Understanding Treatment Adherence in Chronic Diseases: Challenges, Consequences, and Strategies for Improvement
Abstract
1. Introduction
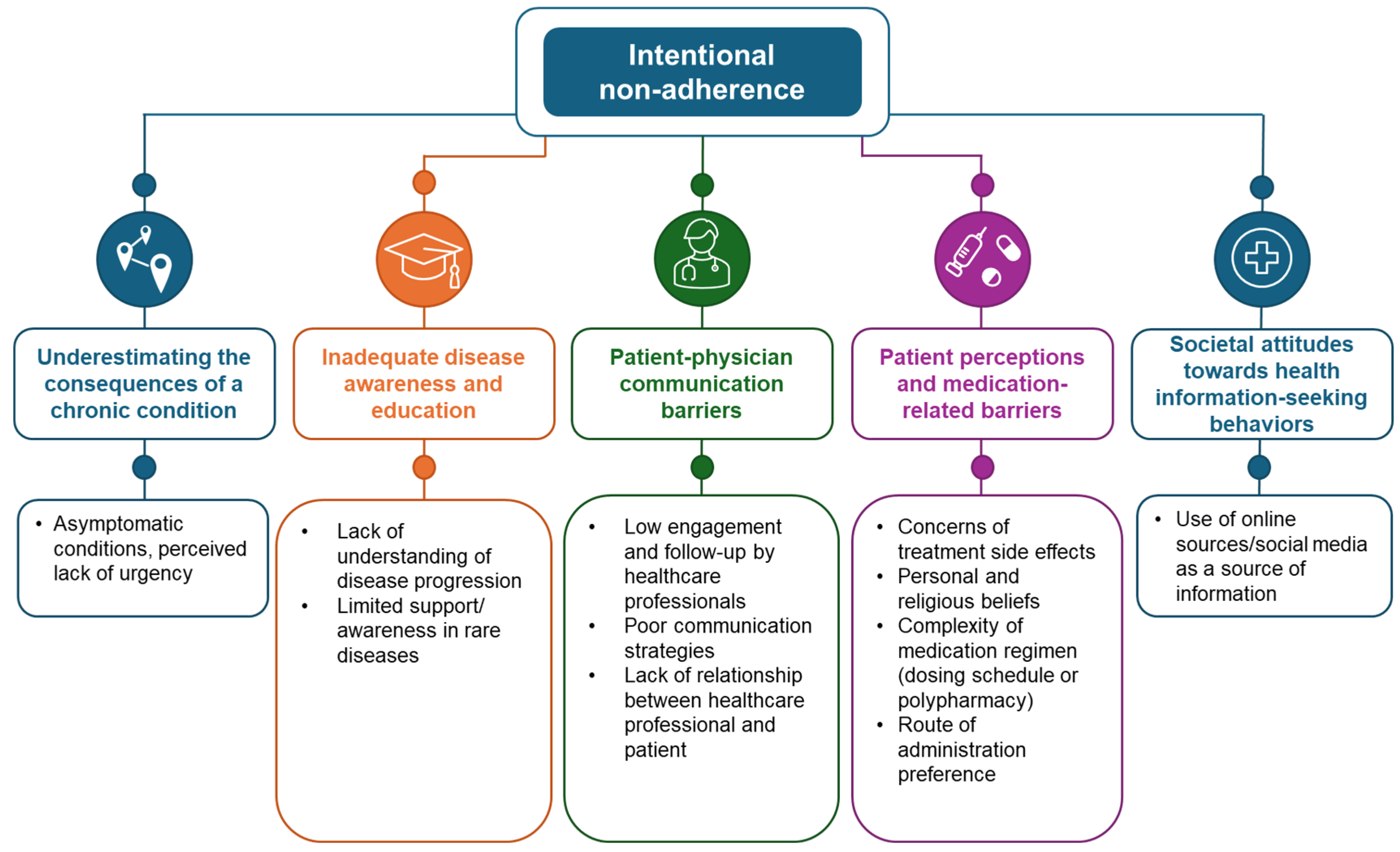
2. Intentional Non-Adherence
2.1. Underestimating Consequences of a Chronic Condition
2.2. Inadequate Disease Awareness and Education
2.3. Patient–Physician Communication Barriers
2.4. Patient Perceptions and Medication-Related Barriers
2.5. Societal Attitudes Towards Health Information-Seeking Behaviors
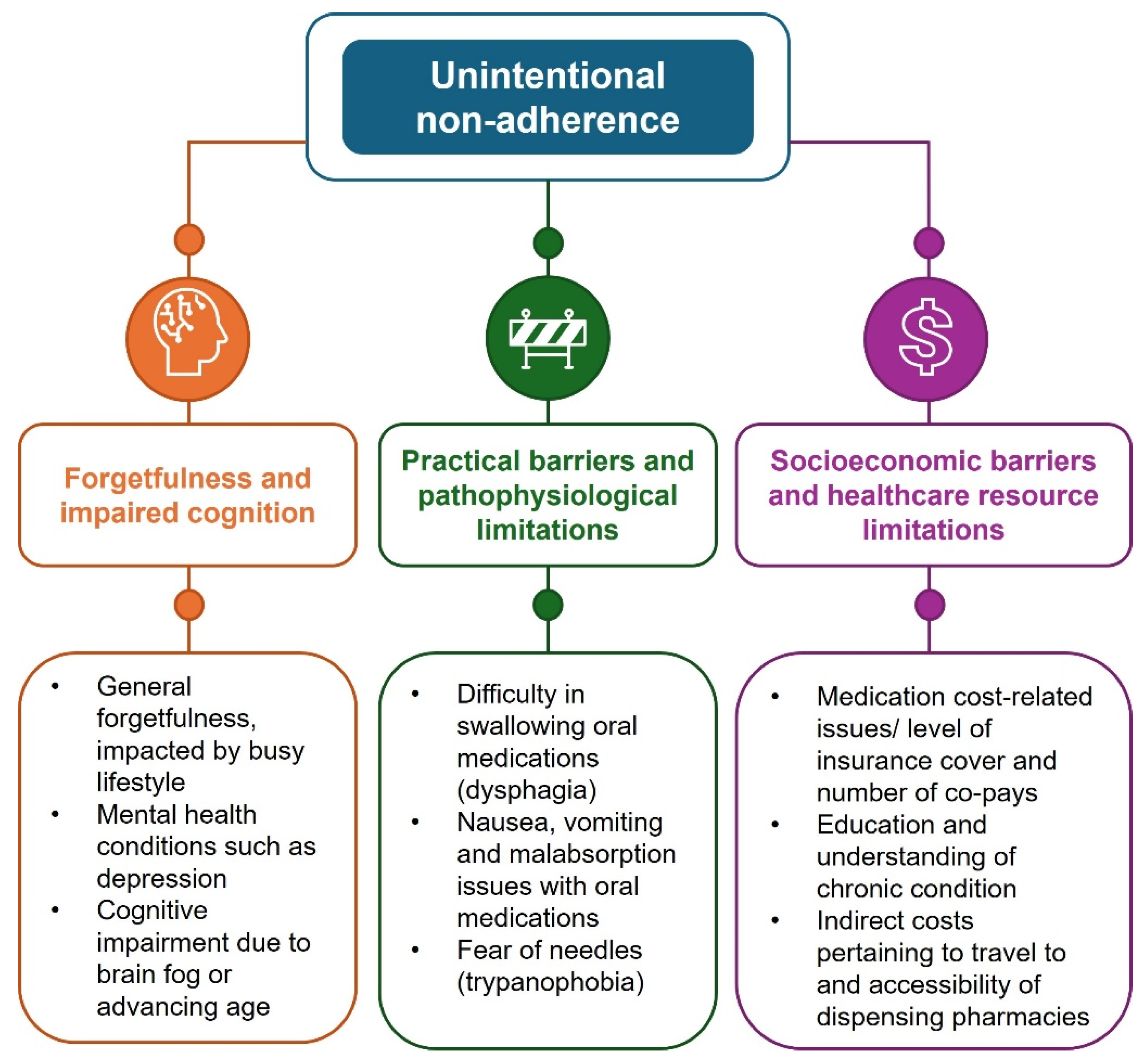
3. Unintentional Non-Adherence
3.1. Forgetfulness and Impaired Cognition
3.2. Practical Barriers and Pathophysiological Limitations
3.3. Socioeconomic Barriers and Healthcare Resource Limitations
4. Special Focus on Adolescent Populations
4.1. Intentional Non-Adherence
4.2. Unintentional Non-Adherence
5. Discussion and Strategies to Improve Adherence
5.1. Building Trusting Relationships Between Patient and Healthcare Providers
5.2. Expanding Treatment Options
5.3. Practical and Technology-Based Interventions
5.4. Disease Education and Support
5.5. Health Policy and Socioeconomic Drivers
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Bussell, J.K. Medication adherence: WHO cares? Mayo Clin Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef]
- Burnier, M. The role of adherence in patients with chronic diseases. Eur. J. Intern. Med. 2024, 119, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Buchman-Wildbaum, T.; Váradi, E.; Schmelowszky, Á.; Griffiths, M.D.; Demetrovics, Z.; Urbán, R. Targeting the problem of treatment non-adherence among mentally ill patients: The impact of loss, grief and stigma. Psychiatry Res. 2020, 290, 113140. [Google Scholar] [CrossRef] [PubMed]
