Abstract
Intermittent claudication is the most common manifestation of peripheral arterial disease as well as a lifestyle-limiting disease with a favorable prognosis. Despite societal guideline recommendations, most claudicants do not trial optimal medical therapy (OMT) and supervised exercise therapy (SET) or receive a quality-of-life (QoL) assessment prior to intervention. In this review, we discuss the components of OMT and SET and the trials establishing their clear benefits in claudicants. We assess adherence rates to OMT/SET and qualitative and quantitative studies attempting to understand the barriers to adoption. We also review how patient-reported outcome metrics were developed to assess QoL in claudicants and reasons for their underutilization in daily clinical practice. Last, we describe novel initiatives seeking to improve adherence to OMT, SET, and QoL assessment.
1. Introduction
Peripheral arterial disease (PAD) affects more than 230 million individuals globally [1]. Its most common manifestation is intermittent claudication, defined as exertion-induced muscle pain of the lower extremities resolved with rest [2].
The prognosis of IC is favorable. Over two-thirds of claudicants will have symptoms that remain stable or improve without intervention. Furthermore, the rate of limb loss is less than 1% per year [3]. Despite this, intervention rates for claudication have risen over the past decade [4]. There is no clinical evidence suggesting that surgical intervention improves walking distance and quality of life (QoL) compared with non-surgical options like supervised exercise therapy (SET) and optimal medical therapy (OMT) [5,6]. Premature surgical intervention is also a predictor for progression to CLTI [7].
Subsequently, multiple surgical societies have published guidelines stating that intervention for claudication should be reserved for patients with severe lifestyle-limiting symptoms that have failed OMT and SET [3,8,9]. Adherence to such guidelines lacks consistency across healthcare systems [10]. An analysis of the Vascular Quality Initiative (VQI) data found that only one-third of claudicants who underwent elective revascularization were medically optimized prior to intervention [11]. Additionally, a US-based survey found that almost half of vascular specialists had never referred a patient to an SET program [12]. Additionally, it is unclear the degree to which surgeons assess the severity of lifestyle limitation and quality of life (QoL) in claudicants. Patient-reported outcome measures (PROMs), such as the Vascular Quality of Life Questionnaire (VascuQoL) or the Walking Impairment Questionnaire (WIQ), are gold standards in measuring QoL changes but are not yet widely utilized clinically.
Adherence to guideline-directed medical therapy (GDMT) and reporting of lifestyle limitation/QoL remains sub-optimal. This article provides a contemporary review on the benefits of OMT, SET, and PROMs usage, qualitative/quantitative analyses exploring their adherence, and innovative quality improvement interventions seeking to improve their utilization.
2. Optimal Medical Therapy
2.1. Current Guideline Recommendations
OMT is the cornerstone of non-surgical management for claudication and reduces disease progression, major adverse limb events (MALEs), and major adverse cardiac events (MACEs). OMT, compared with surgical intervention, also demonstrates greater improvements in patient-reported outcome measures [13]. Per the ACC/AHA Guideline for the Management of Lower Extremity Peripheral Arterial Disease, OMT involves antiplatelet and antithrombotic therapy, lipid-lowering therapy, smoking cessation, antihypertensive therapy, diabetes management, and symptom management therapy (Figure 1) [9].
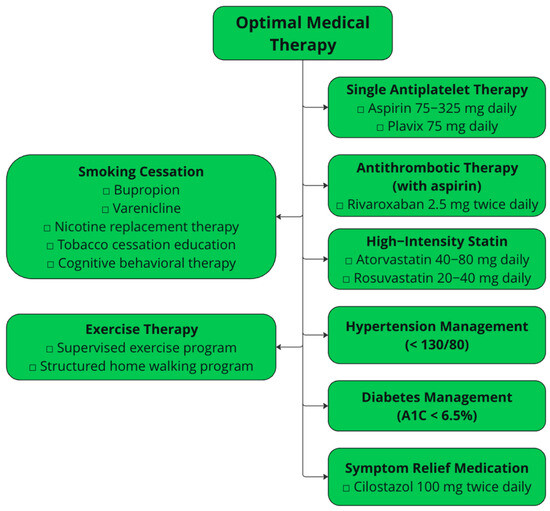
Figure 1.
The tenets of optimal medical therapy per the ACC/AHA Guideline for the Management of Lower Extremity Peripheral Arterial Disease.
Antiplatelet therapy involves a single antiplatelet agent, typically aspirin alone (75–325 mg daily) or clopidogrel (75 mg daily). Antiplatelet therapy provides cardioprotective benefits, as it reduces the risk of MACEs in patients with symptomatic PAD [14,15]. Dual antiplatelet therapy in patients prior to revascularization is not recommended due to higher rates of bleeding.
Antithrombotic therapy refers to low-dose rivaroxaban (2.5 mg twice daily) in conjunction with single antiplatelet therapy. These recommendations are based on the COMPASS and VOYAGER randomized clinical trials, which showed improved cardiovascular outcomes and MALEs in patients with PAD taking both ASA and rivaroxaban compared with ASA alone [16,17,18]. A sub-group analysis of the COMPASS trial also investigated the impact of rivaroxaban 2.5 mg plus aspirin versus rivaroxaban 5 mg alone and aspirin alone in patients with PAD. This analysis revealed that the primary outcome (composite of cardiovascular death, myocardial infarction, and stroke) was significantly reduced in the rivaroxaban plus aspirin group versus the aspirin alone group. No differences were seen in the rivaroxaban alone group compared with the aspirin alone group [19]. A sub-analysis from the VOYAGER PAD trial investigated whether the addition of clopidogrel impacted the effects of rivaroxaban plus aspirin in patients with revascularization for PAD. This study revealed that the addition of clopidogrel did not change the efficacy outcome of acute limb ischemia, major amputation, myocardial infarction, ischemia stroke, and cardiovascular death. However, there was a trend toward increased bleeding risk if clopidogrel was used over 30 days [20].
Lipid-lowering therapies include treatment with high-intensity statins, such as atorvastatin 40–80 mg or rosuvastatin 20–40 mg, with the goal of reducing low-density lipoprotein cholesterol (LDL-C) by >50%. Both RCTs and large database studies have observed decreases in MACEs, MALEs, and death rates in patients with PAD on statin therapies. In patients maximally tolerated on statin therapy with LDL-C levels greater than 70 mg/dL, or those unable to tolerate statins, other options include ezetimibe and PCSK9-inhibitor therapy. Multiple RCTs demonstrate the benefit of alirocumab and evolocumab in lowering rates of MACEs and MALEs compared with placebo in patients with PAD [21,22]. For ezetimibe, an RCT of patients with extracoronary atherosclerotic arterial disease showed that patients with the addition of ezetimibe to simvastatin had reduced MACEs [23]. No studies have shown the impact of ezetimibe on MALEs.
Smoking cessation, including exposure to secondhand tobacco smoke, is recommended in all patients with PAD. Smoking cessation has been shown to reduce MALEs, particularly bypass failures and amputations [24,25]. Some smoking cessation methods include pharmacotherapy such as varenicline, bupropion, and nicotine patches and behavioral therapies such as cognitive behavioral therapy and motivational interviewing [26].
