Temporary Passive Shunt for Visceral Protection During Open Thoracoabdominal Aortic Repair Under Intraoperative Advanced Hemodynamic and Perfusion Monitoring: Tertiary Hospital Institutional Bundle and Preliminary Mid-Term Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Protocol for the Management of Thoracoabdominal Aortic Disease
- Use of sequential aortic cross-clamping;
- Use of blood perfusion for the celiac trunk and superior mesenteric artery and cold (4 °C) perfusion with Custodiol solution (Custodiol; Dr Franz-Kohler Chemie GmbH, Bensheim, Germany) for renal arteries;
- Separate reattachment of visceral and renal arteries when anatomically feasible;
- Reimplantation of at least two patent intercostal arteries whenever doable.
2.2. Passive Shunting: Indication
- TAAA anatomy
- -
- Dissecting aneurysm: Yes 0 point; No 1 point;
- -
- TAAA extent: Type II 0 point; Type II with clamping site distal to LSA: 1 point; Type I or V: 2 points; Type III: 3 points; Type IV: 4 points;
- Cardiac status
- -
- Left Ventricle Ejection Fraction (LVEF) <30%: 0 point; >50%: 1 point; >60%: 2 points;
- -
- No valvular disease: 1 point;
- -
- No atrial fibrillation: 1 point;
- -
- Sizes of cardiac chamber within the normal range: 1 point;
- -
- Preoperative derivative of pressure over time (dP/dt) > 500: 1 point.
- Comorbidities
- -
- Infection: 2 points;
- -
- Cancer: 2 points;
- -
- Contraindication to systemic heparinization: 1 point.
- Prior bypasses
- -
- Prior femoro-femoral cross-over bypass: 1 point;
- -
- Prior axillofemoral bypass: 2 points.
2.3. Passive Shunting: Surgical Technique
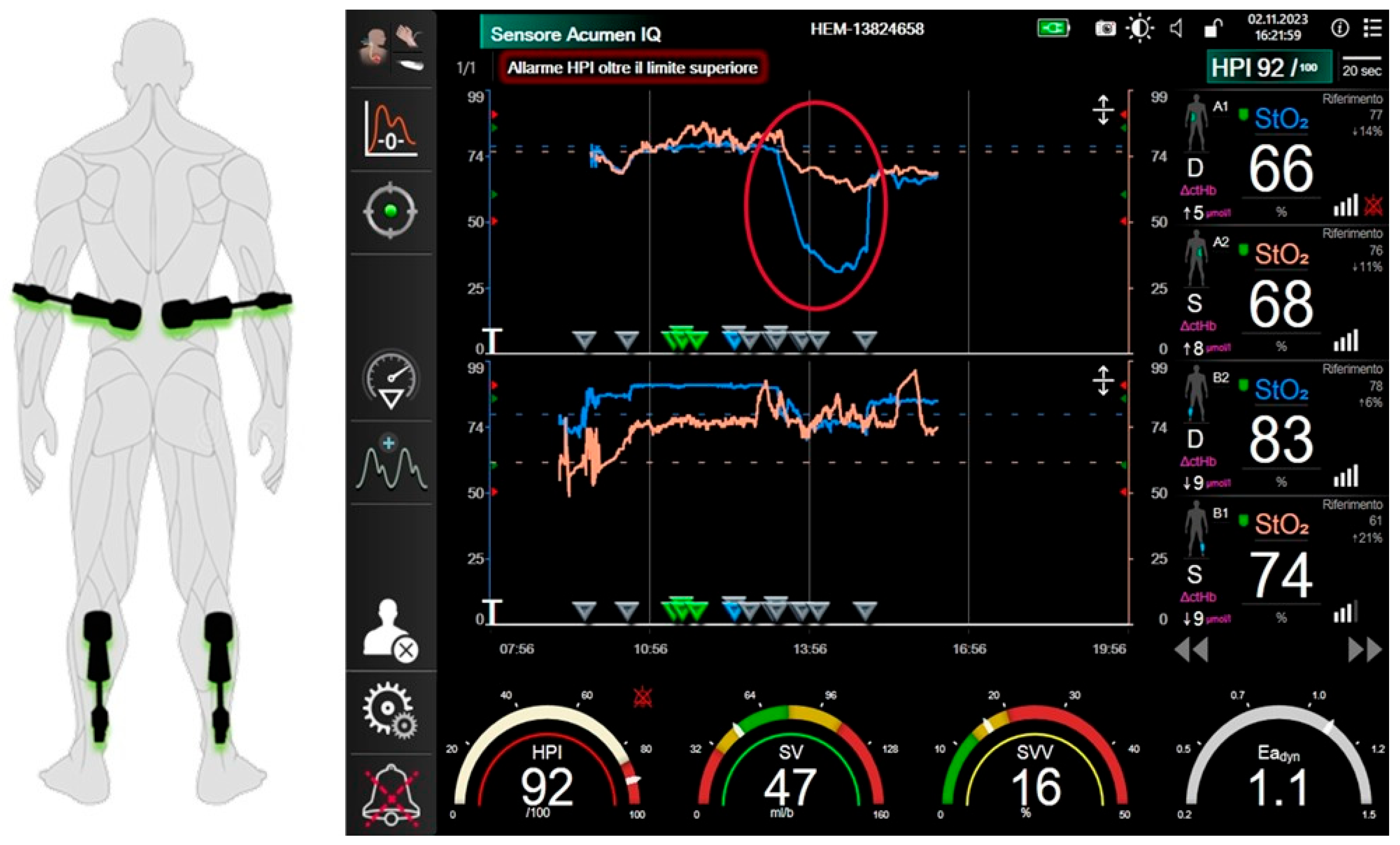
2.4. Intraoperative Monitoring
2.5. Ethical Statement
3. Results
3.1. Single-Center Experience
3.2. Intraoperative Outcomes
3.3. Postoperative Outcomes
4. Discussion
- It allows blood to flow directly from the aorta or one of its branches (e.g., the axillary artery) into the visceral and spinal arteries during aortic clamping, reducing left ventricular afterload and thereby limiting proximal hypertension.
