Abstract
Background and Objectives: Morning halitosis undermines social well-being, yet the combined influence of basal salivary flow and tongue coating in healthy adults is unclear. Methods: In a cross-sectional study of 92 university students (18–35 years), we measured unstimulated salivary flow rate (uSFR), tongue-coating index (TCI), total volatile sulfur compounds (VSCs; Halimeter®), organoleptic score (0–5), and self-perceived halitosis (yes/no) under standardized early-morning conditions. Results: Thirty-seven participants (40.2%) reported morning halitosis and showed lower uSFR (0.2 ± 0.1 vs. 0.3 ± 0.1 mL·min−1) and higher TCI (2.3 ± 0.5 vs. 1.9 ± 0.4), with higher organoleptic scores (3.4 ± 0.6 vs. 2.1 ± 0.7) and VSCs (272.9 ± 39.8 vs. 163.7 ± 45.9 ppb; all p < 0.001). VSCs correlated inversely with uSFR (ρ = −0.58) and positively with TCI (ρ = 0.44). In multivariable models, uSFR (β = −0.53) and TCI (β = 0.31) explained 54% of VSC variance; each 0.1 mL·min−1 fall in uSFR increased the odds of self-perceived halitosis 1.9-fold (p = 0.001). Conclusions: Even among healthy young adults, lower basal saliva and heavier tongue coating are independent contributors to morning malodor. Hydration, daily tongue cleaning, and addressing mouth breathing are pragmatic, first-line strategies.
1. Introduction
Halitosis affects an estimated 22–50% of the population, with the prevalence varying by age, geography, and assessment method [1]. Beyond the sensory stigma, individuals with bad breath report clinically meaningful decrements in oral-health-related quality of life [2]. Clinical management has shifted from masking odor to modifying its ecological drivers. Microbiome-targeted approaches show promise, although evidence remains short-term and low-certainty [3].
Day-to-day control still relies on chemotherapeutic rinses and mechanical tongue cleaning, which reduce organoleptic scores and VSCs in controlled trials [4,5,6,7]. Etiologically, the dorsum of the tongue and inflamed periodontal niches are central: patients with gingivitis/periodontitis often show two- to three-fold-higher VSCs than healthy controls [8,9,10,11]. At the same time, saliva provides natural defense by diluting substrates, buffering pH, and delivering antimicrobial factors; reduced unstimulated flow is linked to a higher VSC burden [12,13].
Saliva provides the principal physiological antidote: flow dilutes sulfur precursors, buffers pH, and delivers antimicrobial peptides. Classic work defined normal unstimulated whole saliva as ≥0.3 mL·min−1 [12], while epidemiological data show that elders with flow ≤ 0.1 mL·min−1 are four times more likely to report xerostomia and carry 8-fold higher VSC burdens [13]. Recognizing the need for analytical rigor, newly published guidelines now recommend standardized pre-sampling fasting, duplicate halimetry, and gas-chromatography calibration to improve inter-study comparability [14].
Beyond routine scraping, one-stage full-mouth disinfection has demonstrated faster (≤2 months) organoleptic improvement versus quadrant scaling [15], whereas antimicrobial photodynamic therapy achieves immediate but transient VSC reductions and awaits longer-term validation [16].
The COVID-19 era added fresh variables: wearing face masks for ≥4 h daily increased self-rated dry mouth and halitosis scores by 1.5–2.0 points in crossover trials [17], and microbiological sampling of mask interiors confirmed elevated VSCs alongside Prevotella spp. enrichment [18]. Notably, self-perceived malodor correlates imperfectly with objective measures and is modulated by concurrent oral complaints, such as dryness or dysgeusia [19]. Large online surveys during the pandemic further showed that women and younger adults were most affected by “mask-mouth” anxiety [20].
Collectively, these observations indicate that salivary hypofunction and tongue biofilm remain the two modifiable pillars of genuine morning malodor, yet their joint influence has seldom been quantified in the same healthy cohort. Addressing this gap, the present cross-sectional study simultaneously measured unstimulated salivary flow, tongue-coating burden, instrumental VSCs, organoleptic ratings, and self-perception in 92 university adults. By clarifying their relative and combined contributions, we aim to reinforce simple, low-tech preventive advice—hydration and mechanical tongue cleaning—before recourse to pharmacological or probiotic adjuncts.
