High On-Treatment Platelet Reactivity as a Tool for Risk Stratification in STEMI Patients
Abstract
1. Introduction
2. Materials and Methods
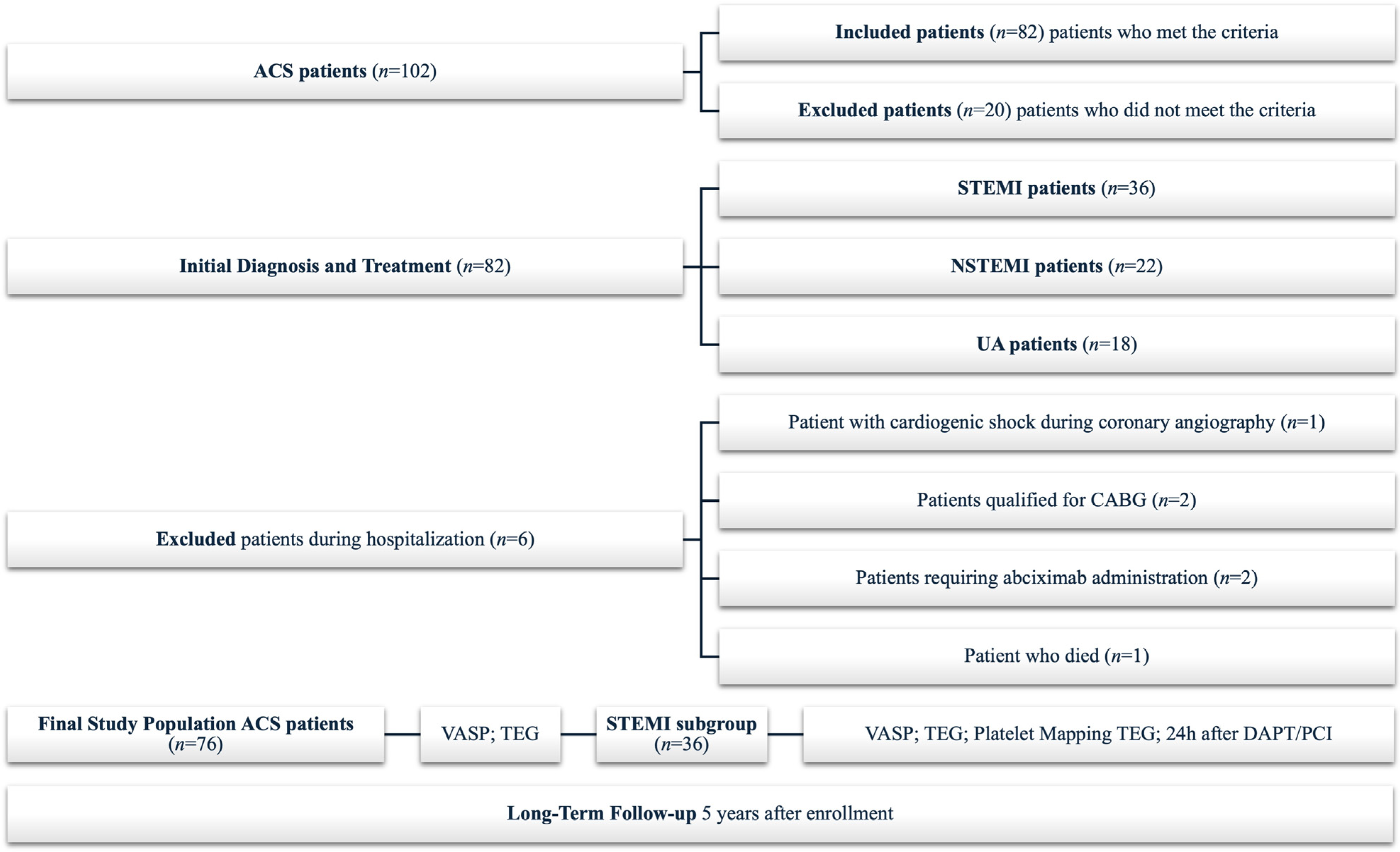
2.1. Study Group
2.2. Study Outcomes