- It avoids recirculation of contaminated blood (e.g., in case of preoperative coagulopathies, cancer, mycotic aortic aneurysm, or infection) and risk of SIRS form contact with tubing.
- Thanks to the intuitive functioning and handiness, it could be considered in urgent settings even in peripheral centers where ECC systems and a perfusionist team are not routinely available.
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICU | Intensive Unit Care |
| FU | Follow-Up |
| SD | Standard Deviation |
| ASA | American Society Anaesthesiology |
| CKD | Chromic Kidney Disease |
| AAA | Abdominal Aortic Aneurysm |
| AR | Amputation Rate |
| DUS | Doppler Ultrasound |
| DVT | Deep Vein Thrombosis |
| CTA | Computed Tomography Angiography |
| PAAs | Popliteal Artery Aneurysms |
| OR | Open Repair |
| ER | Endovascular Repair |
References
- Coselli, J.S.; LeMaire, S.A.; Preventza, O.; de la Cruz, K.I.; Cooley, D.A.; Price, M.D.; Stolz, A.P.; Green, S.Y.; Arredondo, C.N.; Rosengart, T.K. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J. Thorac. Cardiovasc. Surg. 2016, 151, 1323–1338. [Google Scholar] [CrossRef]
- Ferrer, C.; Gallitto, E.; Borghese, O.; Lodato, M.; Cappiello, A.; Cao, P.; Gargiulo, M.; Giudice, R. Long-term results of fenestrated and branched endovascular aneurysm repair for complex abdominal and thoracoabdominal aortic aneurysms in young and fit patients. J. Vasc. Surg. 2024, 80, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.Z.; Eagleton, M.J.; Roselli, E.E.; Blackstone, E.H.; Xiang, F.; Ibrahim, M.; Johnston, D.R.; Soltesz, E.G.; Bakaeen, F.G.; Lyden, S.P.; et al. Outcomes of Open Versus Endovascular Repair of Descending Thoracic and Thoracoabdominal Aortic Aneurysms. Ann. Thorac. Surg. 2022, 113, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Gott, V.L.; Whiffen, J.D.; Dutton, R.C. Heparin Bonding on Colloidal Graphite Surfaces. Science 1963, 142, 1297–1298. [Google Scholar] [CrossRef]
- Verdant, A.G.; Mercier, C.H.; Pagé, A.A.; Cossette, R.G.; Dontigny, L. Aneurysms of the descending thoracic aorta: Treatment with the Gott shunt. Can. J. Surg. 1981, 24, 594–596. [Google Scholar]
- Comerota, A.J.; White, J.V. Reducing morbidity of thoracoabdominal aneurysm repair by preliminary axillofemoral bypass. Am. J. Surg. 1995, 170, 218–222. [Google Scholar] [CrossRef]