Antihypertensive therapy is recommended in claudicants with a systolic blood pressure goal of <130 mmHg and diastolic blood pressure goal of <80 mmHg. Blood pressure control reduces the risk of stroke, cardiovascular death, and even MALEs, especially for angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs) [27,28,29].
Diabetes management, notably with glucagon-like peptide-1 (GLP-1) agonists and sodium–glucose transport protein 2 (SGLT2) inhibitors, minimizes the MACEs in claudicants [30,31]. No hemoglobin A1C value is recommended, although studies have investigated outcomes with values below 6.5% [32].
Medications to manage leg symptoms include cilostazol, a phosphodiesterase III (PDE3) inhibitor causing vasodilation and platelet aggregation inhibition. Cilostazol improves leg symptoms, quality of life, and walking distance, as shown in multiple RCTs [33,34,35]. Cilostazol is contraindicated in patients with congestive heart failure since it is a PDE3 inhibitor, which is known to worsen mortality rates in this population [36]. Pentoxifylline is another medical therapy used historically to improve pain-free walking distance. While well tolerated, the literature investigating pentoxifylline in claudicants is highly variable, and the assessment of effectiveness is uncertain [37]. An RCT of 698 patients compared mean maximal walking distance in those treated with cilostazol, pentoxifylline, or placebo. At 24 weeks, improvement in maximal walking distance was significantly higher in the cilostazol group compared with the pentoxifylline and control group [38]. As a result, pentoxifylline is not currently recommended by the ACC and AHA for the treatment of claudication [9]. Similarly, prostaglandins, which have vasodilatory and antiplatelet formation effects, have not been shown to improve walking distance or quality of life in claudicants compared with cilostazol. There remains insufficient evidence investigating the impact of prostaglandins on intermittent claudication, and thus, remains unrecommended by claudication guidelines [39].
According to the ACA/AHA guidelines, healthy nutrition is vital for preventing the development of atherosclerotic cardiovascular disease and reducing MACEs. The recommended diet consists of vegetables, fruits, legumes, nuts, whole grains, and fish. This is supported by the PREDIMED (Prevención con Dieta Mediterránea) trial, which found that a Mediterranean diet supplemented with extra-virgin olive oil and mixed nuts had a reduced number of cardiovascular events compared with a standard reduced-fat diet [40].
2.2. Adherence Rates to OMT
Adherence rates to OMT are varied, but in general are low. A study of 39,088 claudicants with surgical intervention in the Vascular Quality Initiative (VQI) revealed that only 38.1% were on complete OMT, defined as antiplatelet, statin, and smoking cessation. Furthermore, 54.6% were on partial OMT, defined as meeting 1–2 components. Both complete and partial OMT decreased the odds of reintervention and MALEs compared with no OMT. For individual components, 76.3% were on an aspirin, 76.2% were on a statin, and 53.4% demonstrated smoking cessation [11]. Similarly, the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease) registry investigated adherence to guideline-recommended therapy in 1275 patients with PAD across 16 international centers from 2011 to 2015. They documented four measures: antiplatelets, statins, smoking cessation counseling/therapy, and SET referral. A total of 77.2% were adherent to two quality measures, while 19.7% were adherent to four quality measures [41]. Furthermore, only a third of smokers with PAD were ever offered smoking cessation counseling or pharmacologic treatment [42].
For cilostazol, in a VQI study of 245,309 patients undergoing peripheral vascular intervention for claudication or CLTI, only 6.6% of patients were on cilostazol prior to intervention. Patients on cilostazol ultimately had lower rates of long-term mortality and major amputation [43]. In terms of antihypertensive regiments, a UK-based cohort study found prescription rates of ACE inhibitors/ARBs to be above 40% in patients two months after a diagnosis of PAD [44]. For diabetes, a VQI study of diabetics with lower extremity bypass demonstrated that 25% had no documented A1C, 29% had a documented A1C of <7%, and 46% had an A1C of above 7% [45]. These results reveal that many patients are not medically optimized prior to undergoing surgical intervention.
There are qualitative studies attempting to understand patient factors in low medication adherence. Lampridou et al. explored patient themes regarding medication adherence. One theme was the lack of perceived symptom benefits with GDMT, as its dominant impact is its cardioprotective and limb-protective effects rather than symptom relief or functional improvement. Another theme was lack of clarity regarding the purpose of the numerous medications and the fear of polypharmacy. Certain patients also complained about the side effects of certain medications, such as muscle pain from statin therapy or bleeding from antiplatelets/anticoagulation. Lastly, adequate social support from family/friends and partnering with their healthcare provider appeared to be a key factor in maintaining medication adherence [46].
2.3. Current and Upcoming Interventions
Multiple qualitative improvement initiatives aiming to improve GDMT adherence have been published, with varying levels of success (Table 1). Haile et al. developed a nursing-led follow-up program to maintain adherence to lipid-lowering medications, antiplatelets, and anticoagulants in claudicants following intervention. The intervention involved three nursing visits and two telephone calls by a vascular nurse in the first year after revascularization. Their RCT revealed no difference in medication adherence between the intervention and control group. They noted their baseline adherence rates were high, making it difficult to achieve any significant improvements [47]. Another RCT assessed the ability of medication counseling via telephone calls to improve medication adherence among 1446 participants with abdominal aortic aneurysms, peripheral arterial disease, or hypertension. Their primary outcome was composite adherence to statin, antithrombotic, and antihypertensive treatment. While an increase in statin prescriptions was seen at 6 months, there was no change in adherence among the composite of three medications at 12 and 60 months of follow-up [48].
Mobile health interventions have been developed to modify patient behavior. A multicenter RCT of 822 patients investigated the impact of 6 text messages a week for 6 months regarding motivational content and education related to disease knowledge, risk factor control, and medication adherence for hypertension. Although patients found the messages useful, there was no change in systolic blood pressure, cholesterol level, or physical activity between the intervention and control groups [49]. Similarly, another RCT found that weekly text messages for 6 months regarding risk factor modification in patients with percutaneous coronary interventions increased the level of physical activity, fruit/vegetable consumption, and medication adherence. Despite this, no changes in blood pressure, cholesterol levels, or body-mass index were seen [50].
Virani et al. utilized a natural language processing algorithm to analyze clinical documentation and identify reasons for non-adherence to high-intensity statins in patients with cardiovascular disease. Medication side effects were the largest barrier. Thus, a clinical decision support tool was designed that alerted physicians before clinic visits whether a patient needed a statin, any existing side effects, and guideline resources [51]. Baseline adherence rates of high-intensity statins at the intervention clinics (n = 14) were 53.6%, and they increased by 10.1% post-intervention. In the control clinic (n = 13), the baseline rate was 55.9%, and this decreased by 0.18% post-intervention [52]. This study reveals the impact of developing interventions based on identified root issues.
Beyond electronic-based interventions, innovations in drug delivery may also impact adherence. Castellano et al. investigated the impact of a polypill containing ASA 100 mg, simvastatin 40 mg, and ramipril 2.5/5/10 mg on medication adherence for secondary prevention post-myocardial infarction, compared with the three drugs given separately. They performed an RCT with a five-country cohort of 2118 patients and found that the polypill group (50.8%) demonstrated improved adherence after 9 months from baseline (41.0%) [53].
