. Cell Biosci. 2021, 11, 78. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.-L. Molecular regulation of Gal-3 expression and therapeutic implication in cancer progression. Biomed. Pharmacother. 2016, 78, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Gal-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef] [PubMed]
- Bouffette, S.; Botez, I.; De Ceuninck, F. Targeting Gal-3 in inflammatory and fibrotic diseases. Trends Pharmacol. Sci. 2023, 44, 8. [Google Scholar] [CrossRef]
- Kruk, L.; Braun, A.; Cosset, E.; Gudermann, T.; Mammadova-Bach, E. Galectin functions in cancer-associated inflammation and thrombosis. Front. Cardiovasc. Med. 2023, 10, 105295. [Google Scholar] [CrossRef]
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.G. Increased circulation of Gal-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin. Cancer Res. 2013, 19, 1693–1704. [Google Scholar] [CrossRef]
- Meijers, W.C.; van der Velde, A.R.; Ruifrok, W.P.; Schroten, N.F.; Dokter, M.M.; Damman, K.; Assa, S.; Franssen, C.F.; Gansevoort, R.T.; van Gilst, W.H.; et al. Renal Handling of Gal-3 in the General Population, Chronic Heart Failure, and Hemodialysis. J. Am. Heart Assoc. 2014, 3, e000962. [Google Scholar] [CrossRef]
- Baggen, V.J.M.; van den Bosch, A.E.; Eindhoven, J.A.; Menting, M.E.; Witsenburg, M.; Cuypers, J.A.A.E.; Boersma, E.; Roos-Hesselink, J.W. Prognostic value of Gal-3 in adults with congenital heart disease. Heart 2018, 104, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, E.; Kuśmierczyk-Droszcz, B.; Klisiewicz, A.; Wróbel, A.; Lutyńska, A.; Gawor, M.; Niewiadomska, J.; Lipczyńska, M.; Biernacka, E.K.; Grzybowski, J.; et al. Gal-3 Plasma Levels in Adult Congenital Heart Disease and the Pressure Overloaded Right Ventricle: Reason Matters. Biomark. Med. 2020, 14, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.A.; Gafar, H.S.; Hussien, N.R. Galectin-3 as Early Detector of Heart Failure in Children with Congenital Acyanotic Heart Disease. Clin. Med. Diagn. 2014, 4, 90–98. [Google Scholar]
- Făgărășan, A.; Săsăran, M.; Gozar, L.; Crauciuc, A.; Bănescu, C. The Role of Gal-3 in Predicting Congenital Heart Disease Outcome: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 10511. [Google Scholar] [CrossRef]
- Frogoudaki, A.A.; Pantelakis, I.; Bistola, V.; Kroupis, C.; Birba, D.; Ikonomidis, I.; Alexopoulos, D.; Filippatos, G.; Parissis, J. Global Longitudinal Strain of the Systemic Ventricle Is Correlated with Plasma Gal-3 and Predicts Major Cardiovascular Events in Adult Patients with Congenital Heart Disease. Medicina 2020, 56, 305. [Google Scholar] [CrossRef]
- Pietrzak, R.; Książczyk, T.M.; Górska, E.; Małek, Ł.A.; Werner, B. Evaluation of Gal-3 Plasma Concentration in Adolescents with Ventricular Arrhythmia. Int. J. Environ. Res. Public. Health 2021, 18, 2410. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Kanayama, T.; Noguchi, K.; Niwa, A.; Matsuo, M.; Kuroda, T.; Hatano, Y.; Okada, H.; Tomita, H. Gal-3: A Potential Prognostic and Diagnostic Marker for Heart Disease and Detection of Early Stage Pathology. Biomolecules 2020, 10, 1277. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; la Rosa, N.L.D.; la Mora, M.S.D.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Gal-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a Biomarker Linking Oxidative Stress and Inflammation with the Clinical Outcomes of Patients with Atherothrombosis. J. Am. Heart Assoc. 2014, 3, e000785. [Google Scholar] [CrossRef]
- Akin, Y.; Demirkan, B.; Suljevic, S.; Ipek, E.G. Gal-3: A New Biomarker for Atherosclerosis Management. Angiology 2020, 71, 873–967. [Google Scholar] [CrossRef]