- Kuniyoshi, Y.; Koja, K.; Miyagi, K.; Uezu, T.; Yamashiro, S.; Arakaki, K.; Nagano, T.; Mabuni, K.; Senaha, S. Selective visceral perfusion during thoracoabdominal aortic aneurysm repair. Ann. Thorac. Cardiovasc. Surg. 2004, 10, 367–372. [Google Scholar]
- Taylor, P.R. Visceral and renal perfusion during thoracoabdominal aneurysm repair. Cardiovasc. Surg. 1999, 7, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Borghese, O.; Mastracci, T.M. Thoracoabdominal aortic aneurysm in women: Many questions remain regarding their poor outcome. Ann. Cardiothorac. Surg. 2023, 12, 549–557. [Google Scholar] [CrossRef]
- Aftab, M.; Coselli, J.S. Renal and visceral protection in thoracoabdominal aortic surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 2963–2966. [Google Scholar] [CrossRef]
- Brisard, L.; El Batti, S.; Borghese, O.; Maurel, B. Risk Factors for Spinal Cord Injury during Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score. J. Clin. Med. 2023, 12, 7520. [Google Scholar] [CrossRef]
- Sueda, T.; Morita, S.; Okada, K.; Orihashi, K.; Shikata, H.; Matsuura, Y. Selective intercostal arterial perfusion during thoracoabdominal aortic aneurysm surgery. Ann. Thorac. Surg. 2000, 70, 44–47. [Google Scholar] [CrossRef]
- Chung, J.; Ouzounian, M.; Lindsay, T. Motor Evoked Potential Monitoring During Thoracoabdominal Aortic Surgery: Useful or Not? Anesth. Analg. 2018, 126, 741–742. [Google Scholar] [CrossRef]
- Borghese, O.; Brisard, L.; Le Corvec, T.; Hauguel, A.; Guimbretière, G.; Maurel, B. Spinal Cord Protection During Thoracic and Thoracoabdominal Endovascular Aortic Repair: 5-Year Results of a Preventive Protocol Including Prophylactic Cerebrospinal Fluid Drainage in High-Risk Patients. J. Endovasc. Ther. 2023, 15266028231215972. [Google Scholar] [CrossRef] [PubMed]
- Tshomba, Y.; Leopardi, M.; Mascia, D.; Kahlberg, A.; Carozzo, A.; Magrin, S.; Melissano, G.; Chiesa, R. Automated pressure-controlled cerebrospinal fluid drainage during open thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 2017, 66, 37–44. [Google Scholar] [CrossRef]
- Monnot, A.; Dusseaux, M.M.; Godier, S.; Plissonnier, D. Passive Temporary Visceral Shunt from the Axillar Artery as an Adjunct Method during the Open Treatment of Thoracoabdominal Aortic Aneurysm. Ann. Vasc. Surg. 2016, 36, 127–131. [Google Scholar] [CrossRef]