Improving medication adherence remains a difficult challenge, as many interventions are unable to achieve sustained patient benefits. These likely reflect the difficulties in designing an effective intervention and changing patient behaviors. Ultimately, promising interventions will emphasize understanding the root causes of medication non-adherence and focus on improving the partnership between the provider and patient to achieve improved care within claudicants.

Table 1.
Interventions to improve adherence to optimal medical therapy.
Table 1.
Interventions to improve adherence to optimal medical therapy.
| Study Title and Year | Intervention | Control | Study Sample | Primary Outcome | Results |
|---|---|---|---|---|---|
| Effects of a person−centered, nurse−led follow−up programme on adherence to prescribed medication among patients surgically treated for intermittent claudication: randomized clinical trial (2022) [47] | Three visits and two telephone calls by a specially trained vascular nurse over one year after revascularization | Two visits after surgery to vascular surgeon or nurse | Two centers, 214 patients | Proportion of days covered (PDC) (number of available dispensed doses via registry data/number of days patient prescribed the medication) | No difference in PDC for lipid−modifying agents, antiplatelets, anticoagulants |
| Randomised trial of telephone counselling to improve participants' adherence to prescribed drugs in a vascular screening trial (2020) [48] | 5−15 min sessions of telephone counseling by nurse regarding refilling prescriptions, side effects, and health advice | No telephone counseling | 1446 patients with AAA, PAD, or hypertension | Proportion of days covered (PDC) by statin, antithrombotic, and antihypertensive agents at 6−month follow-up | Increase in PDC for statins at 6 months. No other differences noted at 6, 12, and 60 months. |
| Effect of Text Messaging on Risk Factor Management in Patients With Coronary Heart Disease: The CHAT Randomized Clinical Trial (2019) [49] | Six texts/week of education information related to disease knowledge, physical activity, and medication adherence | Two thank you text messages a month | 37 hospitals, 822 patients with coronary heart disease | Systolic blood pressure at six months | No differences in blood pressure at six months. No differences in LDL, physical activity, or smoking activity. |
| Cluster Randomized Trial of a Personalized Clinical Decision Support Intervention to Improve Statin Prescribing in Patients With Atherosclerotic Cardiovascular Disease (2023) [52] | Clinical decision support tool to alerts physicians before clinic visits about current statin and dose, date of last fill, type of side effect | Usual clinician access to patient dashboard detailing compliance with statin | 27 primary care clinics, 36,641 patients with cardiovascular disease | Statin adherence (defined as proportion of days covered for statin > 80%) | Absolute change in statin adherence was +10.1% in intervention group vs −0.18% decrease in control group |
| A polypill strategy to improve adherence: results from the FOCUS project (2014) [53] | Polypill containing aspirin 100 mg, simvastatin 40 mg, and ramipril 2.5/5/10 mg | Three drugs given separately | 2118 patients | Medication adherence via Morisky−Green Questionnaire (MAQ) and pill count after nine months | Adherence was 50.8% in intervention group versus 41% (p = 0.02). No difference in blood pressure or cholesterol levels. |
Alongside OMT, supervised exercise therapy (SET) is recommended as a first-line intervention in claudicants prior to surgical revascularization. SET involves walking on a treadmill at a speed that induces moderate claudication pain within several minutes, guided by a physical therapist. The patient stops and rests until the pain improves, upon which they will resume walking. This cycle is repeated for at least 30 min to 1 hour and should be performed at least three times a week. The Centers for Medicare and Medicaid Services currently covers up to 36 separate 30–60 min sessions over a 12-week treatment period [54].
3. Supervised Exercise Therapy
3.1. Mechanisms and Benefits of Supervised Exercise Therapy
There are several mechanisms by which SET provides patient benefit. SET causes exercise-induced angiogenesis, creating collateral blood supply around atherosclerotic occlusions. SET also causes nitric oxide-dependent vasodilation of the arterial microcirculation, improving perfusion. Studies have also shown that the metabolism of glucose and fatty acids by skeletal myocytes is improved during SET. These mechanisms all improve functional outcomes within claudicants.
Multiple RCTs showcase the durable benefits of SET. In the CLEVER (Claudication: Exercise Versus Endoluminal Revascularization) study by Murphy et al., SET and OMT showed comparable improvements in claudication-onset time and peak walking time to surgical revascularization at 18 months post-intervention [55]. Fakhry et al. performed an RCT of 212 claudicants assessing the effectiveness of endovascular revascularization with SET versus SET alone. At the one-year follow-up, combination therapy demonstrated greater improvements in maximum walking distance, pain-free walking distance, VascuQol scores, and the SF-36 physical functioning scores [56].
3.2. Adherence to Supervised Exercise Therapy
Despite the benefits of SET, its utilization rate in real-world practice is poor. Published rates of SET completion range anywhere between 5% and 55% [57]. A systematic review of 23 RCTs for SET found that of 7517 screened total patients, only 24.2% were recruited. Of those recruited, only 75% adequately completed the program. The top reasons for screen failure included lack of interest in SET, comorbidities affecting the ability to exercise, and the inability to attend SET due to distance/timing. The top reasons for incomplete adherence were a lack of motivation, comorbidities, lack of results, and patient choice [58]. Likewise, in a questionnaire of 516 patients with PAD, 85.1% revealed their physician never prescribed or recommended SET, despite 73.2% willing to travel several times a week to participate. However, only 41% were willing to pay the $11 per session copay. Patients unwilling to travel to SET felt it was too time-consuming, too inconvenient, and overall lacked interest [59]. Similarly, in a survey of 135 vascular surgeons, vascular medicine physicians, and cardiologists, 54% had no SET program at their facility, 49% had never referred a patient to SET, and 26% were unaware that SET sessions were covered by CMS. These are daunting statistics showcasing a lack of fundamental knowledge of SET even among vascular specialists [12]. Despite being covered by Medicaid, SET adherence remains greatly impacted by the lack of awareness around SET, interference with daily life due to session frequency/timing, and lack of motivation to attend dozens of sessions.
3.3. Interventions to Enhance and Facilitate SET
Recent initiatives have attempted to address exercise therapy adherence (Table 2). One strategy is performing exercise therapy at home through structured, community-based walking programs. In these programs, patients follow a guided program with the aid of a virtual coach/physical therapist. The Group Oriented Arterial Leg Study (GOALS) compared outcomes of a home-based group-mediated cognitive behavioral walking intervention versus a control group exposed to general health education. The experimental group received six months of PAD- and SET-specific weekly group education from a trained facilitator as well as a paper form to track weekly walking goals and distance. Compared with the control, patients within the intervention group reported significant increases in six-minute walking distance, maximum treadmill walking distance, and walking impairment questionnaire distance and speed score [60]. The LITE RCT investigated the effectiveness of various intensities of a home-based walking program. They compared a low-intensity program where ischemic symptoms were not induced to a high-intensity group that walked at a pace to induce moderate to severe ischemic leg symptoms. The high-intensity walking group demonstrated a higher six-minute walk distance at 12 months [61]. These studies indicate the effectiveness of allowing patients to walk at their convenience without the need to travel. However, a facilitator is required to provide motivation and ensure patients are walking at a sufficient intensity.

Table 2.