- Sadlonova, M.; Meyer, T.; Binder, L.; Wachter, R.; Edelmann, F.; Herrmann-Lingen, C. Higher Gal-3 levels are independently associated with lower anxiety in patients with risk factors for heart failure. Biopsychosoc. Med. 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Boutin, L.; Dépret, F.; Gayat, E.; Legrand, M.; Chadjichristos, C.E. Gal-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3124. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, P.L. The role of Gal-3 in the kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, V.; Desmedt, S.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Gal-3 in renal pathology: More than just an innocent bystander. Am. J. Nephrol. 2016, 43, 305–317. [Google Scholar] [CrossRef]
- Ou, S.-M.; Tsai, M.-T.; Chen, H.-Y.; Li, F.-A.; Tseng, W.-C.; Lee, K.-H.; Chang, F.-P.; Lin, Y.-P.; Yang, R.-B.; Tarng, D.-C. Identification of Gal-3 as potential biomarkers for renal fibrosis by RNA-sequencing and clinicopathologic findings of kidney biopsy. Front. Med. 2021, 8, 748225. [Google Scholar] [CrossRef]
- Fadella, A.; Harun, H.; Priyono, D.; Viotra, D. Plasma Gal-3 Biomarkers in Chronic Kidney Disease. Indones. J. Multidiscip. Sci. 2023, 2, 2440–2452. [Google Scholar] [CrossRef]
- Tan, R.; Liu, X.; Wang, J.; Lu, P.; Han, Z.; Tao, J.; Yin, C.; Gu, M. Alternations of galectin levels after renal transplantation. Clin. Biochem. 2014, 47, 83–88. [Google Scholar] [CrossRef]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Gal-3, renal function, and clinical outcomes: Results from the luric and 4D studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef]
- La Jolla Pharmaceutical Company Reports Positive, Top-Line Results from Phase 2 Clinical Trial of GCS-100 in Chronic Kidney Disease. Press Release Data Mar 10. Available online: https://www.sec.gov/Archives/edgar/data/920465/000092046514000012/pressreleasedatamar10.htm (accessed on 22 August 2025).
- Schwerg, M.; Eilers, B.; Wienecke, A.; Baumann, G.; Laule, M.; Knebel, F.; Stangl, K.; Stangl, V. Gal-3 and prediction of therapeutic response to renal sympathetic denervation. Clin. Exp. Hypertens. 2016, 38, 399–403. [Google Scholar] [CrossRef]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Gal-3 in Heart Failure—The Diagnostic, Prognostic and Therapeutic Potential—Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef]
- Seropian, I.M.; Cassaglia, P.; Miksztowicz, V.; González, G.E. Unraveling the role of Gal-3 in cardiac pathology and physiology. Front. Physiol. 2023, 14, 1304735. [Google Scholar] [CrossRef]
- Sonkawade, S.D.; Pokharel, S.; Karthikeyan, B.; Kim, M.; Xu, S.; Kc, K.; Sexton, S.; Catalfamo, K.; Spernyak, J.A.; Sharma, U.C. Small endogeneous peptide mitigates myocardial remodeling in a mouse model of cardioselective Gal-3 overexpression. Circ. Heart Fail. 2021, 14, e008510. [Google Scholar] [CrossRef]
- Chen, B.; Li, R.; Hernandez, S.C.; Hanna, A.; Su, K.; Shinde, A.V.; Frangogiannis, N.G. Differential effects of Smad2 and Smad3 in regulation of macrophage phenotype and function in the infarcted myocardium. J. Mol. Cell Cardiol. 2022, 171, 1–15. [Google Scholar] [CrossRef]
- Kubota, A.; Frangogiannis, N.G. Macrophages in myocardial infarction. Am. J. Physiol. Cell Physiol. 2022, 323, C1304–C1324. [Google Scholar] [CrossRef]
- Cassaglia, P.; Penas, F.; Betazza, C.; Estevez, F.F.; Miksztowicz, V.; Naya, N.M.; Llamosas, M.C.; Truant, S.N.; Wilensky, L.; Volberg, V.; et al. Genetic deletion of Gal-3 alters the temporal evolution of macrophage infiltration and healing affecting the cardiac remodeling and function after myocardial infarction in mice. Am. J. Pathol. 2020, 190, 1789–1800. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021, 18, 400–423. [Google Scholar] [CrossRef]