- Cambria, R.P.; Davison, J.K.; Giglia, J.S.; Gertler, J.P. Mesenteric shunting decreases visceral ischemia during thoracoabdominal aneurysm repair. J. Vasc. Surg. 1998, 27, 745–749. [Google Scholar] [CrossRef]
- Bouziane, Z.; Settembre, N.; Saba, C.; Malikov, S. FT10. Open Repair of Type IV Thoracoabdominal Aortic Aneurysm: Results of Passive Visceral Shunt and Two-Graft Technique. J. Vasc. Surg. 2018, 67, e78–e79. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef] [PubMed]
- de Borst, G.J.; Boyle, J.R.; Dick, F.; Kakkos, S.K.; Mani, K.; Mills, J.L.; Björck, M. Editor’s Choice–European Journal of Vascular and Endovascular Surgery Publication Standards for Reporting Vascular Surgical Research. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 9–22. [Google Scholar] [CrossRef] [PubMed]
- ASIA and ISCoS International Standards Committee. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)-What’s new? Spinal Cord 2019, 57, 815–817. [Google Scholar] [CrossRef]
- Kahlberg, A.; Tshomba, Y.; Baccellieri, D.; Bertoglio, L.; Rinaldi, E.; Ardita, V.; Colombo, E.; Moscato, U.; Melissano, G.; Chiesa, R.; et al. Renal perfusion with histidine-tryptophan-ketoglutarate compared with Ringer’s solution in patients undergoing thoracoabdominal aortic open repair. J. Thorac. Cardiovasc. Surg. 2023, 165, 569–579.e5. [Google Scholar] [CrossRef]
- Loschi, D.; Melloni, A.; Kahlberg, A.; Chiesa, R.; Melissano, G. Kidney protection in thoracoabdominal aortic aneurysm surgery. J. Cardiovasc. Surg. 2021, 62, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Schepens, M.A.; Vermeulen, F.E.; Morshuis, W.J.; Dossche, K.M.; van Dongen, E.P.; Ter Beek, H.T.; Boezeman, E.H. Impact of left heart bypass on the results of thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 1999, 67, 1963–1967. [Google Scholar] [CrossRef]
- Coselli, J.S.; LeMaire, S.A. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 1999, 67, 1931–1934. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Amigoni, P.; Sangalli, F.; Paolini, G. Systemic and myocardial inflammatory response in coronary artery bypass graft surgery with miniaturized extracorporeal circulation: Differences with a standard circuit and off-pump technique in a randomized clinical trial. ASAIO J. 2013, 59, 600–606. [Google Scholar]