Novel interventions to enhance supervised exercise therapy adherence.
Mobile health interventions have been an increasingly popular method to deliver and facilitate walking programs. Normahani et al. conducted an RCT investigating the impact of a wearable activity monitor (WAM) to assist the delivery of a home-based walking program. The intervention group received 3 months of SET followed by 9 months of home walking program monitored by a WAM; the control group received only SET. At one year, there were higher improvements in mean walking distance and VascuQoL score in the intervention group compared with the control [62]. Paldan et al. developed a smartphone app, TrackPAD, to record training duration/frequency, step count, and pain levels during SET. In addition, the app sets weekly goals and “gamifies” the experience through achievement medals and leaderboards to compete with other users. In their RCT, the intervention group increased their six-minute walking distance at three months, with the control group demonstrating no improvement [63]. Mobile health programs are an effective way to deliver personalized coaching and track walking progress, especially with the ubiquity of smartphones and wearable devices.
Financial incentives can also be a creative way to tackle SET adherence. Cleary et al. utilized a three-pronged method involving financial incentives up to $180, individualized coaching, and informational materials to improve their SET adherence rate from 54% to 76.7% over a two-year period. This study revealed that the hidden associated costs of SET (copays, transportation costs, unpaid leave from work) are underrecognized barriers to adherence [64].
In summary, SET adherence remains a difficult challenge. Successful interventions include health coaching and physician partnership to provide continuous education and maintain accountability. SET access is also a major issue; SET facilities need to be expanded, and access to quality home walking programs need to be provided to patients. Ultimately, the future of exercise therapy may involve home-based programs delivered through mobile apps and financial incentives to offset the hidden costs of SET.
An important metric when providing guideline-directed care for claudicants is the assessment of the quality of life and the degree of lifestyle limitation. Given that claudication is primarily a lifestyle disease, there has been increasing interest in utilizing patient-reported outcome measures (PROMs) to assess the functional outcomes of surgical intervention. Despite a host of validated surveys and recommendations from the AHA/ACC guidelines recommending periodic assessment of health-related quality of life, PROMs remain underutilized.
4. Patient-Reported Outcome Measures
4.1. Existing PROMs for Claudication
According to the FDA, PROMs are tools that serve as a report of the status of a patient’s health condition, health behavior, or experience with healthcare that comes directly from the patient, without interpretation by a clinician or anyone else [65]. PROMs are frequently utilized in comparative effectiveness research and to monitor disease states. PROMs feature questions that represent a “domain” of assessment, such as pain, psychological impact, impact on social activities, and impact on daily living. These domains will be scored to present an overall assessment of the patient’s quality of life (QoL). PROMs are developed through a conceptual framework inspired by the existing literature and focus groups/patient interviews. Once a draft instrument is developed, PROMs are repeatedly tested among hundreds of patients to ensure survey validity, reliability, and responsiveness. PROMs also undergo translation and cultural adaptation so they can be utilized among patients from diverse socioeconomic and health backgrounds [66].
Most randomized clinical trials regarding peripheral arterial disease utilize one generic and disease-specific PROM to assess QoL and surgical outcomes. The most commonly used generic PROMs include the 36-Item Short Form Survey (SF-36) and the European Quality of Life 5-Dimension (EQ-5D). The SF-36, developed in 1992, is a survey of 36 questions assessing 8 health domains: (1) limitations in physical activities, (2) limitations in social activities, (3) limitations in usual role activities, (4) bodily pain, (5) general mental health, (6) limitations in usual role activities due to emotional problems, (7) vitality, and (8) general health perceptions [67]. A score from 0 to 100 is counted, with 100 being the highest possible score. Studies have found that patients with claudication report ultimately lower scores in the physical functioning/limitation components of the SF-36, as well as bodily pain, when compared with healthy controls [68]. The EQ-5D is a generic PROM developed by the EuroQol Group, aiming to measure five dimensions of health states: mobility, usual activities, self-care, pain and discomfort, and anxiety and depression. Compared with the SF-36, the EQ-5D is shorter and can generate utility scores for use in cost analysis research [69].
Both the SF-36 and EQ-5D have been widely utilized to examine quality of life outcomes in several major clinical trials for PAD. For example, the ERASE clinical trial compared the effectiveness of endovascular surgery with SET versus SET alone in 212 patients. They found that the combination therapy demonstrated a higher SF-36 score in the physical functioning domain but not in the physical role functioning, bodily pain, and general health perception [70]. The Disrupt PAD III RCT compared the use of intravascular lithotripsy versus balloon angioplasty in severely calcified femoropopliteal arteries prior to stenting or drug-coated balloon treatment. EQ-5D values were a secondary outcome in this study, and results showed no differences in the increase from baseline at one year, indicating a similar quality of life improvement between the two interventions [71].
One of the most popular PROMs designed specifically for PAD is the Vascular Quality of Life Questionnaire (VascuQoL). The VascuQol is a 25-question survey assessing 5 quality-of-life domains: pain, symptoms, activities, social impact, and emotional impact. The survey is designed to take 9 min to answer, and its shorter version, the VascuQoL-6, is a six-question, shortened version designed to take 1.4 min. The VascuQoL is a popular PROM for PAD research. Compared with the SF-37 and EQ-5D, the VascuQoL-6 has been shown to have a higher receiver operating characteristic area under the curve when detecting improvements in Rutherford classification at six-month follow-up in a prospective study of 450 patients with PAD [72]. Compared with the VascuQoL, the Walking Impairment Questionnaire (WIQ) is a more specific measurement of functional status and directly assesses the walking ability of patients. The WIQ is a 22-question survey designed to measure self-reported walking distance, walking speed, and stair-climbing limitation (Figure 2). The WIQ has been validated in patients with claudication and was shown to be highly correlated with the six-minute walk and measured walking velocity in both men and women [73]. In fact, the WIQ has even been shown to predict mortality, as a study by Jain et al. revealed that patients with PAD in the lowest quartile of WIQ stair-climbing scores had higher all-cause and cardiovascular mortality than those in the highest quartile [74].

Figure 2.
Walking distance and stair climbing component of the Walking Impairment Questionnaire [73].
4.2. Barriers to PROM Implementation
The validation and utility of PROMs for assessing outcomes research for PAD and claudication is evident. However, its adoption in real-world clinical practice is low, given that these outcome measures have been used primarily in research settings. There is an ongoing effort by the Society of Vascular Surgery to improve the implementation of PROMs in the community.
In 2021, the SVS distributed a survey to 106 SVS members serving on the Policy and Advocacy Council, with 78 respondents ultimately. They found that while 80.8% were aware of PROMs, and 100% felt they would be useful in assessing vascular surgery patients, only 23.1% indicated that their practice or institution used PROMs. Of those utilizing PROMs, the most commonly cited reasons for using them were research/quality improvement initiatives followed by institutional requirements and quality reporting. Furthermore, 70% of respondents indicated they would consider using PROMs if incorporated into electronic medical records. Reasons for not collecting PROMs included existing PROMs not being specific to the patient’s problem, difficulty obtaining results, or inability to analyze the collected data [66].