- Berezin, A.E.; Lichtenauer, M.; Berezin, A.A. Heart failure among patients with prediabetes and type 2 diabetes mellitus: Diagnostic and predictive biomarkers: A narrative review. J. Lab. Precis. Med. 2022, 7, 5. [Google Scholar] [CrossRef]
- Wu, C.-K.; Su, M.-Y.; Lee, J.-K.; Chiang, F.-T.; Hwang, J.-J.; Lin, J.-L.; Chen, J.-J.; Liu, F.-T.; Tsai, C.-T. Gal-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci. Rep. 2015, 5, 17007. [Google Scholar]
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Adourian, A.; Muntendam, P.; Cohn, J.N. Baseline serial measurements of Gal-3 in patients with heart failure: Relationship to prognosis effect of treatment with valsartan in the Val-HeFT. Eur. J. Heart Fail. 2013, 15, 511–518. [Google Scholar] [CrossRef]
- Medvedeva, E.A.; Berezin, I.I.; Surkova, E.A.; Yaranov, D.M.; Shchukin, Y.V. Gal-3 in patients with chronic heart failure: Association with oxidative stress, inflammation, renal dysfunction and prognosis. Minerva Cardioangiol. 2016, 64, 595–602. [Google Scholar]
- Lala, R.I.; Darabantiu, D.; Pilat, L.; Puschita, M. Gal-3: A link between myocardial and arterial stiffening in patients with acute decompensated heart failure. Arq. Bras. Cardiol. 2016, 106, 121–129. [Google Scholar]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Gal-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar]
- Jiang, J.; Yang, B.; Sun, Y.; Jin, J.; Zhao, Z.; Chen, S. Diagnostic Value of Serum Concentration of Gal-3 in Patients with Heart Failure with Preserved Ejection Fraction. Front. Cardiovasc. Med. 2022, 8, 829151. [Google Scholar] [CrossRef]
- Kanukurti, J.; Mohammed, N.; Sreedevi, N.N.; Khan, S.A.; Baba, K.S.; Bhaskar, M.V.; Satish, O.S.; Naushad, S.M.; Mohan, I.K. Evaluation of Gal-3 as a novel diagnostic biomarker in patients with heart failure with preserved ejection fraction. J. Lab. Physicians. 2020, 12, 126–132. [Google Scholar]
- Trippel, T.D.; Mende, M.; Düngen, H.; Hashemi, D.; Petutschnigg, J.; Nolte, K.; Herrmann-Lingen, C.; Binder, L.; Hasenfuss, G.; Pieske, B.; et al. The diagnostic and prognostic value of Gal-3 in patients at risk for heart failure with preserved ejection fraction: Results from the DIAST-CHF study. ESC Heart Fail. 2021, 8, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Sudharshan, S.; Novak, E.; Hock, K.; Scott, M.G.; Geltman, E.M. Use of biomarkers to predict readmission for congestive heart failure. Am. J. Cardiol. 2017, 119, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Ansari, U.; Behnes, M.; Hoffmann, J.; Natale, M.; Fastner, C.; El-Battrawy, I.; Rusnak, J.; Kim, S.-H.; Lang, S.; Hoffmann, U.; et al. Gal-3 reflects the echocardiographic grades of left ventricular diastolic dysfunction. Ann. Lab. Med. 2018, 38, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, B.; Sygitowicz, G.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A. Gal-3 is related to right ventricular dysfunction in heart failure patients with reduced ejection fraction and may affect exercise capacity. Sci. Rep. 2020, 10, 16682. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, R.-C.; Chen, S.-P.; Li, T.; Wang, Y.; Xue, Y.-B.; Liu, J.; Han, X.; Su, Y.-D.; Bai, L.; et al. The Diagnostic and Prognostic Value of Plasma Galectin 3 in HFrEF Related to the Etiology of Heart Failure. Front. Cardiovasc. Med. 2021, 8, 748875. [Google Scholar] [CrossRef]
- Zile, M.R.; O’MEara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.; Shi, V.; Lefkowitz, M.; et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806. [Google Scholar] [CrossRef]