- Maisat, W.; Yuki, K. Narrative review of systemic inflammatory response mechanisms in cardiac surgery and immunomodulatory role of anesthetic agents. Ann. Card. Anaesth. 2023, 26, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Halle, D.R.; Benhassen, L.L.; Søberg, K.L.; Nielsen, P.F.; Kimose, H.H.; Bauer, A.; Hasenkam, J.M.; Modrau, I.S. Impact of minimal invasive extracorporeal circulation on systemic inflammatory response–a randomized trial. J. Cardiothorac. Surg. 2024, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.; Karkouti, K. Managing the coagulopathy associated with cardiopulmonary bypass. J. Thromb. Haemost. 2021, 19, 617–632. [Google Scholar] [CrossRef]
- Authors/Task Force Members; Kunst, G.; Milojevic, M.; Boer, C.; De Somer, F.M.J.J.; Gudbjartsson, T.; van den Goor, J.; Jones, T.J.; Lomivorotov, V.; Merkle, F.; et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Br. J. Anaesth. 2019, 123, 713–757. [Google Scholar]
- Moulakakis, K.G.; Karaolanis, G.; Antonopoulos, C.N.; Kakisis, J.; Klonaris, C.; Preventza, O.; Coselli, J.S.; Geroulakos, G. Open repair of thoracoabdominal aortic aneurysms in experienced centers. J. Vasc. Surg. 2018, 68, 634–645.e12. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Orihashi, K.; Shikata, H.; Murakami, H.; Fukunaga, S.; Sueda, T.; Matsuura, Y. Comparison studies of passive and active shunting during cross-clamping of the descending thoracic aorta. Panminerva Med. 1994, 36, 160–167. [Google Scholar] [PubMed]
- Wahba, A.; Kunst, G.; De Somer, F.; Kildahl, H.A.; Milne, B.; Kjellberg, G.; Bauer, A.; Beyersdorf, F.; Ravn, H.B.; Debeuckelaere, G.; et al. 2024 EACTS/EACTAIC/EBCP Guidelines on cardiopulmonary bypass in adult cardiac surgery. Br. J. Anaesth. 2025, 134, 917–1008. [Google Scholar] [CrossRef]
- Chiesa, R.; Tshomba, Y.; Mascia, D.; Rinaldi, E.; Logaldo, D.; Civilini, E. Open repair for juxtarenal aortic aneurysms. J. Cardiovasc. Surg. 2013, 54 (Suppl. S1), 35–45. [Google Scholar] [PubMed]
- Kunihara, T.; Shiiya, N.; Wakasa, S.; Matsuzaki, K.; Matsui, Y. Assessment of hepatosplanchnic pathophysiology during thoracoabdominal aortic aneurysm repair using visceral perfusion and shunt. Eur. J. Cardio-Thorac. Surg. 2009, 35, 677–683. [Google Scholar] [CrossRef]
- Kalder, J.; Keschenau, P.; Hanssen, S.J.; Greiner, A.; Vermeulen Windsant, I.C.; Kennes, L.N.; Tolba, R.; Prinzen, F.W.; Buurman, W.A.; Jacobs, M.J.; et al. The impact of selective visceral perfusion on intestinal macrohemodynamics and microhemodynamics in a porcine model of thoracic aortic cross-clamping. J. Vasc. Surg. 2012, 56, 149–158. [Google Scholar] [CrossRef]