- Greener, M. Wasted medicines and avoidable adverse events: A multibillion pound problem. J. Med. Econ. 2006, 9, 27–44. [Google Scholar] [CrossRef]
- Morgan, T.M. The economic impact of wasted prescription medication in an outpatient population of older adults. J. Fam. Pract. 2001, 50, 779–781. [Google Scholar]
- Achterbosch, M.; Aksoy, N.; Obeng, G.D.; Ameyaw, D.; Ágh, T.; van Boven, J.F.M. Clinical and economic consequences of medication nonadherence: A review of systematic reviews. Front. Pharmacol. 2025, 16, 1570359. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Foley, L.; Larkin, J.; Lombard-Vance, R.; Murphy, A.W.; Hynes, L.; Galvin, E.; Molloy, G.J. Prevalence and predictors of medication non-adherence among people living with multimorbidity: A systematic review and meta-analysis. BMJ Open 2021, 11, e044987. [Google Scholar] [CrossRef]
- Aremu, T.O.; Oluwole, O.E.; Adeyinka, K.O.; Schommer, J.C. Medication Adherence and Compliance: Recipe for Improving Patient Outcomes. Pharmacy 2022, 10, 106. [Google Scholar] [CrossRef]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication compliance and persistence: Terminology and definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef]
- Hichborn, J.; Kaganoff, S.; Subramanian, N.; Yaar, Z. Improving Patient Adherence Through Data-Driven Insights. 2018. Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/improving-patient-adherence-through-data-driven-insights (accessed on 24 July 2025).