- Du, X.J.; Zhao, W.B.; Nguyen, M.N.; Lu, Q.; Kiriazis, H. beta-Adrenoceptor activation affects Gal-3 as a biomarker and therapeutic target in heart disease. Br. J. Pharmacol. 2019, 176, 2449–2464. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Targeting redox imbalance as an approach for Diabetic kidney Disease. Biomedicines 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ranawat, C.S.; Bhandiwad, C.; Arya, H.; Mali, M.; Singh, C.P.; Sharma, N.; Lathwal, N.; Wasim, S. Gal-3 as a Potential Biomarker of Microvascular Complications in Patients with Type 2 Diabetes. Indian J. Endocrinol. Metab. 2022, 26, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jin, H.; Ning, J.; Cui, D.; Zhang, M.; Yang, H. Elevated Gal-3 levels detected in women with hyperglycemia during early and mid-pregnancy antagonizes high glucose − induced trophoblast cells apoptosis via Gal-3/foxc1 pathway. Mol. Med. 2023, 29, 115. [Google Scholar] [CrossRef]
- Yilmaz, H.; Cakmak, M.; Inan, O.; Darcin, T.; Akcay, A. Increased levels of Gal-3 were associated with prediabetes and diabetes: New risk factor? J. Endocrinol. Investig. 2015, 38, 527–533. [Google Scholar] [CrossRef]
- Mensah-Brown, E.P.K.; Al Rabesi, Z.; Shahin, A.; Al Shamsi, M.; Arsenijevic, N.; Hsu, D.K.; Liu, F.T.; Lukic, M.L. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009, 130, 83–88. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.F.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-derived galectin-3Gal-3 causes cellular and systemic insulin resistance. Cell 2016, 167, 973–984. [Google Scholar] [CrossRef]
- Pejnovic, N.N.; Pantic, J.M.; Jovanovic, I.P.; Radosavljevic, G.D.; Milovanovic, M.Z.; Nikolic, I.G.; Zdravkovic, N.S.; Djukic, A.L.; Arsenijevic, N.N.; Lukic, M.L. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes 2013, 62, 1932–1944. [Google Scholar] [CrossRef]
- Darrow, A.L.; Shohet, R.V. Galectin-3 deficiency exacerbates hyperglycemia and the endothelial response to diabetes. Cardiovasc Diabetol. 2015, 14, 73. [Google Scholar] [CrossRef]
- Atalar, M.N.; Abuşoğlu, S.; Ünlü, A.; Tok, O.; İpEkçi, S.H.; Baldane, S.; Kebapcılar, L. Assessment of serum Gal-3, methylated arginine and Hs-CRP levels in type 2 diabetes and prediabetes. Life Sci. 2019, 231, 116577. [Google Scholar] [CrossRef]
- Nayor, M.; Wang, N.; Larson, M.G.; Vasan, R.S.; Levy, D.; Ho, J.E. Circulating Gal-3 Is Associated with Cardiometabolic Disease in the Community. J. Am. Heart Assoc. 2015, 5, e002347. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-H.; Kim, S.-J.; Kang, H.G.; Lee, H.-W.; Kim, J.-H.; Hwang, K.-A.; Song, J.; Chun, K.-H. Galectin-3 activates PPARγ and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology 2015, 156, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.B.; Cheung, C.-L.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Gal-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia 2018, 61, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.O.; Park, S.-Y.; Lee, S.B.; Kang, N.-R.; Cho, D.H.; Chung, D.J.; Chung, M.Y. Plasma Gal-3 concentration and estimated glomerular filtration rate in patients with type 2 diabetes with and without albuminuria. Sci. Rep. 2022, 12, 16328. [Google Scholar] [CrossRef]
- Hodeib, H.; Hagras, M.M.; Abdelhai, D.; Watany, M.M.; Selim, A.; Tawfik, M.; Elsebaey, M.; Elshweikh, S. Gal-3 as a prognostic biomarker for diabetic nephropathy. Diabetes Metab. Syndr. Obes. 2022, 12, 325–331. [Google Scholar] [CrossRef]
- Vora, A.; de Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of Gal-3 with Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 4449–4458. [Google Scholar] [CrossRef]