- Frankort, J.; Doukas, P.; Mees, B.; Gombert, A.; Jacobs, M. Contemporary operative strategies and technical nuances for open thoracoabdominal aortic aneurysm repair. JVS-Vasc. Insights 2024, 2, 100109. [Google Scholar] [CrossRef]
- Coselli, J.S.; de la Cruz, K.I.; Preventza, O.; LeMaire, S.A.; Weldon, S.A. Extent II thoracoabdominal aortic aneurysm repair: How I do it. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 221–237. [Google Scholar] [CrossRef]
- Chiesa, R.; Civilini, E.; Melissano, G.; Logaldo, D.; Calliari, F.M.; Bertoglio, L.; Carozzo, A.; Mennella, R. Management of thoracoabdominal aortic aneurysms. HSR Proc. Intensive Care Cardiovasc. Anesth. 2009, 1, 45–53. [Google Scholar]
- Köksoy, C.; LeMaire, S.A.; Curling, P.E.; Raskin, S.A.; Schmittling, Z.C.; Conklin, L.D.; Coselli, J.S. Renal perfusion during thoracoabdominal aortic operations: Cold crystalloid is superior to normothermic blood. Ann. Thorac. Surg. 2007, 73, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Palombo, D.; Valenti, D.; Gaggiano, A.; Lupo, M.; Borin, P. Early experience with the minimal extracorporeal circulation system (MECC) during thoracoabdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 324–326. [Google Scholar] [PubMed]
- Hassoun, H.T.; Miller, C.C., 3rd; Huynh, T.T.; Estrera, A.L.; Smith, J.J.; Safi, H.J. Cold visceral perfusion improves early survival in patients with acute renal failure after thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 2004, 39, 506–512. [Google Scholar] [CrossRef] [PubMed]
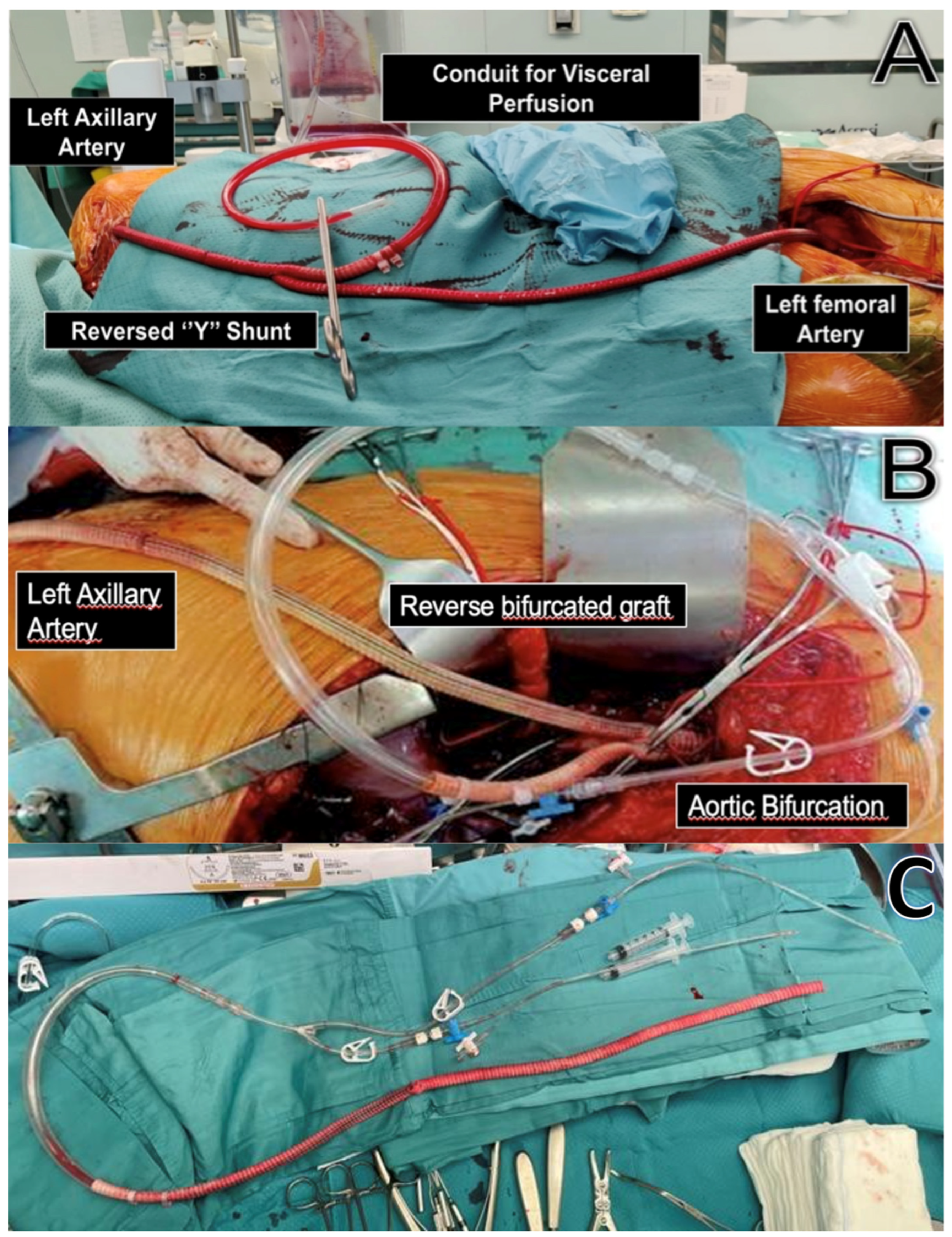
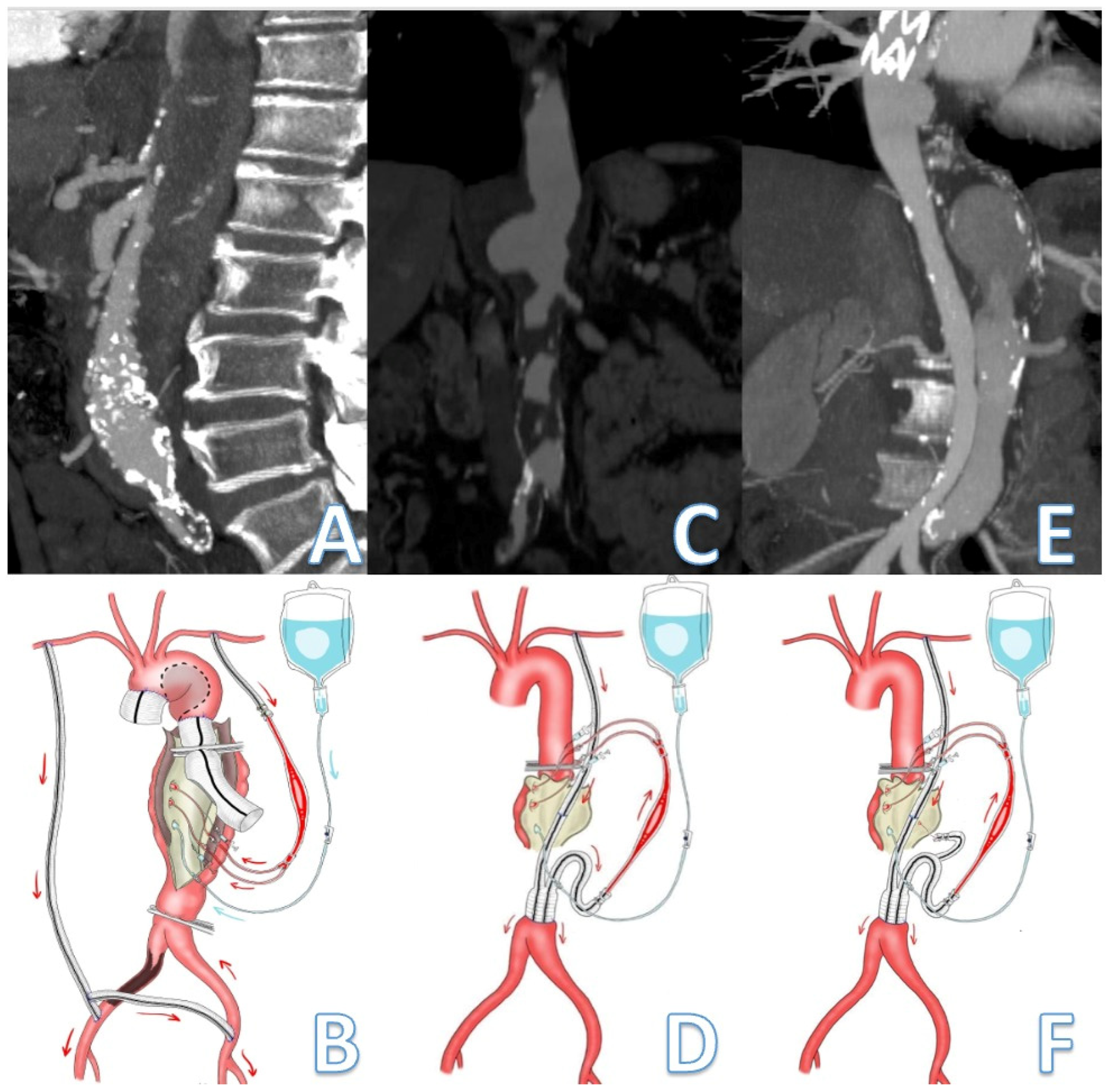
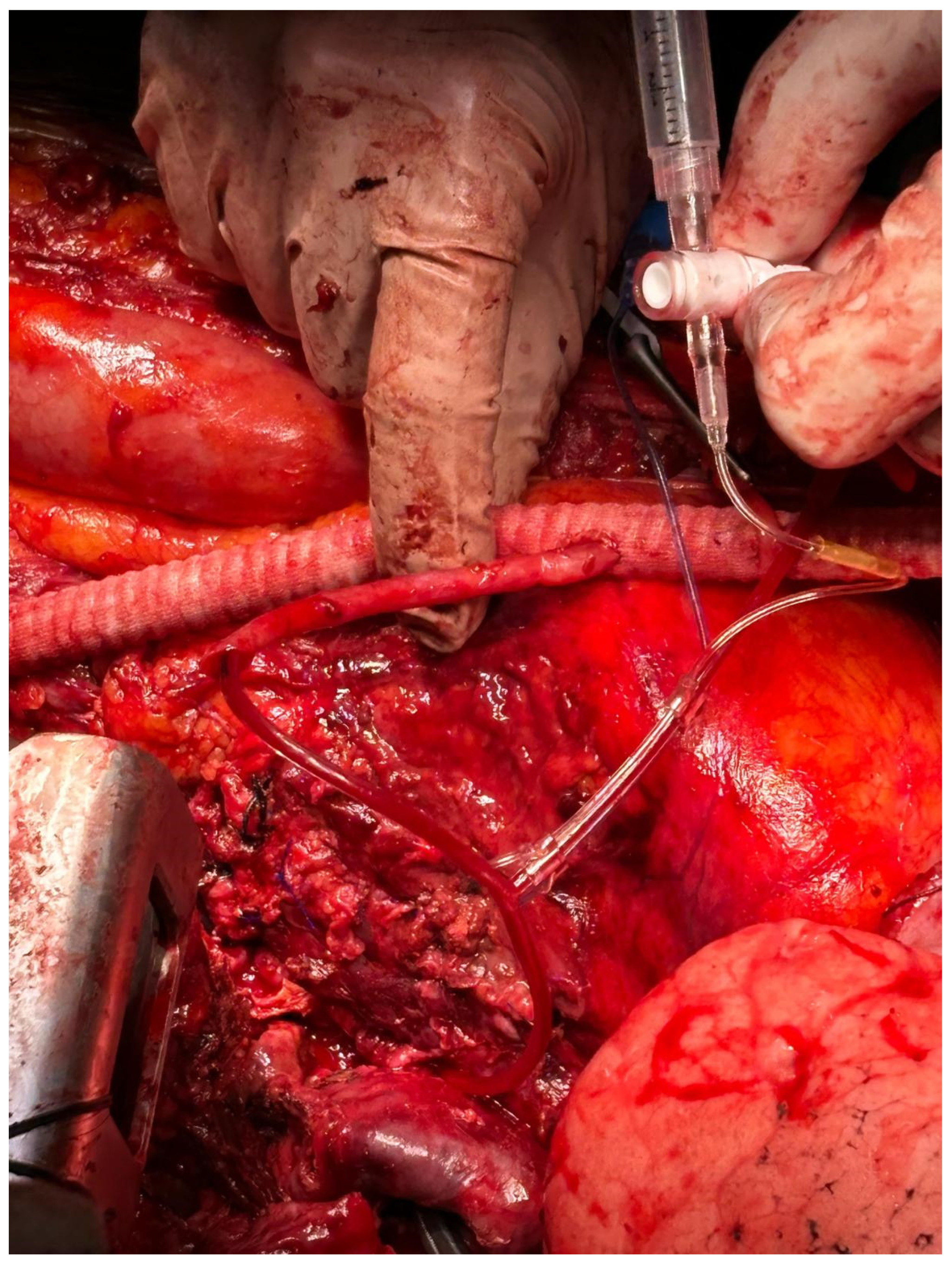
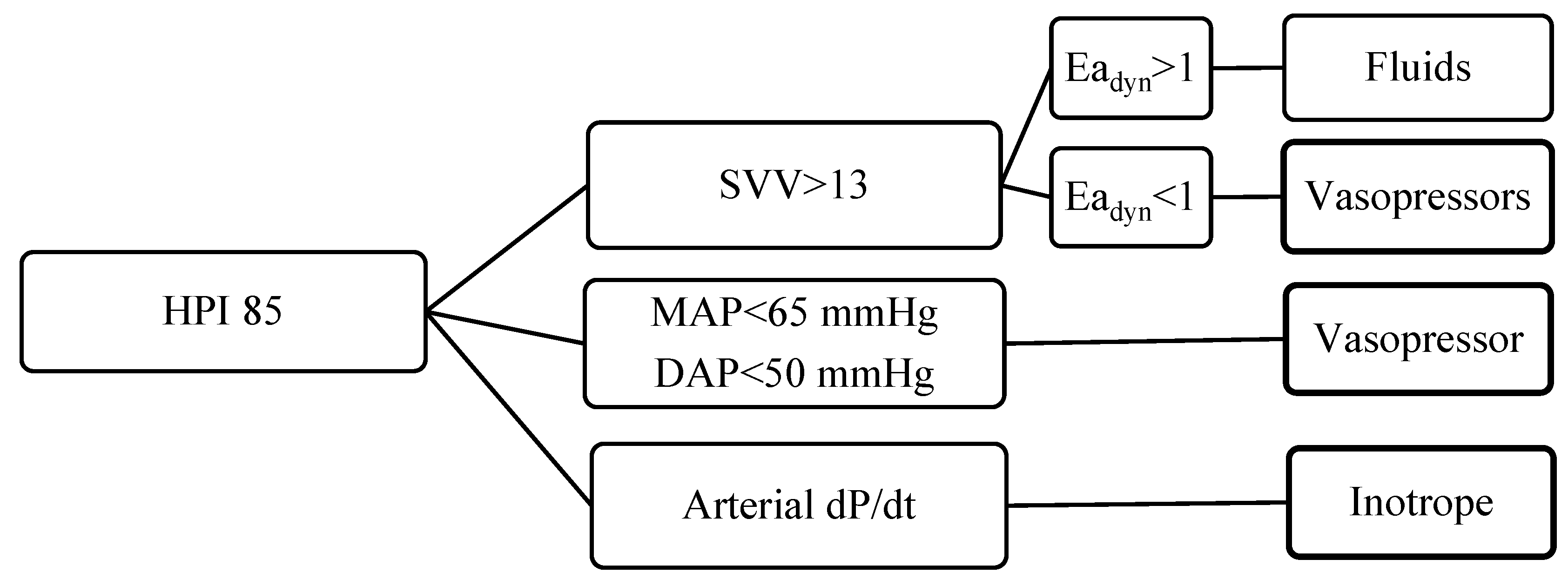
| Patients | Total (n/%) |
|---|---|
| Sex | |
| Male | 8 (73.6) |
| Female | 4 (36.4) |
| Mean Age | 67 years (range 63–79) |
| TAAA Extent (Crawford Classification) | |
| Type I | 1 (9) |
| Type II | 2 (18.2) |
| Type III | - |
| Type IV | 6 (54.5) |
| Type V | 2 (18.2) |
| Comorbidities | |
| Hypertension | 11 (100) |
| Dyslipidemia | 9 (82) |
| Diabetes mellitus | - |
| CAD | 3 (27.3) |
| COPD | 2 (18.2) |
| Chronic kidney disease | 5 (45.5) |
| Tobacco use | 7 (63.7) |
| ASA class | |
| 1 | - |
| 2 | 2 (18.2) |
| 3 | 9 (82) |
| 4 | - |
| Clinical presentation | |
| Asymptomatic | 10 (91) |
| Thoracic Pain | 1 (9) |