- Carls, G.S.; Tuttle, E.; Tan, R.-D.; Huynh, J.; Yee, J.; Edelman, S.V.; Polonsky, W.H. Understanding the Gap Between Efficacy in Randomized Controlled Trials and Effectiveness in Real-World Use of GLP-1 RA and DPP-4 Therapies in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 1469–1478. [Google Scholar] [CrossRef]
- Haygarova, I.; Pavlikyanova, P.; Pesheva, M.; Ganov, N.; Kamusheva, M. Assessment of medication adherence in patients with rare diseases: A systematic review. Pharmacia 2024, 71, 1–13. [Google Scholar] [CrossRef]
- Gast, A.; Mathes, T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Syst. Rev. 2019, 8, 112. [Google Scholar] [CrossRef]
- Huber, C.A.; Rapold, R.; Brüngger, B.; Reich, O.; Rosemann, T. One-year adherence to oral antihyperglycemic medication and risk prediction of patient outcomes for adults with diabetes mellitus: An observational study. Medicine 2016, 95, e3994. [Google Scholar] [CrossRef]
- Simard, P.; Presse, N.; Roy, L.; Dorais, M.; White-Guay, B.; Räkel, A.; Perreault, S. Persistence and adherence to oral antidiabetics: A population-based cohort study. Acta Diabetol. 2015, 52, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Shani, M.; Lustman, A.; Vinker, S. Adherence to oral antihypertensive medications, are all medications equal? J. Clin. Hypertens. 2019, 21, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.A.; Sansone, L.A. Antidepressant adherence: Are patients taking their medications? Innov. Clin. Neurosci. 2012, 9, 41–46. [Google Scholar]
- Schmid, H.; Hartmann, B.; Schiffl, H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: A critical review of the literature. Eur. J. Med. Res. 2009, 14, 185–190. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Kloosterboer, S.M.; Dierckx, B.; Koch, B.C. Assessing methods of measuring medication adherence in chronically ill children-a narrative review. Patient Prefer. Adherence 2019, 13, 1175–1189. [Google Scholar] [CrossRef]
- Lee, E.K.P.; Poon, P.; Yip, B.H.K.; Bo, Y.; Zhu, M.-T.; Yu, C.-P.; Ngai, A.C.H.; Wong, M.C.S.; Wong, S.Y.S. Global Burden, Regional Differences, Trends, and Health Consequences of Medication Nonadherence for Hypertension During 2010 to 2020: A Meta-Analysis Involving 27 Million Patients. J. Am. Heart Assoc. 2022, 11, e026582. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Price, D. Treatment Adherence in Adolescents with Asthma. J. Asthma Allergy 2020, 13, 39–49. [Google Scholar] [CrossRef]
- Taddeo, D.; Egedy, M.; Frappier, J.-Y. Adherence to treatment in adolescents. Paediatr. Child Health 2008, 13, 19–24. [Google Scholar] [CrossRef]
- Miller, N.H. Compliance with treatment regimens in chronic asymptomatic diseases. Am. J. Med. 1997, 102, 43–49. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Copp, H.L.; Nelson, C.P.; Shortliffe, L.D.; Lai, J.; Saigal, C.S.; Kennedy, W.A. Compliance with antibiotic prophylaxis in children with vesicoureteral reflux: Results from a national pharmacy claims database. J. Urol. 2010, 183, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.; Moayyeri, A.; Ali, L.; Taylor, A.; Feudjo-Tepie, M.; Hamilton, L.; Bayly, J. Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos. Int. 2020, 31, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Hafron, J. Adherence With Oral Anticancer Therapies: Clinical Trial vs Real-world Experiences With a Focus on Prostate Cancer. J. Urol. 2023, 209, 485–493. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Lee, H.; Yano, Y.; Cho, S.M.J.; Heo, J.E.; Kim, D.-W.; Park, S.; Lloyd-Jones, D.M.; Kim, H.C. Adherence to Antihypertensive Medication and Incident Cardiovascular Events in Young Adults with Hypertension. Hypertension 2021, 77, 1341–1349. [Google Scholar] [CrossRef]
- Dean, Y.E.; Motawea, K.R.; Shebl, M.A.; Elawady, S.S.; Nuhu, K.; Abuzuaiter, B.; Awayda, K.; Fouad, A.M.; Tanas, Y.; Batista, R.; et al. Adherence to antihypertensives in the United States: A comparative meta-analysis of 23 million patients. J. Clin. Hypertens. 2024, 26, 303–313. [Google Scholar] [CrossRef]
- Kripalani, S.; Henderson, L.E.; Jacobson, T.A.; Vaccarino, V. Medication use among inner-city patients after hospital discharge: Patient-reported barriers and solutions. Mayo Clin. Proc. 2008, 83, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Meranius, M.S.; Engstrom, G. Experience of self-management of medications among older people with multimorbidity. J. Clin. Nurs. 2015, 24, 2757–2764. [Google Scholar] [CrossRef]