- Flores-Ramírez, R.; Azpiri-López, J.R.; González-González, J.G.; Ordaz-Farías, A.; González-Carrillo, L.E.; Carrizales-Sepúlveda, E.F.; Vera-Pineda, R. Global longitudinal strain as a biomarker in diabetic cardiomyopathy.A comparative study with Gal-3 in patients with preserved ejection fraction. Arch. Cardiol. Mex. 2017, 87, 278–285. [Google Scholar]
- Schmitt, V.H.; Prochaska, J.H.; Föll, A.S.; Schulz, A.; Keller, K.; Hahad, O.; Koeck, T.; Tröbs, S.-O.; Rapp, S.; Beutel, M.; et al. Gal-3 for prediction of cardiac function compared to NT-proBNP in individuals with prediabetes and type 2 diabetes mellitus. Sci. Rep. 2021, 11, 19012. [Google Scholar] [CrossRef]
- Saeed, M.; Tapia, G.; Ariansen, I.; Stene, L.C.; Seljeflot, I.; Tell, G.S.; Skrivarhaug, T.; Joner, G. Serum Gal-3 and Subsequent Risk of Coronary Heart Disease in Subjects with Childhood-Onset Type 1 Diabetes: A Cohort Study. Diabetes Care 2021, 44, 810–816. [Google Scholar] [CrossRef]
- Ozturk, D.; Celik, O.; Satilmis, S.; Aslan, S.; Erturk, M.; Cakmak, H.A.; Kalkan, A.K.; Ozyilmaz, S.; Diker, V.; Gul, M. Association between serum galectin-3 levels and coronary atherosclerosis and plaque burden/structure in patients with type 2 diabetes mellitus. Coron. Artery Dis. 2015, 26, 396–401. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhou, Z.; Xiao, Y. Emerging roles of Gal-3 in diabetes and diabetes complications: A snapshot. Rev. Endocr. Metab. Disord. 2022, 23, 569–577. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- House, A.A.; Anand, I.; Bellomo, R.; Cruz, D.; Bobek, I.; Anker, S.D.; Aspromonte, N.; Bagshaw, S.; Berl, T.; Daliento, L.; et al. Definition and classification of cardio-renal syndromes: Workgroup statements from the 7th ADQI Consensus Conference. Nephrol. Dial. Transplant. 2010, 25, 1416–1420. [Google Scholar] [CrossRef]
- Horiuchi Yu Wettersten, N.; VANVeldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Müeller, G.A.; Birkhahn, R.; et al. Gal-3, Acute Kidney Injury and Myocardial Damage in Patients with Acute Heart Failure. J. Card. Fail. 2023, 29, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.L.; Katz, R.; Bellovich, K.A.; Bhat, Z.Y.; Brosius, F.C.; de Boer, I.H.; Gadegbeku, C.A.; Gipson, D.S.; Hawkins, J.J.; Himmelfarb, J.; et al. Soluble ST2 and Gal-3 and Progression of CKD. Kidney Int. Rep. 2019, 4, 103–111. [Google Scholar] [CrossRef]
- Ozyildirim, S.; Dogan, O.; Barman, H.A.; Tanyolac, S.; Atici, A.; Enar, R.; Dogan, S.M. Gal-3 as a Biomarker to Predict Cardiorenal Syndrome in Patients with Acute Heart Failure. Acta Cardiol. Sin. 2023, 39, 862–870. [Google Scholar]
- Iacoviello, M.; Aspromonte, N.; Leone, M.; Paradies, V.; Antoncecchi, V.; Valle, R.; Caldarola, P.; Ciccone, M.M.; Gesualdo, L.; Di Serio, F. Gal-3 serum levels are independently associated with microalbuminuria in chronic heart failure outpatients. Res. Cardiovasc. Med. 2016, 5, 28952. [Google Scholar] [CrossRef]
- Gopal, D.M.; Kommineni, M.; Ayalon, N.; Koelbl, C.; Ayalon, R.; Biolo, A.; Dember, L.M.; Downing, J.; Siwik, D.A.; Liang, C.S.; et al. Relationship of plasma galectin-3 to renal function in patients with heart failure: Effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J. Am. Heart Assoc. 2012, 1, e000760. [Google Scholar] [CrossRef]
- Brisco, M.A.; Testani, J.M. Novel renal biomarkers to assess cardiorenal syndrome. Curr. Heart Fail. Rep. 2014, 11, 485–499. [Google Scholar] [CrossRef]
- Goffredo, G.; Barone, R.; Di Terlizzi, V.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Biomarkers in Cardiorenal Syndrome. J. Clin. Med. 2021, 10, 3433. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.J.; Van Der Meer, P.; de la Porte, P.W.B.A.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic value of Gal-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef]