2. Materials and Methods
2.1. Study Design and Ethics
We undertook an observational, analytical cross-sectional study at the Faculty of Dental Medicine from “Victor Babeș” University of Medicine and Pharmacy, Timișoara. The protocol conformed to the Declaration of Helsinki and was approved by the “Victor Babeș” University Ethics Committee (approval code E-787, dated 8 February 2023). Reporting follows STROBE guidance for cross-sectional studies.
The study’s PICO framework was defined as follows: the population comprised medically healthy university adults aged 18–35 years. The exposure of interest was a lower unstimulated salivary flow rate (uSFR) combined with a greater tongue-coating burden (TCI), while the comparator groups included participants from the same cohort exhibiting higher uSFR and/or lighter tongue coating, as well as those reporting negative versus positive self-perception of morning halitosis. The outcomes were 2-fold: first, objective measures of morning malodor—namely, total volatile sulfur-compound concentration (VSC, parts-per-billion) and an organoleptic score ranging from 0 to 5; and second, the subjective experience of morning breath odor, recorded as self-perceived halitosis (yes/no).
All volunteers provided written informed consent and could withdraw at any point without penalty. Measures were non-invasive; no adverse events occurred. Personal identifiers were replaced with study IDs, and the de-identified dataset was stored on an encrypted university server accessible only to the research team.
2.2. Study Participants
Inclusion criteria were age 18–35 years, full natural dentition (≥24 teeth), and self-reported good general health with no diagnosed salivary gland disorder. Exclusion criteria comprised (i) systemic disease or medication affecting saliva; (ii) antibiotic, antiseptic mouthwash, or professional dental cleaning within four weeks; (iii) active upper-respiratory infection, untreated dental caries, or probing pocket depths > 4 mm; (iv) smoking > 10 cigarettes∙day−1; (v) pregnancy or lactation; and (vi) allergy to H2S calibration gas. A priori sample-size calculation (G*Power 3.1) for detecting |ρ| ≥ 0.35 between uSFR and VSC with 80% power at α = 0.05 indicated 90 participants; 95 were enrolled to compensate for attrition, and 92 completed all assessments (3 no-shows).
2.3. Examination Standards and Variables
Participants were instructed to refrain from food, drinks other than water, oral hygiene, chewing gum, smoking, vigorous exercise, and scented products after 00:00 h.
Primary exposures: uSFR (mL∙min−1) measured by 5 min passive drool into pre-weighed, low-evaporation polypropylene tubes; values < 0.24 mL∙min−1 were classified as “low” saliva, 0.24–0.30 as “medium”, and >0.30 as “high”. The TCI employed the modified Winkel index: six dorsal tongue zones scored 0 = none, 1 = thin, 2 = moderate, and 3 = thick; the mean of the six constituted the participant’s TCI (0–3).
Primary outcomes: (i) VSC, quantified with a portable sulfide monitor (Halimeter®, Interscan Corp., Los Angeles, CA, USA) and expressed in parts per billion (ppb) as a single total VSC value; (ii) Organoleptic score, graded on Rosenberg’s 0–5 scale after participants exhaled slowly at 10 cm, two blinded, calibrated examiners rated simultaneously and reached consensus, and weighted κ = 0.82; (iii) Self-perceived halitosis captured by the single dichotomous item: “Do you believe your breath usually smells unpleasant when you wake up?”
Covariates: Age, sex, body-mass index (BMI, kg/m2), smoking status (current/never), mouth-breathing habit during sleep (yes/no, assessed by the validated Sleep Breathing Questionnaire), Silness–Löe plaque index (PI), and time since last dental visit.
Unstimulated saliva was collected first to avoid stimulation artifacts. Immediately thereafter, tongue coating was photographed macroscopically for archival purposes and scored in real time. The plaque index was assessed using a UNC-15 probe and mouth mirror. Organoleptic assessment and VSC measurement were performed last, separated by a 2 min rest to allow gas equilibrium.