This survey was followed by a one-hour focus group among respondents. One major theme around PROMs was knowledge gaps. Specifically, there was lack of awareness of all the various types of PROM and how PROMs are developed and validated. Many clinicians were unsure of how to utilize PROMs in their practice and how they should guide patient care. It was also unclear which ones to use, as surgical societies do not currently recommend a specific PROM. Another theme was the actual implementation barriers of PROMs. RCTs have the dedicated staff and resources to collect PROM data and input them into database registries, which are not typically performed in daily clinical practice. While electronic methods could be a good alternative, it was felt that the vascular surgery population, which is primarily elderly, may be limited in their ability to respond. Another concern was how PROM results would be interpreted by payers and policy makers and how this may ultimately affect reimbursement [66].
Beyond physician perception, patients themselves face burdens when filling out PROMs. Holeman et al. performed a study investigating patient perceptions of PROMs through interviewing 23 patients with PAD. In terms of PROM timing, patients prefer to receive them prior to the appointment through email or text. Some patients also felt the PROM questions were not specific enough to their direct symptoms or overall health. Despite this, patients felt that PROMs improved communication with their providers and felt they helped track progress over time. Lastly, only 65% of the patients felt that the PROMs were valuable. Those that disagreed with its value felt that the questions were repetitive and focused on previously discussed information with the provider. Others questioned their utility to the surgeon [75].
4.3. Keys to PROM Adoption
Efforts to improve the adoption rate of PROMs need to accelerate, especially as Medicare reimbursement for certain elective surgical procedures will begin to require collection of PROMs as early as 2026 [76]. Improving the adoption of PROMs will require consideration of patient and clinician factors.
First, there should be a standardized, singular PROM that caters to the claudication patient’s symptoms and concern. In a systematic review of 31 studies that recorded PROM usage for claudicants, there were 14 different questionnaires. The most common were the SF-36, EQ-5D, VascuQoL, and WIQ [77]. While these individually assess different aspects of claudication, it is challenging to implement all these PROMs clinically. Surgical societies should provide guidance on which PROMs should be used to help standardize the process of recording QoL metrics.
Second, the designs of the PROMs need to be intuitive and cannot create significant patient and clinician burden to fill out. Several existing PROMs take over 10 min to fill out [66]. Studies have shown that PROM response rates tend to be higher when questionnaires are shorter [78]. Thus, there needs to be a balance between PROM comprehensiveness and efficiency. Furthermore, in the setting of shortened appointment times, PROMs should be delivered to patients prior to appointments via email/smartphone at home. If performed at the clinic, patients should be provided with sufficient time to complete the PROMs prior to seeing the physician as well as with devices that may facilitate the ease of data collection, such as iPads. Qualitative studies have found that electronic PROMs can minimize responder burden and improve compliance [58,79]. Electronic PROMs can also feature additional functionalities to facilitate completion. For example, using the concept of computerized adaptive testing, PROMs can become dynamic, where certain questions only appear depending on the answer to the previous one, preventing the patient from answering irrelevant questions [80].
Third, there needs to be increasing clarity in when and how PROMs for claudicants should be used, for both the patient and physician. Patient education should be provided regarding what PROMs are used for and how certain scores may correlate with certain surgical outcomes. Furthermore, giving patients and physicians the ability to track their PROMs over time through numerical and visual graphics can increase engagement and response compliance [81,82]. This allows both parties to understand the impact of the existing disease and the impact of medical/surgical interventions on overall QoL. On the physician end, there should be further education on the minimal clinically meaningful difference (MCID) for each PROM to help them understand the minimum change in scores that patients would consider beneficial. MCIDs are different for each PROM, and furthermore, vary depending on the patient’s baseline demographics and disease severity [83]. Without known MCIDs, physicians cannot correlate PROMs with patient outcomes, minimizing their usefulness.
5. Conclusions
Despite their obvious benefits for claudicants, adherence to OMT, SET, and QoL assessment remains an extremely difficult challenge. While numerous RCTs and qualitative/quantitative studies have sought to address these issues, many are unsuccessful due to patient behaviors or perceptions not being directly addressed. Successful interventions will involve a root-cause analysis of the existing patient challenges to adherence and recurring patient education and health coaching. Future research should continue to emphasize exciting and novel approaches to OMT, SET, and QoL assessment adherence.
Author Contributions
Conceptualization, A.T.; investigation, R.S.; writing—original draft preparation, R.S., N.B. and A.T.; writing—review and editing, R.S. and A.T.; supervision, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Meru, A.V.; Mittra, S.; Thyagarajan, B.; Chugh, A. Intermittent claudication: An overview. Atherosclerosis 2006, 187, 221–237. [Google Scholar] [CrossRef]
- Conte, M.S.; Pomposelli, F.B.; Clair, D.G.; Geraghty, P.J.; McKinsey, J.F.; Mills, J.L.; Moneta, G.L.; Murad, M.H.; Powell, R.J.; Reed, A.B.; et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. J. Vasc. Surg. 2015, 61 (Suppl. S3), 2S–41S. [Google Scholar] [CrossRef]
- Sachs, T.; Pomposelli, F.; Hamdan, A.; Wyers, M.; Schermerhorn, M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: Angioplasty vs bypass graft. J. Vasc. Surg. 2011, 54, 1021–1031.e1. [Google Scholar] [CrossRef] [PubMed]