- Lopez-Andrès, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.G.; Zannad, F.; Lacolley, P. Association of Gal-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Ivey-Miranda, J.B.; Cox, Z.L.; Moreno-Villagomez, J.; Testani, J.M. Association of Urine Gal-3 with Cardiorenal Outcomes in Patients with Heart Failure. J. Card. Fail. 2024, 30, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yang, C.; Ballew, S.H.; Kalbaugh, C.A.; McEvoy, J.W.; Salameh, M.; Aguilar, D.; Hoogeveen, R.C.; Nambi, V.; Selvin, E.; et al. Fibrosis and Inflammatory Markers and Long-Term Risk of Peripheral Artery Disease: The ARIC Study. Arter. Thromb. Vasc. Biol. 2020, 40, 2322–2331. [Google Scholar] [CrossRef]
- Felker, G.M.; Fiuzat, M.; Shaw, L.K.; Clare, R.; Whellan, D.J.; Bettari, L.; Shirolkar, S.C.; Donahue, M.; Kitzman, D.W.; Zannad, F.; et al. Gal-3 in ambulatory patients with heart failure: Results from the HF-ACTION study. Circ. Heart Fail. 2012, 5, 72–78. [Google Scholar] [CrossRef]
- Kim, A.J.; Ro, H.; Kim, H.; Chang, J.H.; Lee, H.H.; Chung, W.; Jung, J.Y. Soluble ST2 and Gal-3 as Predictors of Chronic Kidney Disease Progression and Outcomes. Am. J. Nephrol. 2021, 52, 119–130. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Selvin, E.; Liang, M.; Ballantyne, C.M.; Hoogeveen, R.C.; Aguilar, D.; McEvoy, J.W.; Grams, M.E.; Coresh, J. Plasma Gal-3 Levels Are Associated with the Risk of Incident Chronic Kidney Disease. Kidney Int. 2018, 93, 252. [Google Scholar] [CrossRef]
- Wu, C.; Lv, Z.; Li, X.; Zhou, X.; Mao, W.; Zhu, M. Gal-3 in Predicting Mortality of Heart Failure: A Systematic Review and Meta-Analysis. Heart Surg. Forum 2021, 24, E327–E332. [Google Scholar] [CrossRef]
- Lin, D.; Hong, X.; Sun, K.; Zhang, X.; Lian, H.; Wang, J.; Mao, N.; Zhang, X.; Ren, M.; Yan, L.; et al. Gal-3/adiponectin as a new biological indicator for assessing the risk of type 2 diabetes: A cross-sectional study in a community population. Aging 2021, 13, 15433. [Google Scholar] [CrossRef]
- Kramer, T.; Brinkkoetter, P.; Rosenkranz, S. Right Heart Function in Cardiorenal Syndrome. Curr. Heart Fail. Rep. 2022, 19, 386–399. [Google Scholar] [CrossRef]
- Perez, C.; González-Juanatey, J.R.; Nuche, J.; Matute-Blanco, L.; Serrano, I.; Martínez Selles, M.; Vázquez García, R.; Martínez Dolz, L.; Gómez-Bueno, M.; Pascual Figal, D.; et al. Renal Function Impact in the Prognostic Value of Gal-3 in Acute Heart Failure. Front. Cardiovasc. Med. 2022, 9, 861651. [Google Scholar]
- Martinez-Martinez, E.; Calvier, L.; Rossignol, P.; Rousseau, E.; Fernández-Celis, A.; Jurado-López, R.; Laville, M.; Cachofeiro, V.; López-Andrés, N. Gal-3 inhibition prevents adipose tissue remodelling in obesity. Int. J. Obes. 2016, 40, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of Gal-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]


| Diseases | Pathogenic Roles of Gal-3 |
|---|---|
| Chronic inflammation |
|
| Fibrosis |
|
| Cancer |
|
| Cardiovascular diseases |
|
| Kidney diseases |
|
| Disease | Diagnosis Utility | Prognosis Utility | Treatment Decisions |
|---|---|---|---|
| Acute decompensated heart failure | Diagnostic utility when combined with natriuretic peptides |
|
|
| Chronic heart failure | Completes natriuretic peptides |
|
|
| Diabetic nephropathy | Identifies kidney injury early before albuminuria |
|
|
| Author/Study Name | Population | Study Subjects | Results |
|---|---|---|---|
| PRIDE study [83] | ADHF | patients with acute decompensated heart failure | Gal-3 was a prognostic marker for 60-day mortality. |