2.4. Statistical Analysis
Analyses employed SPSS version 27.0 (IBM Corp., Armonk, NY, USA). Normality was checked by Shapiro–Wilk, homogeneity by Levene, and multicollinearity by variance-inflation factor (VIF < 2). Skewed variables (VSC, organoleptic) were log10-transformed. Continuous data are presented as mean ± SD; categorical data as frequency (%). Group differences for continuous variables were tested using independent-samples t-tests or one-way ANOVA with Tukey post hoc; non-parametric alternatives (Mann–Whitney, Kruskal–Wallis) confirmed robustness. Associations employed Spearman coefficients; effect sizes are expressed as Cohen’s d or partial η2 where appropriate. A stepwise multiple linear regression (entry p < 0.10, stay p < 0.05) predicted log10-VSC from uSFR, TCI, PI, sex, BMI, smoking, and mouth breathing. Determinants of self-perceived halitosis were assessed in a multivariable logistic model; model fit was evaluated using a Hosmer–Lemeshow test and Nagelkerke R2. Missing data were < 1% and handled by listwise deletion after confirming a missing-completely-at-random pattern (Little’s MCAR p = 0.71). All tests were two-tailed with α = 0.05; no multiplicity correction was applied because hypotheses were prespecified, and the study was exploratory.
3. Results
Groups did not differ in age, sex, or BMI (all p > 0.45). In contrast, the halitosis ‘Yes’ group showed lower uSFR and higher TCI, with markedly higher organoleptic and VSC values (all p < 0.001). Full descriptive statistics are provided in Table 1.

Table 1.
Baseline characteristics by self-perceived halitosis.
Table 2 reports six pairwise Spearman coefficients that collectively map the inter-relationships of flow, coating, plaque, and odor indices. The inverse correlation between uSFR and VSC (ρ = −0.58, p < 0.001) is the strongest salivary finding, indicating that every incremental 0.1 mL·min−1 increase in basal flow is accompanied by an average 0.26-log decline in sulfur-gas concentration. Tongue coating also emerged as an important, albeit more modest, contributor: its positive correlation with VSC reached ρ = 0.44 (p < 0.001) and with organoleptic score ρ = 0.41 (p < 0.001), implying that heavier dorsum biofilm perceptibly degrades breath quality. Organoleptic assessments mirrored instrumental VSC readings closely (ρ = 0.67, p < 0.001), validating the subjective panel method against objective halimetry. Salivary flow further correlated negatively with organoleptic rating (ρ = −0.52, p < 0.001), reinforcing its dual biochemical and sensory impact. Finally, plaque index displayed a small yet significant association with VSC (ρ = 0.37, p = 0.001), suggesting supragingival deposits modestly intensify malodor but are secondary to dorsum factors (Figure 1).

Table 2.
Spearman correlations among study variables.
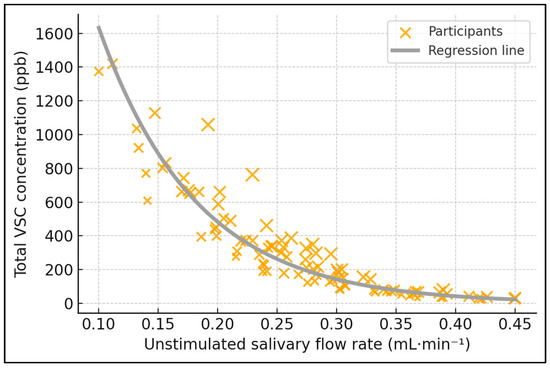
Figure 1.
Salivary flow vs. sulfur-gas output. Relationship between unstimulated salivary flow rate (uSFR) and total volatile sulfur compounds (VSCs). Scatter with linear fit and 95% CI; 92 participants; VSC in ppb (Halimeter®). Spearman ρ = −0.58, p < 0.001.
VSC increased stepwise across uSFR tertiles (ANOVA p < 0.001), with each higher-flow category associated with substantially lower mean VSCs (Table 3 and Figure 2). Post hoc tests confirmed all pairwise differences.

Table 3.
Mean VSC by salivary-flow tertile.