| Patient (Sex/Age) | TAAA Type * | Previous Aortic Surgery | PS Inflow | PS Outflow | Morbidity | Mortality | Preoperative Score # |
|---|---|---|---|---|---|---|---|
| 1 (M—72 years) | IV | AA replacement | LAA | LCIA | - | No | 9 |
| 2 (M—65 years) | IV | Infrarenal aortic surgical repair | LAA | Infrarenal aorta | AKI | No | 8 |
| 3 (M—66 years) | IV | - | LAA | LCIA | AKI, SCI, mesenteric ischemia | Yes | 8 |
| 4 (F—72 years) | I | TEVAR | LAA | LCIA | - | No | 8 |
| 5 (F—51 years) | III | AA replacement | LAA | Infrarenal aorta | - | No | 9 |
| 6 (M—63 years) | II | AA replacement + right axillofemoral and femoro-femoral bypass | LAA | RCFA | - | Yes | 10 |
| 7 (M—66 years) | IV | Axillofemoral | LAA | LCIA | - | No | 10 |
| 8 (M—78 years) | III | EVAR | LAA | RCIA | AKI | No | 8 |
| 9 (M—69 years) | V | - | LAA | Infrarenal aorta | - | No | 8 |
| 10 (F—67 years) | IV | - | LAA | LCFA | - | No | 9 |
| 11 (M—69 years) | V | AA replacement; TEVAR | DTA | LCIA | - | No | 8 |
| Operative Details | Total (n/%) |
|---|---|
| Setting | |
| Urgent/emergent | 1 (9) |
| Elective | 10 (91) |
| Cerebrospinal fluid drainage | |
| Preoperative insertion | 4 (36.4) |
| Postoperative insertion | 1 (9) |
| Outcomes | Total (n/%) |
| In-hospital mortality | 1 (9) |
| Mortality during follow-up | 1 (9) |
| Paraplegia | |
| Transitory | 1 (9) |
| Permanent | - |
| Renal failure | |
| Transitory | 2 (18.2) |
| Permanent | 1 (9) |
| Mesenteric ischemia | 1 (9) |
| Bleeding requiring reoperations | - |
| Mean ICU stay | 5 days (range 1–10) |
| Mean hospital stays | 14 days (range 9–20) |
| Mean follow-up | 18 months (range 10–42) |
| ICU Intensive Care Unit | |
| Author, Year | N Patient/Aneurysm Extent | Type of PS | Setting/Rationale for PS Use | Arteries Perfused with PS | Postoperative AKI | AKI at the Discharge | SCI | Peri-Operative Coagulopathy Major Bleeding Mesenteric Ischemia | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Comerota et al. [6] 1995 | 15 TAAA (4 type I, 3 type II, 5 type III, 3 type IV) | Axillofemoral bypass | Ps was used reduce the cardiac afterload of thoracic aortic clamping | 1 (7%) | 0 (0%) | 1 (7%) | - | 1 (7%) | |
| Monnot et al. [16] 2016 | 10 type III TAAA | Temporary axillary bypass | Severe aortoiliac occlusive disease that would have limited retrograde perfuse to visceral vessels during proximal aortic anastomosis | Both SMA and RRA (LRA perfused with 4 °C Lactate Ringer solution) | 1 (10%) | 1 (10%) | - | - | - |