| DEAL-HF study [84] | CHF | patients with moderate-to-advanced chronic HF | Gal-3 was an independent predictor of mortality. |
| Val-HeFT study [40] | CHF | the effect of valsartan in patients with heart failure | Patients with low Gal-3 levels have a reduction in repeat HF hospitalizations. |
| Care-hf trial [85] | CHF | patients with heart failure, left ventricular dysfunction, and dyssynchrony | Elevated Gal-3 levels were significantly associated with long-term cardiovascular outcomes. |
| TOPCAT trial [86] | CHF | patients with HFpEF | There are higher Gal-3 levels for this population. |
| ARIC study [87] | No PAD | 9851 participants free of peripheral artery disease | Higher levels of Gal-3 measured were significantly associated with an elevated risk of PAD and critical limb ischemia over 17.4 years of follow-up. |
| HF-ACTION study [88] | CHF | 900 ambulatory patients with HF | Patients with high levels of both NT-proBNP and Gal-3 showed a hazard ratio of 2.19 for hospitalization at 4 years of follow-up compared to patients with low levels of both markers. |
| Chung, J.O. et al. [66] | T2DM | 334 patients with T2DM | Gal-3 concentration was negatively associated with eGFR in patients with T2DM. Moreover, this association was independent of albuminuria status. |
| Kim, A.J et al. [89] | CKD | 352 patients with chronic kidney disease | Gal-3 plasma levels were associated with elevated serum creatinine and urine protein/creatinine ratio and were independently associated with CKD progression. |
| Rebholz, C.M. et al. [90] | NO CKD and NO CHF | 9148 patients with no chronic kidney disease and no chronic heart failure | Gal-3 was higher for low estimated glomerular filtration rate and low urine albumin-to-creatinine ratio and was associated with CKD with an OR of 2.22 95% CI [1.89, 2.60]. |
| Wu, C. et al. [91] | ADFH and CHF | 9217 patients with chronic and acute HF | The diagnostic hazard ratios of Gal-3 in predicting mortality in chronic HF patients were 1.13 (95% CI: 1.07–1.21) and 2.17 (95% CI: 1.27–3.08) in AHF patients. |
| Vora A. et al. [68] | Free of CVD | 6586 participants from the Dallas Heart Study | Gal-3 was associated with diabetes prevalence and incidence. |
| Lin D. et al. [92] | T2DM | 405 patients (135 newly diagnosed patients with type 2 diabetes and 270 age- and sex-matched nondiabetic patients) | Gal-3 was increased in T2D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buliga-Finis, O.N.; Ouatu, A.; Tanase, D.M.; Badescu, M.C.; Dima, N.; Gosav, E.M.; Popescu, D.; Rezus, C. The Role of Galectin-3 as a Biomarker in the Cardio–Renal–Metabolic Pathology Axis. J. Clin. Med. 2025, 14, 6071. https://doi.org/10.3390/jcm14176071
Buliga-Finis ON, Ouatu A, Tanase DM, Badescu MC, Dima N, Gosav EM, Popescu D, Rezus C. The Role of Galectin-3 as a Biomarker in the Cardio–Renal–Metabolic Pathology Axis. Journal of Clinical Medicine. 2025; 14(17):6071. https://doi.org/10.3390/jcm14176071
Chicago/Turabian StyleBuliga-Finis, Oana Nicoleta, Anca Ouatu, Daniela Maria Tanase, Minerva Codruta Badescu, Nicoleta Dima, Evelina Maria Gosav, Diana Popescu, and Ciprian Rezus. 2025. "The Role of Galectin-3 as a Biomarker in the Cardio–Renal–Metabolic Pathology Axis" Journal of Clinical Medicine 14, no. 17: 6071. https://doi.org/10.3390/jcm14176071
APA StyleBuliga-Finis, O. N., Ouatu, A., Tanase, D. M., Badescu, M. C., Dima, N., Gosav, E. M., Popescu, D., & Rezus, C. (2025). The Role of Galectin-3 as a Biomarker in the Cardio–Renal–Metabolic Pathology Axis. Journal of Clinical Medicine, 14(17), 6071. https://doi.org/10.3390/jcm14176071