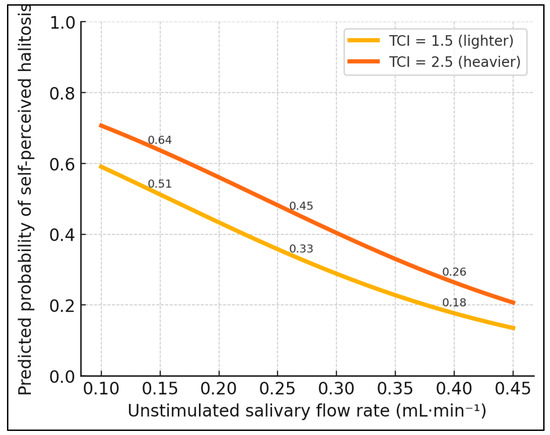
Figure 2.
Influence of flow and tongue coating on halitosis perception. Morning malodor by salivary-flow tertile. Mean (±SD) VSC for low (<0.24 mL·min−1), medium (0.24–0.30), and high (>0.30) uSFR groups. ANOVA F = 35.6, p < 0.001; Tukey post hoc: all pairwise comparisons p ≤ 0.002.
Table 4 summarizes a multivariable model explaining over half of the variability (R2 = 0.54) in log-transformed VSC concentrations. Each 0.1 mL·min−1 rise in uSFR corresponded to a β-coefficient of −0.53 (95% CI −0.64 to −0.42, p < 0.001), equating to a 32% reduction in absolute sulfur-gas levels—a magnitude that dwarfs all other predictors. Tongue-coating burden retained independent significance, as a one-unit increment on the 0–3 scale raised the log10-VSC by 0.31 (p = 0.002), equivalent to a 104 ppb increase in the cohort mean. The plaque index contributed a smaller yet still significant β of 0.18 (p = 0.008), suggesting that the supragingival biofilm amplifies malodor once flow and dorsum factors are controlled. Male sex entered the stepwise selection but failed to reach significance (β = 0.09, p = 0.164), indicating that gender did not materially influence the breath chemistry when oral parameters were accounted for. The model’s standard error of estimate was 0.14 log units, and variance-inflation factors remained below 1.6, excluding multicollinearity.

Table 4.
Stepwise linear regression predicting log10-VSC.
Table 5 dissects how habitual oro-nasal airflow modifies the flow–odor nexus, comparing 28 self-identified mouth breathers with 64 nasal breathers. Mouth breathers displayed a uSFR almost identical to the low-flow tertile (0.2 ± 0.1 mL·min−1) and 33% lower than nose breathers (0.3 ± 0.1 mL·min−1, p < 0.001), substantiating physiological desiccation due to increased evaporative loss (Figure 3). Concordantly, their TCI averaged 2.4 ± 0.5 versus 1.9 ± 0.5 (p < 0.001), indicating a thicker dorsum biofilm likely promoted by reduced salivary clearance. These combined deficits translated into a 1.5-fold surge in VSCs (281.5 ± 38.4 ppb vs. 185.6 ± 47.2 ppb, p < 0.001) and an organoleptic escalation of 1.2 points (3.5 ± 0.6 vs. 2.3 ± 0.7, p < 0.001), pushing many mouth breathers into the socially unacceptable odor range (>3). Effect sizes (Cohen’s d = 1.9 for VSC, 1.8 for organoleptic score) exceed thresholds for “large,” underscoring the clinical relevance.

Table 5.
Subgroup analysis by breathing pattern.
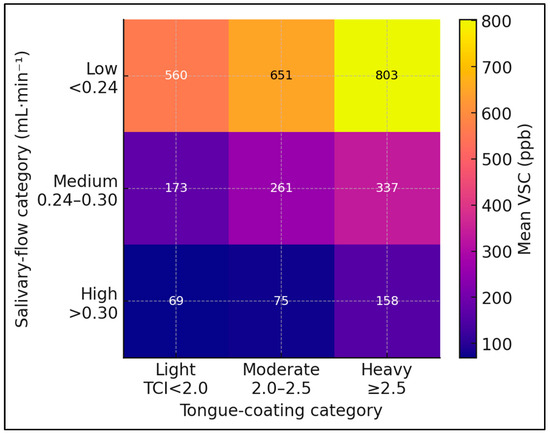
Figure 3.