- Thanigaimani, S.; Phie, J.; Sharma, C.; Wong, S.; Ibrahim, M.; Huynh, P.; Moxon, J.; Jones, R.; Golledge, J. Network Meta-Analysis Comparing the Outcomes of Treatments for Intermittent Claudication Tested in Randomized Controlled Trials. J. Am. Heart Assoc. 2021, 10, e019672. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Banerjee, S.; Ngo, C.; Mody, P.; Marso, S.P.; Brilakis, E.S.; Armstrong, E.J.; Giri, J.; Bonaca, M.P.; Pradhan, A.; et al. Comparative Efficacy of Endovascular Revascularization Versus Supervised Exercise Training in Patients with Intermittent Claudication: Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2017, 10, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, V.; Davenport, D.; Jones, S.; Khoudoud, S.A.; Orr, N.; Minion, D.; Endean, E.; Tyagi, S. Revascularization of intermittent claudicants leads to more chronic limb-threatening ischemia and higher amputation rates. J. Vasc. Surg. 2021, 74, 771–779. [Google Scholar] [CrossRef]
- Woo, K.; Siracuse, J.J.; Klingbeil, K.; Kraiss, L.W.; Osborne, N.H.; Singh, N.; Tan, T.-W.; Arya, S.; Banerjee, S.; Bonaca, M.P.; et al. Society for Vascular Surgery appropriate use criteria for management of intermittent claudication. J. Vasc. Surg. 2022, 76, 3–22.e1. [Google Scholar] [CrossRef]
- Gornik, H.L.; Aronow, H.D.; Goodney, P.P.; Arya, S.; Brewster, L.P.; Byrd, L.; Chandra, V.; Drachman, D.E.; Eaves, J.M.; Ehrman, J.K.; et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS Guideline for the Management of Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2024, 83, 2497–2604. [Google Scholar] [CrossRef]
- Bath, J.; Lawrence, P.F.; Neal, D.; Zhao, Y.; Smith, J.B.; Beck, A.W.; Conte, M.; Schermerhorn, M.; Woo, K. Endovascular interventions for claudication do not meet minimum standards for the Society for Vascular Surgery efficacy guidelines. J. Vasc. Surg. 2021, 73, 1693–1700.e3. [Google Scholar] [CrossRef]
- Jarosinski, M.C.; Hafeez, M.S.; Sridharan, N.D.; Andraska, E.A.; Meyer, J.M.; Khamzina, Y.; Tzeng, E.; Reitz, K.M. Markers of optimal medical therapy are associated with improved limb outcomes after elective revascularization for intermittent claudication. J. Vasc. Surg. 2025, 81, 200–209.e3. [Google Scholar] [CrossRef]
- Dua, A.; Gologorsky, R.; Savage, D.; Rens, N.; Gandhi, N.; Brooke, B.; Corriere, M.; Jackson, E.; Aalami, O. National assessment of availability, awareness, and utilization of supervised exercise therapy for peripheral artery disease patients with intermittent claudication. J. Vasc. Surg. 2020, 71, 1702–1707. [Google Scholar] [CrossRef]
- Holeman, T.A.; Chester, C.; Hales, J.B.; Zhang, Y.; Johnson, C.E.; Brooke, B.S. Long-term patient-reported outcomes among patients undergoing revascularization vs medical therapy for intermittent claudication. J. Vasc. Surg. 2024, 80, 466–477.e4. [Google Scholar] [CrossRef]
- Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Bmj 2002, 324, 71–86. [CrossRef]
- Berger, J.S.; Krantz, M.J.; Kittelson, J.M.; Hiatt, W.R. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: A meta-analysis of randomized trials. JAMA 2009, 301, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.H.; Keltai, K.; Bhatt, D.L.; et al. Major Adverse Limb Events and Mortality in Patients with Peripheral Artery Disease: The COMPASS Trial. J. Am. Coll. Cardiol. 2018, 71, 2306–2315. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef]
- Anand, S.S.; Bosch, J.; Eikelboom, J.W.; Connolly, S.J.; Diaz, R.; Widimsky, P.; Aboyans, V.; Alings, M.; Kakkar, A.K.; Keltai, K.; et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 219–229. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Bonaca, M.P.; Patel, M.R.; Nehler, M.R.; Debus, E.S.; Anand, S.S.; Capell, W.H.; Brackin, T.; Jaeger, N.; Hess, C.N.; et al. Rivaroxaban and Aspirin in Peripheral Artery Disease Lower Extremity Revascularization: Impact of Concomitant Clopidogrel on Efficacy and Safety. Circulation 2020, 142, 2219–2230. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef]
- Jukema, J.W.; Szarek, M.; Zijlstra, L.E.; de Silva, H.A.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; et al. Alirocumab in Patients with Polyvascular Disease and Recent Acute Coronary Syndrome: ODYSSEY OUTCOMES Trial. J. Am. Coll. Cardiol. 2019, 74, 1167–1176. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Gutierrez, J.A.; Cannon, C.; Giugliano, R.; Blazing, M.; Park, J.G.; White, J.; Tershakovec, A.; Braunwald, E. Polyvascular disease, type 2 diabetes, and long-term vascular risk: A secondary analysis of the IMPROVE-IT trial. Lancet Diabetes Endocrinol. 2018, 6, 934–943. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Wu, J.; Singh, G.D.; Dawson, D.L.; Pevec, W.C.; Amsterdam, E.A.; Laird, J.R. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2014, 60, 1565–1571. [Google Scholar] [CrossRef]
- Selvarajah, S.; Black, J.H.; Malas, M.B.; Lum, Y.W.; Propper, B.W.; Abularrage, C.J. Preoperative smoking is associated with early graft failure after infrainguinal bypass surgery. J. Vasc. Surg. 2014, 59, 1308–1314. [Google Scholar] [CrossRef]
- Barua, R.S.; Rigotti, N.A.; Benowitz, N.L.; Cummings, K.M.; Jazayeri, M.A.; Morris, P.B.; Ratchford, E.V.; Sarna, L.; Stecker, E.C.; Wiggins, B.S. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2018, 72, 3332–3365. [Google Scholar] [CrossRef] [PubMed]
- Piller, L.B.; Simpson, L.M.; Baraniuk, S.; Habib, G.B.; Rahman, M.; Basile, J.N.; Dart, R.A.; Ellsworth, A.J.; Fendley, H.; Probstfield, J.L.; et al. Characteristics and long-term follow-up of participants with peripheral arterial disease during ALLHAT. J. Gen. Intern. Med. 2014, 29, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Diehm, C.; Pittrow, D.; Lawall, H. Effect of nebivolol vs. hydrochlorothiazide on the walking capacity in hypertensive patients with intermittent claudication. J. Hypertens. 2011, 29, 1448–1456. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Chen, D.C.; Singh, G.D.; Amsterdam, E.A.; Laird, J.R. Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use is associated with reduced major adverse cardiovascular events among patients with critical limb ischemia. Vasc. Med. 2015, 20, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Resnick, H.E.; Carter, E.A.; Sosenko, J.M.; Henly, S.J.; Fabsitz, R.R.; Ness, F.K.; Welty, T.K.; Lee, E.T. Incidence of lower-extremity amputation in American Indians: The Strong Heart Study. Diabetes Care 2004, 27, 1885–1891. [Google Scholar] [CrossRef]
- Bedenis, R.; Stewart, M.; Cleanthis, M.; Robless, P.; Mikhailidis, D.P.; Stansby, G. Cilostazol for intermittent claudication. Cochrane Database Syst. Rev. 2014, 2014, CD003748. [Google Scholar] [CrossRef]
- Mohammed, M.; Gosch, K.; Safley, D.; Jelani, Q.U.; Aronow, H.D.; Mena, C.; Shishehbor, M.H.; Spertus, J.A.; Abbott, J.D.; Smolderen, K.G. Cilostazol and peripheral artery disease-specific health status in ambulatory patients with symptomatic PAD. Int. J. Cardiol. 2020, 316, 222–228. [Google Scholar] [CrossRef]