- Zolnierek, K.B.H.; Dimatteo, M.R. Physician communication and patient adherence to treatment: A meta-analysis. Med. Care 2009, 47, 826–834. [Google Scholar] [CrossRef]
- Hsu, K.L.; Fink, J.C.; Ginsberg, J.S.; Yoffe, M.; Zhan, M.; Fink, W.; Woods, C.M.; Diamantidis, C.J. Self-reported Medication Adherence and Adverse Patient Safety Events in CKD. Am. J. Kidney Dis. 2015, 66, 621–629. [Google Scholar] [CrossRef]
- Stewart, S.-J.F.; Moon, Z.; Horne, R. Medication nonadherence: Health impact, prevalence, correlates and interventions. Psychol. Health 2023, 38, 726–765. [Google Scholar] [CrossRef]
- Gadkari, A.S.; McHorney, C.A. Unintentional non-adherence to chronic prescription medications: How unintentional is it really? BMC Health Serv. Res. 2012, 12, 98. [Google Scholar] [CrossRef]
- Dijkstra, N.E.; Sino, C.G.M.; Schuurmans, M.J.; Schoonhoven, L.; Heerdink, E.R. Medication self-management: Considerations and decisions by older people living at home. Res. Soc. Adm. Pharm. 2022, 18, 2410–2423. [Google Scholar] [CrossRef]
- Grant, R.W.; Devita, N.G.; Singer, D.E.; Meigs, J.B. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care 2003, 26, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.; Alavijeh, F.Z.; Salahshouri, A.; Mahaki, B. The psychosocial barriers to medication adherence of patients with type 2 diabetes: A qualitative study. Biopsychosoc. Med. 2021, 15, 1. [Google Scholar] [CrossRef]
- Rezaei, M.; Valiee, S.; Tahan, M.; Ebtekar, F.; Ghanei Gheshlagh, R. Barriers of medication adherence in patients with type-2 diabetes: A pilot qualitative study. Diabetes Metab. Syndr. Obes. 2019, 12, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Goh, I.; Lai, O.; Chew, L. Prevalence and Risk of Polypharmacy Among Elderly Cancer Patients Receiving Chemotherapy in Ambulatory Oncology Setting. Curr. Oncol. Rep. 2018, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Iskedjian, M.; Einarson, T.R.; MacKeigan, L.D.; Shear, N.; Addis, A.; Mittmann, N.; Ilersich, A.L. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: Evidence from a meta-analysis. Clin. Ther. 2002, 24, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Geynisman, D.M.; Wickersham, K.E. Adherence to targeted oral anticancer medications. Discov. Med. 2013, 15, 231–241. [Google Scholar]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Limone, B.; Sobieraj, D.M.; Lee, S.; Roberts, M.S.; Kaur, R.; Alam, T. Dosing frequency and medication adherence in chronic disease. J. Manag. Care Pharm. 2012, 18, 527–539. [Google Scholar] [CrossRef]
- Horii, T.; Iwasawa, M.; Kabeya, Y.; Atuda, K. Polypharmacy and oral antidiabetic treatment for type 2 diabetes characterised by drug class and patient characteristics: A Japanese database analysis. Sci. Rep. 2019, 9, 12992. [Google Scholar] [CrossRef]
- Stoehr, G.P.; Lu, S.-Y.; Lavery, L.; Bilt, J.V.; Saxton, J.A.; Chang, C.-C.H.; Ganguli, M. Factors associated with adherence to medication regimens in older primary care patients: The Steel Valley Seniors Survey. Am. J. Geriatr. Pharmacother. 2008, 6, 255–263. [Google Scholar] [CrossRef]
- Pasina, L.; Brucato, A.L.; Falcone, C.; Cucchi, E.; Bresciani, A.; Sottocorno, M.; Taddei, G.C.; Casati, M.; Franchi, C.; Djade, C.D.; et al. Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging 2014, 31, 283–289. [Google Scholar] [CrossRef]
- Perez, C.L.S.; Araki, F.S.; Graf, H.; de Carvalho, G.A. Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid 2013, 23, 779–784. [Google Scholar] [CrossRef]
- Silverii, G.A. Optimizing metformin therapy in practice: Tailoring therapy in specific patient groups to improve tolerability, efficacy and outcomes. Diabetes Obes. Metab. 2024, 26 (Suppl. 3), 42–54. [Google Scholar] [CrossRef]
- Prats, J.V.T.; Rodríguez, F.S.; Parra, E.G.; Román, L.E.; Fuster, J.M.B.; Monteagud-Marrahí, E.; Serrano, V.M.N. Distar Renal Tubular Acidosis (dRTA): Epidemiological, diagnostics, clinical follow-up and therapeutical issues. Nephrologists cohort survey outcome. Nefrol. (Engl. Ed.) 2021, 41, 62–68. [Google Scholar] [CrossRef]
- VandenBerg, C.J.; Adams, A.; Bockrath, R.; Kim, S.; Rodriguez, G.; Fawcett, A.; Jhaveri, R. Hard to Swallow: A Review of Interventions to Improve Swallowing Solid Medication. Hosp. Pediatr. 2023, 13, e123–e132. [Google Scholar] [CrossRef]
- National Osteoporosis Guideline Group (NOGG). Clinical Guideline for the Prevention and Treatment of Osteoporosis; National Osteoporosis Guideline Group: 2024. Available online: https://www.nogg.org.uk/sites/nogg/download/NOGG-Guideline-2024.pdf?v4 (accessed on 19 August 2025).