Heat-map displaying the combined influence of salivary-flow and tongue-coating strata on mean sulfur-gas concentration. Heat-map of combined uSFR and tongue-coating index (TCI) strata versus mean VSC. Darker shading indicates higher VSC (ppb). Model R2 = 0.54 after adjustment for plaque index and sex.
Table 6 translates biochemical and behavioral metrics into subjective experience by modeling the odds of reporting bad breath. A 0.1 mL·min−1 increase in the uSFR halved the likelihood of self-perceived malodor (OR = 0.53, 95% CI 0.37–0.76, p = 0.001), confirming the protective role of adequate hydration. Conversely, each unit rise in the TCI (roughly one-third of full scale) boosted odds by 67% (p = 0.014), while every 50-ppb increment in VSC raised them 41% (p < 0.001), quantitatively linking chemical load to conscious awareness. Mouth breathing emerged as a potent contextual factor: habitual oral airflow nearly tripled the odds (OR = 2.84, 95% CI 1.06–7.59, p = 0.038), independent of other variables. The multivariate Nagelkerke R2 of 0.48 indicates that the model explains almost half of the variance in self-report, and good calibration (Hosmer–Lemeshow p = 0.62) suggests predictive reliability across probability strata.

Table 6.
Logistic regression for self-perceived halitosis (Dependent = “Yes”).
4. Discussion
4.1. Literature Findings
Early-morning halitosis, though transient, can undermine social confidence. Our cross-sectional analysis of 92 healthy students demonstrates that physiological variations in basal salivary secretion and tongue-coating thickness meaningfully influence both objective VSC concentrations and subjective breath assessment. Unlike interventional trials, our design captured naturalistic behavior, thereby revealing real-life determinants amenable to everyday self-care.
These findings align with earlier clinical studies showing inverse correlations between salivary flow and VSCs, yet extend them to a younger, medication-free population. The strong dose–response across salivary-flow tertiles suggests that, even within “normal” ranges, individuals at the lower end are predisposed to higher sulfur output. Combined with the independent effect of tongue coating, the data endorse a preventive, dual-target strategy that emphasizes hydration as vigorously as biofilm removal.
The breathing pattern emerged as an under-appreciated contributor. Mouth breathers exhibited both lower saliva and thicker coatings, yielding VSC levels comparable to those recorded in gingivitis cohorts. This synergy underscores the need for interdisciplinary collaboration between dentistry and otolaryngology. Future longitudinal work should investigate whether correcting nasal obstruction or implementing nocturnal humidification sustainably reduces morning malodor.
Our findings confirm that basal salivary output is not merely a binary normal/abnormal variable but a powerful quantitative determinant of sulfur-gas load. Every 0.1 mL·min−1 drop in uSFR was accompanied by an average rise of 55 ppb in VSCs and a near-doubling of self-perceived malodor in our cohort; a virtually identical pattern was seen in a recent Odontology study, in which subjects below the 0.25 mL·min−1 threshold emitted median VSCs of 286 ppb versus 168 ppb above that limit (ρ = −0.42). The close agreement between their correlation coefficient and our own (−0.58) suggests that the inverse salivation–odor relationship is robust across ethnic, age-range, and periodontal-health spectra [21].
The stepwise increase in VSCs across our flow tertiles also aligns well with physiological reference data. In a Jordanian normative survey (n = 243), the mean unstimulated rate was 0.46 ± 0.25 mL·min−1, with the 10th percentile at 0.18 mL·min−1—almost exactly where our “low-flow” tertile began. Such overlap indicates that a sizeable slice of the “healthy” population operates in a zone where odor risk escalates steeply. Bollen and Beikler’s multidisciplinary review further warned that once the flow approaches 0.15 mL·min−1, plaque and tongue deposits rise 2- to 3-fold, amplifying the malodor potential; our data illustrate the early slope of that continuum [22,23].
Tongue biofilm retained an independent, sizeable impact after accounting for saliva and plaque (β = 0.31). Participants in the upper TCI quartile (≥2.5) produced 118 ppb more VSCs than peers with lighter coatings, even though their plaque scores differed by only 0.2 units. This echoes periodontal-clinic evidence that each unit of tongue-coating thickness adds roughly 40 ppb to the sulfur output, underscoring the dorsum as a distinct ecological niche that responds poorly to tooth-focused hygiene alone [24].