| Cambria et al. [17] 1998 | (15 patients: 5 type I, 6 type II, 4 type III TAA) | Aortic prosthetic body | PS was used to reduce metabolic and hemodynamic derangements occurring with reestablishment of visceral perfusion during clamp and sew TAA repair | CT or SMA | - | - | - | - | - |
| Bouziane et al. [18] 2018 | 24 cases of type IV TAAA | NR | SMA, RRA, LRA | 3 (12.5) | - | NA | - | 1(4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghese, O.; Minucci, M.; Jacchia, E.; Annuvolo, P.A.; Scurto, L.; Luparelli, A.; Russo, A.; Aceto, P.; Donati, T.; Tshomba, Y. Temporary Passive Shunt for Visceral Protection During Open Thoracoabdominal Aortic Repair Under Intraoperative Advanced Hemodynamic and Perfusion Monitoring: Tertiary Hospital Institutional Bundle and Preliminary Mid-Term Results. J. Clin. Med. 2025, 14, 6064. https://doi.org/10.3390/jcm14176064
Borghese O, Minucci M, Jacchia E, Annuvolo PA, Scurto L, Luparelli A, Russo A, Aceto P, Donati T, Tshomba Y. Temporary Passive Shunt for Visceral Protection During Open Thoracoabdominal Aortic Repair Under Intraoperative Advanced Hemodynamic and Perfusion Monitoring: Tertiary Hospital Institutional Bundle and Preliminary Mid-Term Results. Journal of Clinical Medicine. 2025; 14(17):6064. https://doi.org/10.3390/jcm14176064
Chicago/Turabian StyleBorghese, Ottavia, Marta Minucci, Elena Jacchia, Pierfrancesco Antonio Annuvolo, Lucia Scurto, Antonio Luparelli, Andrea Russo, Paola Aceto, Tommaso Donati, and Yamume Tshomba. 2025. "Temporary Passive Shunt for Visceral Protection During Open Thoracoabdominal Aortic Repair Under Intraoperative Advanced Hemodynamic and Perfusion Monitoring: Tertiary Hospital Institutional Bundle and Preliminary Mid-Term Results" Journal of Clinical Medicine 14, no. 17: 6064. https://doi.org/10.3390/jcm14176064
APA StyleBorghese, O., Minucci, M., Jacchia, E., Annuvolo, P. A., Scurto, L., Luparelli, A., Russo, A., Aceto, P., Donati, T., & Tshomba, Y. (2025). Temporary Passive Shunt for Visceral Protection During Open Thoracoabdominal Aortic Repair Under Intraoperative Advanced Hemodynamic and Perfusion Monitoring: Tertiary Hospital Institutional Bundle and Preliminary Mid-Term Results. Journal of Clinical Medicine, 14(17), 6064. https://doi.org/10.3390/jcm14176064