- Babos, M.B.; Perry, J.D.; Reed, S.A.; Bugariu, S.; Hill-Norby, S.; Allen, M.J.; Corwell, T.K.; Funck, J.E.; Kabir, K.F.; Sullivan, K.A.; et al. Animal-derived medications: Cultural considerations and available alternatives. J. Osteopath. Med. 2021, 121, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Soni, H.; Mishra, A.; Sarma, P. Are your capsules vegetarian or nonvegetarian: An ethical and scientific justification. Indian. J. Pharmacol. 2017, 49, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Kaba, R.; Sooriakumaran, P. The evolution of the doctor-patient relationship. Int. J. Surg. 2007, 5, 57–65. [Google Scholar] [CrossRef]
- Arbuckle, C.; Tomaszewski, D.; Brown, L.; Schommer, J.; Morisky, D.; Parlett-Pelleriti, C.; Linstead, E. Exploring the relationship of digital information sources and medication adherence. Comput. Biol. Med. 2019, 109, 303–310. [Google Scholar] [CrossRef]
- Grenard, J.L.; Munjas, B.A.; Adams, J.L.; Suttorp, M.; Maglione, M.; McGlynn, E.A.; Gellad, W.F. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. J. Gen. Intern. Med. 2011, 26, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Haskard-Zolnierek, K.; Wilson, C.; Pruin, J.; Deason, R.; Howard, K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin. Nurs. Res. 2022, 31, 445–452. [Google Scholar] [CrossRef]
- Dolansky, M.A.; Hawkins, M.A.W.; Schaefer, J.T.; Sattar, A.; Gunstad, J.; Redle, J.D.; Josephson, R.; Moore, S.M.; Hughes, J.W. Association Between Poorer Cognitive Function and Reduced Objectively Monitored Medication Adherence in Patients With Heart Failure. Circ. Heart Fail. 2016, 9, e002475. [Google Scholar] [CrossRef]
- Kamel, K.; Lottrup, A.M.W.; Piggin, M.; Naylor, A.; Katsof, B.; Brok-Kristensen, M.; Stevens, J.; Rigbolt, M. A Qualitative Study Using a Multi-Grounded Theory-Based Approach to Understand the Lived Experiences of People with Paroxysmal Nocturnal Haemoglobinuria Receiving Complement C5 Inhibitor Treatment in Europe. J. Community Med. Health Educ. 2024, 13, 877. [Google Scholar] [CrossRef]
- Rao, K.V.; Faso, A. Chemotherapy-induced nausea and vomiting: Optimizing prevention and management. Am. Health Drug Benefits 2012, 5, 232–240. [Google Scholar]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations—An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef]
- Nelson-Piercy, C.; Dean, C.; Shehmar, M.; Gadsby, R.; O’Hara, M.; Hodson, K.; Nana, M. The Management of Nausea and Vomiting in Pregnancy and Hyperemesis Gravidarum (Green-top Guideline No. 69). BJOG 2024, 131, e1–e30. [Google Scholar] [CrossRef]
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef]
- Robertson, J.; Chadwick, D.; Baines, S.; Emerson, E.; Hatton, C. People with intellectual disabilities and dysphagia. Disabil. Rehabil. 2018, 40, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Forough, A.S.; Lau, E.T.; Steadman, K.J.; Cichero, J.A.; Kyle, G.J.; Serrano Santos, J.M.; Nissen, L.M. A spoonful of sugar helps the medicine go down? A review of strategies for making pills easier to swallow. Patient Prefer. Adherence 2018, 12, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, A.P.; Penson, P.E.; Tse, Y.; Abdelhafiz, M.A.; Ahmed, S.N.; Lim, E.J. Identifying and addressing pill aversion in adults without physiological-related dysphagia: A narrative review. Br. J. Clin. Pharmacol. 2022, 88, 5128–5148. [Google Scholar] [CrossRef]
- Strachan, I.; Greener, M. Medication-related swallowing difficulties may be more common than we realise. Pharm. Pract. 2005, 15, 411–414. [Google Scholar]
- Alsbrooks, K.; Hoerauf, K. Prevalence, causes, impacts, and management of needle phobia: An international survey of a general adult population. PLoS ONE 2022, 17, e0276814. [Google Scholar] [CrossRef]
- McLenon, J.; Rogers, M.A.M. The fear of needles: A systematic review and meta-analysis. J. Adv. Nurs. 2019, 75, 30–42. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Blecker, S.; Li, X.; Kronish, I.M.; Chunara, R.; Zheng, Y.; Lawrence, S.; Dodson, J.A.; Kozloff, S.; Adhikari, S. Neighborhood-Level Socioeconomic Status and Prescription Fill Patterns Among Patients with Heart Failure. JAMA Netw. Open 2023, 6, e2347519. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Rowan-Martin, M.T.; Levin, R.; Fonseca, V.A.; Schmittdiel, J.A.; Herman, W.H.; Aubert, R.E. Determinants of Adherence to Diabetes Medications: Findings From a Large Pharmacy Claims Database. Diabetes Care 2015, 38, 604–609. [Google Scholar] [CrossRef]