Airway behavior magnified these effects. Mouth-breathing students presented with 52% thicker coatings, 0.1 mL·min−1 lower flow, and almost 100 ppb higher VSCs than nasal breathers. A pediatric RCT, in which annatto-based photodynamic therapy was delivered to mouth-breathing children, reported baseline VSCs (≈280 ppb) virtually identical to ours and achieved a 51% reduction within one week, illustrating both the heightened risk in this subgroup and its modifiability [25].
While plaque index explained a smaller share of variance (β = 0.18), its contribution is clinically meaningful. A 2024 Saudi cross-sectional study found plaque coverage to be twice as high in Halimeter-positive adults (47.5% vs. 27.9%) and a VSC–plaque correlation of r = 0.38—figures mirroring our 0.37. Likewise, a Brazilian university cohort of 5420 individuals showed gingival bleeding and plaque to be the strongest predictors of self-reported halitosis after socioeconomic adjustment (adjusted OR = 1.56), confirming that periodontal hygiene, while secondary to saliva and tongue status, still shapes odor perception [26,27].
Finally, the flow-dependent gradient we observed lends mechanistic support to low-tech hydration measures. In a randomized trial, drinking or rinsing with a single glass of water reduced methyl-mercaptan by ~60% and organoleptic scores by 1 point within 30 s—an effect large enough to erase the 133 ppb gap between our lowest and highest flow tertiles. When coupled with evidence that xerostomic flows (<0.25 mL·min−1) promote plaque accretion and biofilm maturation, routine advice to sip water before social interaction becomes a physiologically grounded first-line intervention [23,24].
In healthy young adults, modest decrements in basal salivary flow and heavier tongue coating are each independently linked to a higher VSC output and to perceiving ‘morning breath.’ Because both determinants are readily modifiable, first-line counseling should prioritize (i) hydration routines before anticipated social interactions, (ii) daily mechanical tongue cleaning (scraper or brush) in addition to toothbrushing, and (iii) screening for nocturnal mouth-breathing or nasal obstruction, with referral when indicated. These low-tech measures align with our dose–response data and can be implemented without pharmacologic adjuncts.
4.2. Limitations
The cross-sectional design precludes causal inference; the associations we report between uSFR/TCI and malodor may reflect bidirectional or unmeasured influences. Generalizability is constrained by convenience sampling of medical science students aged 18–35 years from a single university setting, with relatively homogeneous hygiene habits and diet. As such, the magnitude of effects may differ in older adults, patients with systemic disease, those taking xerogenic medications, or in populations with distinct cultural and dietary practices.
The Halimeter® does not differentiate among individual sulfur species; gas chromatography would allow for compositional profiling. Salivary flow and malodor also exhibit circadian and day-to-day variability that a single morning measurement cannot capture. Since we utilized a validated portable sulfide monitor that provides a combined VSC value rather than gas-specific speciation, compositional differences among H2S, CH3SH, and (CH3)2S could not be resolved. We did not capture psychosocial variables, such as anxiety, social self-consciousness, or halitophobia, which can modulate self-perceived malodor independently of chemical measures. Nor did we quantify recent dietary sulfur intake (e.g., alliums, crucifers) beyond the standardized overnight fast. Future longitudinal studies incorporating validated psychometric scales and dietary recalls are warranted.
5. Conclusions
Within a homogeneous, medication-free cohort, lower unstimulated salivary flow and thicker tongue coating were independently associated with higher sulfur-gas output and greater odds of self-perceived morning malodor. Simply, low-cost strategies, hydration, daily tongue cleaning, and addressing mouth breathing represent practical, first-line measures for dentists to recommend and for patients to adopt.
Author Contributions
Conceptualization, M.P. and S.D.; methodology, M.P. and S.D.; software, M.P. and S.D.; validation, M.M.L.; formal analysis, M.M.L.; investigation, M.M.L.; resources, B.A.B.; data curation, B.A.B.; writing—original draft preparation, M.P. and S.D.; writing—review and editing, B.A.B. and S.T.; visualization, S.T.; supervision, S.T.; project administration, S.T.; All authors have read and agreed to the published version of the manuscript.