- Ma, B.; Fan, X.; Liu, P. Therapeutic Effects of Medication Use on Intermittent Claudication: A Network Meta-analysis. J. Cardiovasc. Pharmacol. 2021, 77, 253–262. [Google Scholar] [CrossRef]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; DiBianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L.; et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef]
- Broderick, C.; Forster, R.; Abdel-Hadi, M.; Salhiyyah, K. Pentoxifylline for intermittent claudication. Cochrane Database Syst. Rev. 2020, 10, CD005262. [Google Scholar] [CrossRef]
- Dawson, D.L.; Cutler, B.S.; Hiatt, W.R.; Hobson, R.W.; Martin, J.D.; Bortey, E.B.; Forbes, W.P.; Strandness, D.E., Jr. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am. J. Med. 2000, 109, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Nordanstig, J.; Behrendt, C.A.; Baumgartner, I.; Belch, J.; Bäck, M.; Fitridge, R.; Hinchliffe, R.; Lejay, A.; Mills, J.L.; Rother, U.; et al. Editor’s Choice -- European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 9–96. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Saxon, J.T.; Safley, D.M.; Mena-Hurtado, C.; Heyligers, J.; Fitridge, R.; Shishehbor, M.; Spertus, J.A.; Gosch, K.; Patel, M.R.; Smolderen, K.G. Adherence to Guideline-Recommended Therapy-Including Supervised Exercise Therapy Referral-Across Peripheral Artery Disease Specialty Clinics: Insights from the International PORTRAIT Registry. J. Am. Heart Assoc. 2020, 9, e012541. [Google Scholar] [CrossRef]
- Kalbaugh, C.A.; Gonzalez, N.J.; Luckett, D.J.; Fine, J.; Brothers, T.E.; Farber, M.A.; Beck, A.W.; Hallett, J.W.; Marston, W.A.; Vallabhaneni, R. The impact of current smoking on outcomes after infrainguinal bypass for claudication. J. Vasc. Surg. 2018, 68, 495–502.e1. [Google Scholar] [CrossRef]
- Alameddine, D.; Damara, F.A.; Pinto Rodriguez, P.; Huttler, J.; Slade, M.D.; Arhuidese, I.; Aboian, E.; Chaar, C.I.O. The Use and Impact of Cilostazol on Patients Undergoing Endovascular Peripheral Interventions. Ann. Vasc. Surg. 2024, 103, 47–57. [Google Scholar] [CrossRef]
- Cea-Soriano, L.; Fowkes, F.G.R.; Johansson, S.; Allum, A.M.; García Rodriguez, L.A. Time trends in peripheral artery disease incidence, prevalence and secondary preventive therapy: A cohort study in The Health Improvement Network in the UK. BMJ Open 2018, 8, e018184. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.Y.; Crawford, A.S.; Nguyen, T.; Judelson, D.; Learned, A.; Chan, J.; Schanzer, A.; Simons, J.P.; Jones, D.W. Hemoglobin A1c monitoring practices before lower extremity bypass in patients with diabetes vary broadly and do not predict outcomes. J. Vasc. Surg. 2022, 76, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Lampridou, S.; Saghdaoui, L.B.; Reguenga, M.; Davies, A.H.; Wells, M. Understanding adherence to guideline-recommended therapy in patients with peripheral artery disease: A qualitative study. J. Vasc. Nurs. 2025, 43, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.T.; Joelsson-Alm, E.; Johansson, U.B.; Lööf, H.; Palmer-Kazen, U.; Gillgren, P.; Linné, A. Effects of a person-centred, nurse-led follow-up programme on adherence to prescribed medication among patients surgically treated for intermittent claudication: Randomized clinical trial. Br. J. Surg. 2022, 109, 846–856. [Google Scholar] [CrossRef]
- Qvist, I.; Lindholt, J.S.; Søgaard, R.; Lorentzen, V.; Hallas, J.; Frost, L. Randomised trial of telephone counselling to improve participants’ adherence to prescribed drugs in a vascular screening trial. Basic. Clin. Pharmacol. Toxicol. 2020, 127, 477–487. [Google Scholar] [CrossRef]
- Zheng, X.; Spatz, E.S.; Bai, X.; Huo, X.; Ding, Q.; Horak, P.; Wu, X.; Guan, W.; Chow, C.K.; Yan, X. Effect of Text Messaging on Risk Factor Management in Patients with Coronary Heart Disease: The CHAT Randomized Clinical Trial. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005616. [Google Scholar] [CrossRef]
- Bae, J.W.; Woo, S.I.; Lee, J.; Park, S.D.; Kwon, S.W.; Choi, S.H.; Yoon, G.-S.; Kim, M.-S.; Hwang, S.-S.; Lee, W.K. mHealth Interventions for Lifestyle and Risk Factor Modification in Coronary Heart Disease: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e29928. [Google Scholar] [CrossRef]
- Gobbel, G.T.; Matheny, M.E.; Reeves, R.R.; Akeroyd, J.M.; Turchin, A.; Ballantyne, C.M.; Petersen, L.A.; Virani, S.S. Leveraging structured and unstructured electronic health record data to detect reasons for suboptimal statin therapy use in patients with atherosclerotic cardiovascular disease. Am. J. Prev. Cardiol. 2022, 9, 100300. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Ramsey, D.J.; Westerman, D.; Kuebeler, M.K.; Chen, L.; Akeroyd, J.M.; Gobbel, G.T.; Ballantyne, C.M.; Petersen, L.A.; Turchin, A.; et al. Cluster Randomized Trial of a Personalized Clinical Decision Support Intervention to Improve Statin Prescribing in Patients with Atherosclerotic Cardiovascular Disease. Circulation 2023, 147, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Sanz, G.; Peñalvo, J.L.; Bansilal, S.; Fernández-Ortiz, A.; Alvarez, L.; Guzmán, L.; Linares, J.C.; García, F.; D'Aniello, F.; et al. A polypill strategy to improve adherence: Results from the FOCUS project. J. Am. Coll. Cardiol. 2014, 64, 2071–2082. [Google Scholar] [CrossRef]
- Treat-Jacobson, D.; McDermott, M.M.; Beckman, J.A.; Burt, M.A.; Creager, M.A.; Ehrman, J.K.; Gardner, A.W.; Mays, R.J.; Regensteiner, J.G.; Salisbury, D.L.; et al. Implementation of Supervised Exercise Therapy for Patients with Symptomatic Peripheral Artery Disease: A Science Advisory from the American Heart Association. Circulation 2019, 140, e700–e710. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.P.; Cerezo, J.; Cutlip, D.E.; Reynolds, M.R.; Regensteiner, J.G.; Mohler, E.R.; Cohen, D.J.; Massaro, J.M.; Lewis, B.A.; Oldenburg, N.C.; et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the claudication: Exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012, 125, 130–139. [Google Scholar] [CrossRef]
- Fakhry, F.; Fokkenrood, H.J.; Spronk, S.; Teijink, J.A.; Rouwet, E.V.; Hunink, M.G.M. Endovascular revascularisation versus conservative management for intermittent claudication. Cochrane Database Syst Rev 2018, 3, CD010512. [Google Scholar] [CrossRef]
- Ehrman, J.K.; Gardner, A.W.; Salisbury, D.; Lui, K.; Treat-Jacobson, D. Supervised Exercise Therapy for Symptomatic Peripheral Artery Disease: A review of current experience and practice-based recommendations. J. Cardiopulm. Rehabil. Prev. 2023, 43, 15–21. [Google Scholar] [CrossRef]
- Harwood, A.E.; Smith, G.E.; Cayton, T.; Broadbent, E.; Chetter, I.C. A Systematic Review of the Uptake and Adherence Rates to Supervised Exercise Programs in Patients with Intermittent Claudication. Ann. Vasc. Surg. 2016, 34, 280–289. [Google Scholar] [CrossRef]