- Wilder, M.E.; Kulie, P.; Jensen, C.; Levett, P.; Blanchard, J.; Dominguez, L.W.; Portela, M.; Srivastava, A.; Li, Y.; McCarthy, M.L. The Impact of Social Determinants of Health on Medication Adherence: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2021, 36, 1359–1370. [Google Scholar] [CrossRef]
- van Alsten, S.C.; Harris, J.K. Cost-Related Nonadherence and Mortality in Patients With Chronic Disease: A Multiyear Investigation, National Health Interview Survey, 2000–2014. Prev. Chronic Dis. 2020, 17, E151. [Google Scholar] [CrossRef]
- Weinstock, R.S.; Trief, P.M.; Burke, B.K.; Wen, H.; Liu, X.; Kalichman, S.; Anderson, B.J.; Bulger, J.D. Antihypertensive and Lipid-Lowering Medication Adherence in Young Adults with Youth-Onset Type 2 Diabetes. JAMA Netw. Open 2023, 6, e2336964. [Google Scholar] [CrossRef]
- Keisler-Starkey, K.; Bunch, L.N.; Lindsrom, R.A. Health Insurance Coverage in the United States: 2022; U.S. Census Bureau, Current Population Reports; U.S. Census Bureau: Suitland, MD, USA, 2023; pp. 60–281.
- Sinnott, S.-J.; Buckley, C.; O’Riordan, D.; Bradley, C.; Whelton, H. The effect of copayments for prescriptions on adherence to prescription medicines in publicly insured populations; a systematic review and meta-analysis. PLoS ONE 2013, 8, e64914. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, H.; Fonseca, V.; Shi, L. Exacerbation of financial burden of insulin and overall glucose-lowing medications among uninsured population with diabetes. J. Diabetes 2023, 15, 215–223. [Google Scholar] [CrossRef]
- Rose, A.M.; Grosse, S.D.; Garcia, S.P.; Bach, J.; Kleyn, M.; Simon, N.-J.E.; Prosser, L.A. The financial and time burden associated with phenylketonuria treatment in the United States. Mol. Genet. Metab. Rep. 2019, 21, 100523. [Google Scholar] [CrossRef]
- Iqbal Khan, M.F.; Shah Khan, I.M.; Khan, S. Cost-Related medication non-adherence in Pakistan: A pervasive public health challenge. J. Pak. Med. Assoc. 2025, 75, 364. [Google Scholar] [CrossRef]
- Khan, S.J.; Asif, M.; Aslam, S.; Khan, W.J.; Hamza, S.A. Pakistan’s Healthcare System: A Review of Major Challenges and the First Comprehensive Universal Health Coverage Initiative. Cureus 2023, 15, e44641. [Google Scholar] [CrossRef]
- Noreen, N.; Bashir, F.; Khan, A.W.; Safi, M.M.; Lashari, W.A.; Hering, D. Determinants of Adherence to Antihypertension Medications Among Patients at a Tertiary Care Hospital in Islamabad, Pakistan, 2019. Prev. Chronic Dis. 2023, 20, E42. [Google Scholar] [CrossRef]
- Ajibola, S.S.; Timothy, F.O. The Influence of National Health Insurance on Medication Adherence Among Outpatient Type 2 Diabetics in Southwest Nigeria. J. Patient Exp. 2018, 5, 114–119. [Google Scholar] [CrossRef]
- Eze, O.I.; Iseolorunkanmi, A.; Adeloye, D. The National Health Insurance Scheme (NHIS) in Nigeria: Current issues and implementation challenges. J. Glob. Health Econ. Policy 2024, 4, e2024002. [Google Scholar] [CrossRef]
- Studer, C.M.; Linder, M.; Pazzagli, L. A global systematic overview of socioeconomic factors associated with antidiabetic medication adherence in individuals with type 2 diabetes. J. Health Popul. Nutr. 2023, 42, 122. [Google Scholar] [CrossRef]
- Hanghøj, S.; Boisen, K.A. Self-reported barriers to medication adherence among chronically ill adolescents: A systematic review. J. Adolesc. Health 2014, 54, 121–138. [Google Scholar] [CrossRef]
- Chandra, S.; Mohammadnezhad, M.; Ward, P. Trust and Communication in a Doctor- Patient Relationship: A Literature Review. J. Healthc. Commun. 2018, 3, 36. [Google Scholar] [CrossRef]
- González, K.; Eixarch, T.; Nuñez, L.; Ariceta, G. Quality of life and mental health status in caregivers of pediatric patients with nephropathic cystinosis. Orphanet J. Rare Dis. 2024, 19, 415. [Google Scholar] [CrossRef]
- Ariceta, G.; Lalanza, S.; Peña, C.; Martínez Montero, M.; Bezos Daleske, C.; Acuña Álvarez, L.; Giner, E. Patient journey in cystinosis: Focus on non-adherence and disease management. Drugs Context 2024, 13. [Google Scholar] [CrossRef]