Funding
The Article Processing Charge (APC) was covered by ‘Victor Babeș’ University of Medicine and Pharmacy Timișoara. No external funding was received for the conduct of this research.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of “Victor Babes” University of Medicine and Pharmacy Timisoara (approval code E-787, dated 8 February 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data availability is subject to hospital approval.
Acknowledgments
The authors used ChatGPT v4.0, an AI language model developed by OpenAI (San Francisco, CA, USA), to exclusively improve the manuscript’s language and readability. All the scientific content, interpretations, and conclusions are the original work of the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Akaji, E.A.; Folaranmi, N.; Ashiwaju, O. Halitosis: A review of the literature on its prevalence, impact and control. Oral. Health Prev. Dent. 2014, 12, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Borg-Bartolo, R.; Roccuzzo, A.; Gambetta-Tessini, K.; Schimmel, M.; Molinero-Mourelle, P.; Sabatini, G.; Ferrillo, M.; Esteves-Oliveira, M.; Giacaman, R.A.; Tennert, C.; et al. Association between oral health-related quality of life and structural determinants of health among elderly populations. a systematic review and meta-analysis. Int. J. Equity Health 2025, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Macedo de Sousa, B.; Rodríguez, C.; Suárez, A.; Aragoneses, J.M. Role of Probiotics in Halitosis of Oral Origin: A Systematic Review and Meta-Analysis of Randomized Clinical Studies. Front. Nutr. 2022, 8, 787908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blom, T.; Slot, D.E.; Quirynen, M.; Van der Weijden, G.A. The effect of mouthrinses on oral malodor: A systematic review. Int. J. Dent. Hyg. 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Van der Sleen, M.I.; Slot, D.E.; Van Trijffel, E.; Winkel, E.G.; Van der Weijden, G.A. Effectiveness of mechanical tongue cleaning on breath odour and tongue coating: A systematic review. Int. J. Dent. Hyg. 2010, 8, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Shin, S.I.; Hong, J.Y. Investigation of volatile sulfur compound level and halitosis in patients with gingivitis and periodontitis. Sci. Rep. 2023, 13, 13175. [Google Scholar] [CrossRef]
- Izidoro, C.; Botelho, J.; Machado, V.; Reis, A.M.; Proença, L.; Alves, R.; Mendes, J.J. Intra-Oral Halitosis in Periodontitis: The Role of Tongue Coating—A Cross-Sectional Study. Med. Sci. Forum 2023, 22, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Lo, K.L.; Liman, A.N.; Feng, X.P.; Ye, W. Tongue-Coating Microbial and Metabolic Characteristics in Halitosis. J. Dent. Res. 2024, 103, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xun, Z.; Wang, Z.; Zhang, Q.; Liu, X.; Zheng, H.; Zhang, Q.; Zhang, Y.; Zhang, L.; Wu, C.; et al. Tongue Coating and the Salivary Microbial Communities Vary in Children with Halitosis. Sci. Rep. 2016, 6, 24481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortelli, J.R.; Barbosa, M.D.; Westphal, M.A. Halitosis: A review of associated factors and therapeutic approach. Braz. Oral. Res. 2008, 22 (Suppl. S1), 44–54. [Google Scholar] [CrossRef] [PubMed]
- Yaegaki, K.; Sanada, K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J. Periodontal Res. 1992, 27 Pt 1, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Fox, P.C.; Baum, B.J. How much saliva is enough? ‘Normal’ function defined. J. Am. Dent. Assoc. 1991, 122, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wiener, R.C.; Wu, B.; Crout, R.; Wiener, M.; Plassman, B.; Kao, E.; McNeil, D. Hyposalivation and xerostomia in dentate older adults. J. Am. Dent. Assoc. 2010, 141, 279–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.-Y.; Kim, J.-R.; Byun, J.-S.; Jung, J.-K. Standardized Protocols for Measuring Volatile Sulfur Compounds: Scientific Foundations and Methodologies. J. Oral. Med. Pain 2024, 49, 5–11. [Google Scholar] [CrossRef]