- Cetlin, M.D.; Polonsky, T.; Ho, K.; Zhang, D.; Tian, L.; Zhao, L.; Greenland, P.; Treat-Jacobson, D.; Kibbe, M.R.; Criqui, M.H.; et al. Barriers to participation in supervised exercise therapy reported by people with peripheral artery disease. J. Vasc. Surg. 2023, 77, 506–514. [Google Scholar] [CrossRef]
- McDermott, M.M.; Domanchuk, K.; Liu, K.; Guralnik, J.M.; Tian, L.; Criqui, M.H.; Ferrucci, L.; Kibbe, M.; Jones, D.-L.; Pearce, W.H.; et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral arterial disease. Contemp. Clin. Trials 2012, 33, 1311–1320. [Google Scholar] [CrossRef]
- McDermott, M.M.; Spring, B.; Tian, L.; Treat-Jacobson, D.; Ferrucci, L.; Lloyd-Jones, D.; Zhao, L.; Polonsky, T.; Kibbe, M.R.; Bazzano, L.; et al. Effect of Low-Intensity vs High-Intensity Home-Based Walking Exercise on Walk Distance in Patients with Peripheral Artery Disease: The LITE Randomized Clinical Trial. JAMA 2021, 325, 1266–1276. [Google Scholar] [CrossRef]
- Normahani, P.; Kwasnicki, R.; Bicknell, C.; Allen, L.; Jenkins, M.P.; Gibbs, R.; Cheshire, N.; Darzi, A.; Riga, C. Wearable Sensor Technology Efficacy in Peripheral Vascular Disease (wSTEP): A Randomized Controlled Trial. Ann. Surg. 2018, 268, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Paldán, K.; Steinmetz, M.; Simanovski, J.; Rammos, C.; Ullrich, G.; Jánosi, R.A.; Moebus, S.; Rassaf, T.; Lortz, J. Supervised Exercise Therapy Using Mobile Health Technology in Patients with Peripheral Arterial Disease: Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e24214. [Google Scholar] [CrossRef] [PubMed]
- Cleary, C.M.; Adajian, A.; Gifford, E.D.; Orosco, E.; Li, Y.H.; Healy, L.; Dawiczyk, S.; Bozeman, P.; Guerin, E.; Farrell, H.; et al. Incentives and individualized coaching improve completion rates of supervised exercise therapy for claudication. J. Vasc. Surg. 2024, 80, 821–830.e3. [Google Scholar] [CrossRef]
- US Food & Drug Administration. Guidance for Industry on Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. In Food & Drug Administration; US Food & Drug Administration: Silver Spring, MD, USA, 2009. [Google Scholar]
- Hicks, C.W.; Vavra, A.K.; Goldsborough, E.; Rebuffatti, M.; Almeida, J.; Duwayri, Y.M.; Haurani, M.; Ross, C.B.; Shah, S.K.; Shireman, P.K.; et al. Current status of patient-reported outcome measures in vascular surgery. J. Vasc. Surg. 2021, 74, 1693–1706.e1. [Google Scholar] [CrossRef]
- Ware, J.E.; Jr Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Myers, S.A.; Johanning, J.M.; Stergiou, N.; Lynch, T.G.; Longo, G.M.; Pipinos, I.I. Claudication distances and the Walking Impairment Questionnaire best describe the ambulatory limitations in patients with symptomatic peripheral arterial disease. J. Vasc. Surg. 2008, 47, 550–555. [Google Scholar] [CrossRef][Green Version]
- Dyer, M.T.; Goldsmith, K.A.; Sharples, L.S.; Buxton, M.J. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual. Life Outcomes 2010, 8, 13. [Google Scholar] [CrossRef]
- Fakhry, F.; Spronk, S.; van der Laan, L.; Wever, J.J.; Teijink, J.A.; Hoffmann, W.H.; Smits, T.M.; van Brussel, J.P.; Stultiens, G.N.; Derom, A.; et al. Endovascular Revascularization and Supervised Exercise for Peripheral Artery Disease and Intermittent Claudication: A Randomized Clinical Trial. JAMA 2015, 314, 1936–1944. [Google Scholar] [CrossRef]
- Tepe, G.; Brodmann, M.; Bachinsky, W.; Holden, A.; Zeller, T.; Mangalmurti, S.; Nolte-Ernsting, C.; Virmani, R.; Parikh, S.A.; Gray, W.A. Intravascular Lithotripsy for Peripheral Artery Calcification: Mid-term Outcomes from the Randomized Disrupt PAD III Trial. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100341. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Ouwendijk, R.; Kessels, A.G.; de Haan, M.W.; Flobbe, K.; Hunink, M.G.; van Engelshoven, J.M.; Nelemans, P.J. Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J. Vasc. Surg. 2005, 41, 261–268. [Google Scholar] [CrossRef]
- McDermott, M.M.; Liu, K.; Guralnik, J.M.; Martin, G.J.; Criqui, M.H.; Greenland, P. Measurement of walking endurance and walking velocity with questionnaire: Validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J. Vasc. Surg. 1998, 28, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Liu, K.; Ferrucci, L.; Criqui, M.H.; Tian, L.; Guralnik, J.M.; Tao, H.; McDermott, M.M. The Walking Impairment Questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J. Vasc. Surg. 2012, 55, 1662–1673.e2. [Google Scholar] [CrossRef]
- Holeman, T.A.; Hales, J.; Cizik, A.M.; Zickmund, S.; Kean, J.; Brooke, B.S. Factors that impact the implementation of patient reported outcomes in routine clinical care for peripheral artery disease from the patient perspective. Qual. Life Res. 2025, 34, 711–723. [Google Scholar] [CrossRef]
- Pasqualini, I.; Piuzzi, N.S. New CMS Policy on the Mandatory Collection of Patient-Reported Outcome Measures for Total Hip and Knee Arthroplasty by 2027: What Orthopaedic Surgeons Should Know. J. Bone Jt. Surg. Am. 2024, 106, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Harwood, A.E.; Totty, J.P.; Broadbent, E.; Smith, G.E.; Chetter, I.C. Quality of life in patients with intermittent claudication. Gefasschirurgie 2017, 22, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Rolstad, S.; Adler, J.; Rydén, A. Response burden and questionnaire length: Is shorter better? A review and meta-analysis. Value Health 2011, 14, 1101–1108. [Google Scholar] [CrossRef]
- Roberts, A.; Benterud, E.; Santana, M.J.; Engbers, J.; Lorenz, C.; Verdin, N.; Pearson, W.; Edgar, P.; Adekanye, J.; Javaheri, P.; et al. APPROACH e-PROM system: A user-centered development and evaluation of an electronic patient-reported outcomes measurement system for management of coronary artery disease. J. Patient Rep. Outcomes 2024, 8, 102. [Google Scholar] [CrossRef]
- Kane, L.T.; Namdari, S.; Plummer, O.R.; Beredjiklian, P.; Vaccaro, A.; Abboud, J.A. Use of Computerized Adaptive Testing to Develop More Concise Patient-Reported Outcome Measures. JBJS Open Access 2020, 5, e0052. [Google Scholar] [CrossRef]
- Wagle, N. Implementing patient-reported outcome measures. NEJM Catal. 2017, 3. [Google Scholar]
- Rotenstein, L.S.; Agarwal, A.; O’Neil, K.; Kelly, A.; Keaty, M.; Whitehouse, C.; Kalinowski, B.; Orio, P.F.; Wagle, N.; E Martin, N. Implementing patient-reported outcome surveys as part of routine care: Lessons from an academic radiation oncology department. J. Am. Med. Inf. Assoc. 2017, 24, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Boers, M.; Wells, G.A. Many faces of the minimal clinically important difference (MCID): A literature review and directions for future research. Curr. Opin. Rheumatol. 2002, 14, 109–114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).