- Nguyen, C.; Dew, M.A.; Irizarry, T.; McNulty, M.; Rennick, J.; Knäuper, B.; Descoteaux, A.; Grenier, A.; Jeannot, L.; Foster, B.J.; et al. Promoting medication adherence from the perspective of adolescent and young adult kidney transplant recipients, parents, and health care professionals: A TAKE-IT TOO study. Pediatr. Transplant. 2020, 24, e13709. [Google Scholar] [CrossRef]
- Deniz, S.; Akbolat, M.; Çimen, M.; Ünal, Ö. The Mediating Role of Shared Decision-Making in the Effect of the Patient-Physician Relationship on Compliance With Treatment. J. Patient Exp. 2021, 8, 23743735211018066. [Google Scholar] [CrossRef]
- Barker, J.M.; Faasse, K. Influence of side effect information on patient willingness to take medication: Consequences for informed consent and medication adherence. Intern. Med. J. 2023, 53, 1692–1696. [Google Scholar] [CrossRef]
- Horvat, M.; Eržen, I.; Vrbnjak, D. Barriers and Facilitators to Medication Adherence among the Vulnerable Elderly: A Focus Group Study. Healthcare 2024, 12, 1723. [Google Scholar] [CrossRef]
- Laranjeira, C.; Carvalho, D.; Valentim, O.; Moutinho, L.; Morgado, T.; Tomás, C.; Gomes, J.; Querido, A. Therapeutic Adherence of People with Mental Disorders: An Evolutionary Concept Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3869. [Google Scholar] [CrossRef]
- Gardezi, S.K.M.; Aitken, W.W.; Jilani, M.H. The Impact of Non-Adherence to Antihypertensive Drug Therapy. Healthcare 2023, 11, 2979. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. 2009. Available online: https://www.nice.org.uk/guidance/ng5 (accessed on 19 February 2025).
- Waddell, A.; Lennox, A.; Spassova, G.; Bragge, P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: A systematic review. Implement. Sci. 2021, 16, 74. [Google Scholar] [CrossRef]
- Losi, S.; Berra, C.C.F.; Fornengo, R.; Potocco, D.; Biricolti, G.; Federici, M.O. The role of patient preferences in adherence to treatment in chronic disease: A narrative review. Drug Target Insights 2021, 15, 13–20. [Google Scholar] [CrossRef]
- Hugo, C.; Weihprecht, H.; Banas, B.; Schröppel, B.; Jank, S.; Arns, W.; Schenker, P.; Rath, T.; Hergesell, O.; Feldkamp, T.; et al. Renal Function and Patient-Reported Outcomes in Stable Kidney Transplant Patients Following Conversion From Twice-Daily Immediate-Release Tacrolimus to Once-Daily Prolonged-Release Tacrolimus: A 12-Month Observational Study in Routine Clinical Practice in Germany (ADAGIO). Transpl. Proc. 2021, 53, 1484–1493. [Google Scholar] [CrossRef]
- Paoli, C.J.; Linder, J.; Gurjar, K.; Thakur, D.; Wyckmans, J.; Grieve, S. Effectiveness of Single-Tablet Combination Therapy in Improving Adherence and Persistence and the Relation to Clinical and Economic Outcomes. J. Health Econ. Outcomes Res. 2024, 11, 8–22. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwon, O.D.; Cho, B.; Oh, S.-W.; Lee, C.M.; Choi, H.-C. Effects of combination drugs on antihypertensive medication adherence in a real-world setting: A Korean Nationwide Study. BMJ Open 2019, 9, e029862. [Google Scholar] [CrossRef]
- Mash, B.R.J.; Bheekie, A.; Jones, P. Inhaled versus oral steroids for adults with chronic asthma. Cochrane Database Syst. Rev. 2001, CD002160. [Google Scholar] [CrossRef]
- Underwood, M.; Ashby, D.; Carnes, D.; Castelnuovo, E.; Cross, P.; Harding, G.; Hennessy, E.; Letley, L.; Martin, J.; Mt-Isa, S.; et al. Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study. Health Technol. Assess. 2008, 12, iii. [Google Scholar] [CrossRef]
- Duncanson, E.; Le Leu, R.K.; Shanahan, L.; Macauley, L.; Bennett, P.N.; Weichula, R.; McDonald, S.; Burke, A.L.J.; Collins, K.L.; Chur-Hansen, A.; et al. The prevalence and evidence-based management of needle fear in adults with chronic disease: A scoping review. PLoS ONE 2021, 16, e0253048. [Google Scholar] [CrossRef]
- Rahman, O.; Desai, M.; Candiotti, K. Beyond the point: Navigating the impact of needles on pain, anxiety and the patient experience. ON Drug Delivery 2024, 156, 40–43. [Google Scholar]
- Al Hayek, A.A.; Al Dawish, M. Evaluating the User Preference and Level of Insulin Self-Administration Adherence in Young Patients With Type 1 Diabetes: Experience With Two Insulin Pen Needle Lengths. Cureus 2020, 12, e8673. [Google Scholar] [CrossRef] [PubMed]
- DiLauri, B. WEARABLE INJECTORS—BD Wearable Drug Delivery Devices: An Attractive Proposal. Drug Dev. Deliv. 2018, 18, 48–53. [Google Scholar]