- Quirynen, M.; Mongardini, C.; van Steenberghe, D. The effect of a 1-stage full-mouth disinfection on oral malodor and microbial colonization of the tongue in periodontitis. A pilot study. J. Periodontol. 1998, 69, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Motta, P.B.; Motta, L.J.; Campos, T.M.; Gonçalves, M.L.L.; Santos, E.M.; Martimbianco, A.L.C.; de Andrade, D.J.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Horliana, A.C.R.T.; et al. Effect of Photodynamic Therapy on Halitosis: A Systematic Review of Randomized Controlled Trials. Sensors 2022, 22, 469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mankia, K.; Cheng, Z.; Do, T.; Hunt, L.; Meade, J.; Kang, J.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Hensor, E.M.A.; et al. Prevalence of Periodontal Disease and Periodontopathic Bacteria in Anti-Cyclic Citrullinated Protein Antibody-Positive At-Risk Adults Without Arthritis. JAMA Netw. Open 2019, 2, e195394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.H.; Kim, H.; Heo, D.W.; Ahn, I.S.; Auh, Q.S. Volatile sulfide compounds and oral microorganisms on the inner surface of masks in individuals with halitosis during COVID-19 pandemic. Sci. Rep. 2023, 13, 2487. [Google Scholar] [CrossRef]
- Kameyama, A.; Ishii, K.; Tomita, S.; Tatsuta, C.; Sugiyama, T.; Ishizuka, Y.; Takahashi, T.; Tsunoda, M. Correlations between Perceived Oral Malodor Levels and Self-Reported Oral Complaints. Int. J. Dent. 2015, 2015, 343527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanzow, P.; Dylla, V.; Mahler, A.M.; Hrasky, V.; Rödig, T.; Barre, F.; Scheithauer, S.; Wiegand, A. COVID-19 Pandemic: Effect of Different Face Masks on Self-Perceived Dry Mouth and Halitosis. Int. J. Environ. Res. Public Health 2021, 18, 9180. [Google Scholar] [CrossRef]
- Silva, M.L.V.; Viana, K.S.S.; de Arruda, J.A.A.; de Miranda, R.D.; Soares, M.C.F.; Calado, H.D.R.; Amorim, M.C.L.; Costa, F.O.; Cota, L.O.M.; Abreu, L.G.; et al. Volatile sulfur compounds, biofilm, and salivary parameters in patients with periodontal disease: A cross-sectional study. Odontology 2025, 113, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Sawair, F.A.; Ryalat, S.; Shayyab, M.; Saku, T. The unstimulated salivary flow rate in a jordanian healthy adult population. J. Clin. Med. Res. 2009, 1, 219–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Bollen, C.; Beikler, T. Halitosis: The multidisciplinary approach. Int. J. Oral Sci. 2012, 4, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluijs, E.; Slot, D.E.; Bakker, E.W.; Van der Weijden, G.A. The effect of water on morning bad breath: A randomized clinical trial. Int. J. Dent. Hyg. 2016, 14, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.H.; Mandetta, A.R.H.; Sobral, A.P.T.; Leal Gonçalves, M.L.; Santos, E.M.; Fossati, A.L.; Gallo, J.M.A.S.; Motta, P.B.; Deana, A.M.; Horliana, A.C.R.T.; et al. Assessment of photodynamic therapy with annatto and led for the treatment of halitosis in mouth-breathing children: Randomized controlled clinical trial. PLoS ONE 2024, 19, e0307957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jazzar, A.; AlDehlawi, H.; Farag, A.; Alhamed, S.; Akeel, S.; Mair, Y.; Flemban, K.; Alqassab, H.; Aljohani, K. Clinical parameters in patients with halitosis: A cross-sectional study. Front. Dent. Med. 2024, 5, 1427280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faria, S.F.S.; Costa, F.O.; Silveira, J.O.; Cyrino, R.M.; Cota, L.O.M. Self-reported halitosis in a sample of Brazilians: Prevalence, associated risk predictors and accuracy estimates with clinical diagnosis. J. Clin. Periodontol. 2020, 47, 233–246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).