- Wasserman, R.L.; Cunningham-Rundles, C.; Anderson, J.; Lugar, P.; Palumbo, M.; Patel, N.C.; Hofmann, J.; Glassman, F.; Rogers, E.; Praus, M.; et al. Systemic IgG exposure and safety in patients with primary immunodeficiency: A randomized crossover study comparing a novel investigational wearable infusor versus the Crono pump. Immunotherapy 2022, 14, 1315–1328. [Google Scholar] [CrossRef]
- Zanni, D.; Nwokoro, E. Enhancing healthcare outcomes and cost efficiency through patient support programs: A comprehensive analysis. Int. J. Pharm. Pract. 2024, 32, ii12–ii13. [Google Scholar] [CrossRef]
- Arnold, L.M.; Kelly, R.J.; Large, J.; Barnfield, C.; Trikha, R.; Stepheson, J.; Patel, S.; Griffin, M. Collaborative Initiative With the National PNH Service: Survey Results From a Pegcetacoplan Patient Support Programme 2025. In Proceedings of the 65th Annual Scientific Meeting of the British Society of Haematology, Glasgow, UK, 27–29 April 2025. [Google Scholar]
- Arnold, L.; Czech, B.; Hillmen, P.; Kelly, R. Injection Site Reactions in Adult Patients with Paroxysmal Nocturnal Hemoglobinuria Who Received Subcutaneous Pegcetacoplan Monotherapy for Up to 3 Years. EHA Library 2024, 8, e104. [Google Scholar] [CrossRef]
- Dowden, A. Do pill organisers improve medication adherence? Prescriber 2020, 31, 24–27. [Google Scholar] [CrossRef]
- Car, J.; Tan, W.S.; Huang, Z.; Sloot, P.; Franklin, B.D. eHealth in the future of medications management: Personalisation, monitoring and adherence. BMC Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Arnold, L.M.; Brondke, H.; Steinitz, K.; Kelly, R.J. Monitoring Patient Reported Outcomes in PNH: Interim Results of a Market Research with orio ® PNH. Blood 2023, 142, 7211. [Google Scholar] [CrossRef]
- Pérez-Jover, V.; Sala-González, M.; Guilabert, M.; Mira, J.J. Mobile Apps for Increasing Treatment Adherence: Systematic Review. J. Med. Internet Res. 2019, 21, e12505. [Google Scholar] [CrossRef]
- Ingerski, L.M.; Hente, E.A.; Modi, A.C.; Hommel, K.A. Electronic measurement of medication adherence in pediatric chronic illness: A review of measures. J. Pediatr. 2011, 159, 528–534. [Google Scholar] [CrossRef]
- Leiz, M.; Pfeuffer, N.; Rehner, L.; Stentzel, U.; van den Berg, N. Telemedicine as a Tool to Improve Medicine Adherence in Patients with Affective Disorders—A Systematic Literature Review. Patient Prefer. Adherence 2022, 16, 3441–3463. [Google Scholar] [CrossRef]
- O’Carroll, R.E.; Chambers, J.A.; Dennis, M.; Sudlow, C.; Johnston, M. Improving adherence to medication in stroke survivors: A pilot randomised controlled trial. Ann. Behav. Med. 2013, 46, 358–368. [Google Scholar] [CrossRef]
- Chapman, S.; Sibelli, A.; St-Clair Jones, A.; Forbes, A.; Chater, A.; Horne, R. Personalised Adherence Support for Maintenance Treatment of Inflammatory Bowel Disease: A Tailored Digital Intervention to Change Adherence-related Beliefs and Barriers. J. Crohns Colitis 2020, 14, 1394–1404. [Google Scholar] [CrossRef]
- Kamerow, D. The pros and cons of generic drugs. BMJ 2011, 343, d4584. [Google Scholar] [CrossRef]
- Viswanathan, M.; Golin, C.E.; Jones, C.D.; Ashok, M.; Blalock, S.J.; Wines, R.C.M.; Coker-Schwimmer, E.J.L.; Rosen, D.L.; Sista, P.; Lohr, K.N. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: A systematic review. Ann. Intern. Med. 2012, 157, 785–795. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Huang, M.; Miliara, S. Understanding Treatment Adherence in Chronic Diseases: Challenges, Consequences, and Strategies for Improvement. J. Clin. Med. 2025, 14, 6034. https://doi.org/10.3390/jcm14176034
Patel S, Huang M, Miliara S. Understanding Treatment Adherence in Chronic Diseases: Challenges, Consequences, and Strategies for Improvement. Journal of Clinical Medicine. 2025; 14(17):6034. https://doi.org/10.3390/jcm14176034
Chicago/Turabian StylePatel, Sheena, Mingyi Huang, and Sophia Miliara. 2025. "Understanding Treatment Adherence in Chronic Diseases: Challenges, Consequences, and Strategies for Improvement" Journal of Clinical Medicine 14, no. 17: 6034. https://doi.org/10.3390/jcm14176034
APA StylePatel, S., Huang, M., & Miliara, S. (2025). Understanding Treatment Adherence in Chronic Diseases: Challenges, Consequences, and Strategies for Improvement. Journal of Clinical Medicine, 14(17), 6034. https://doi.org/10.3390/jcm14176034