2.3. Platelet Reactivity Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and In-Hospital Events
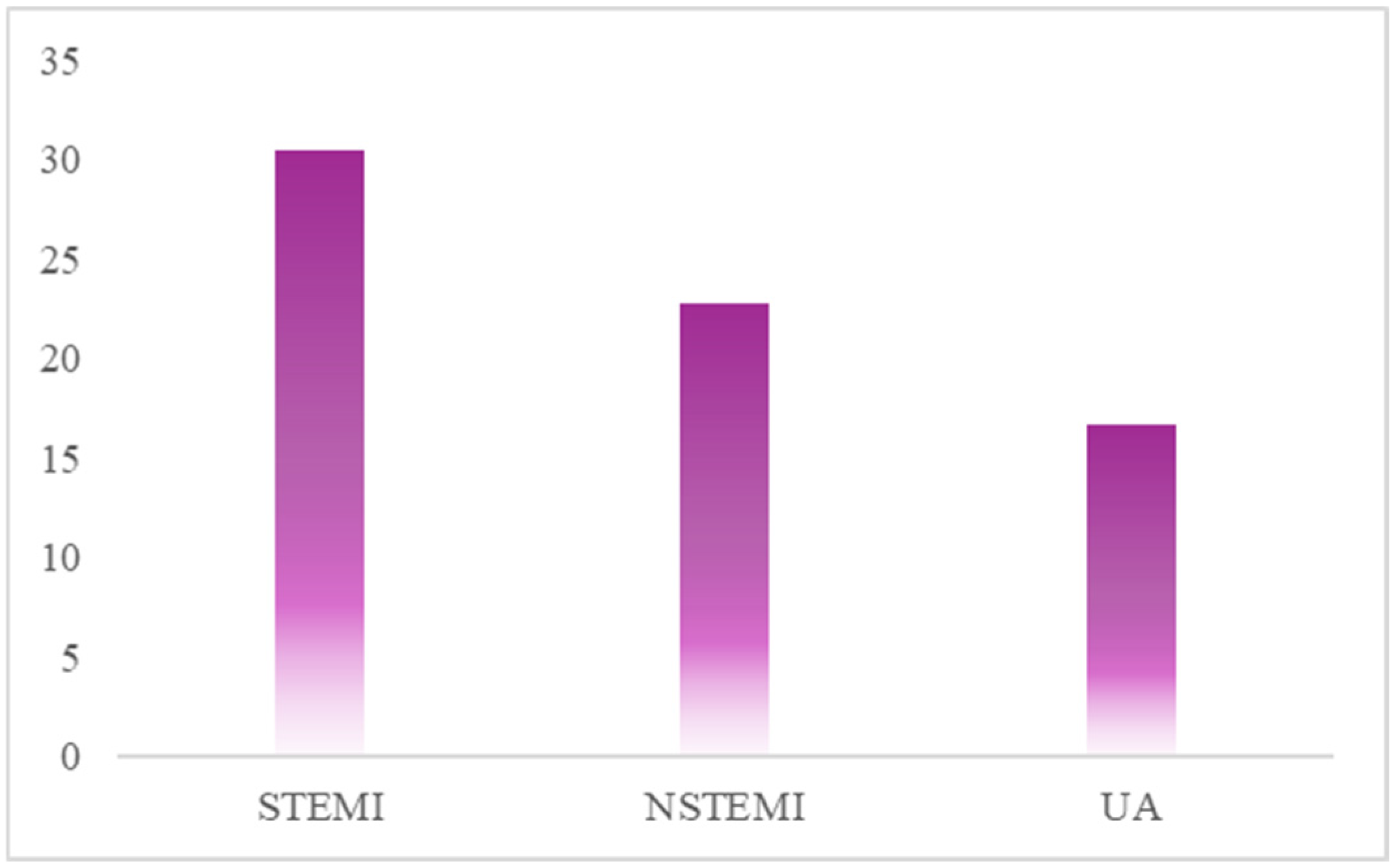
3.2. High On-Treatment Platelet Reactivity in ACS
3.3. High On-Treatment Platelet Reactivity in STEMI
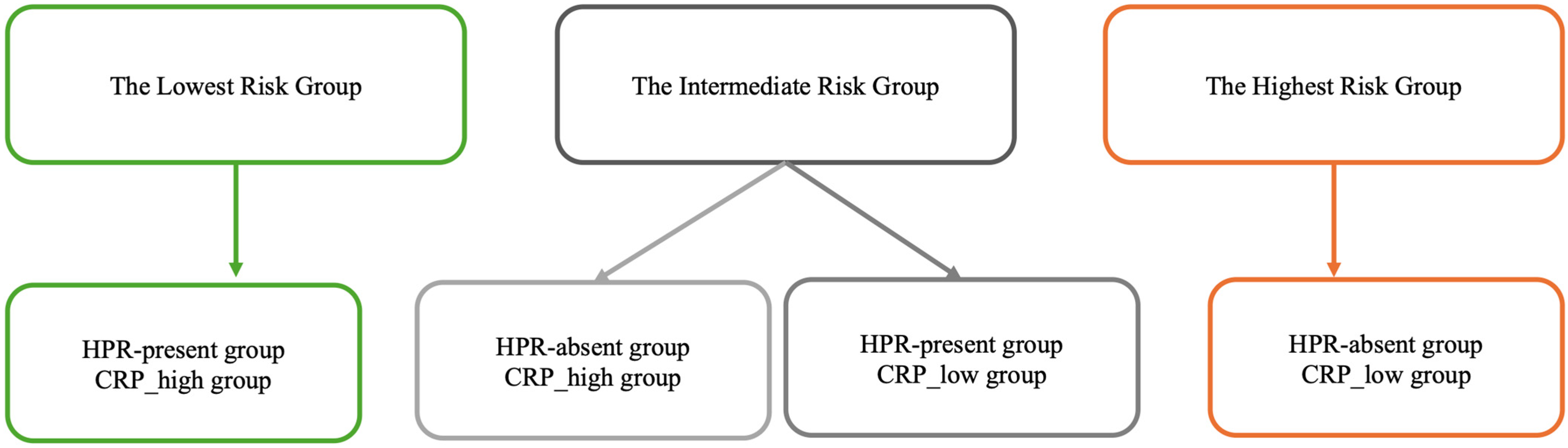
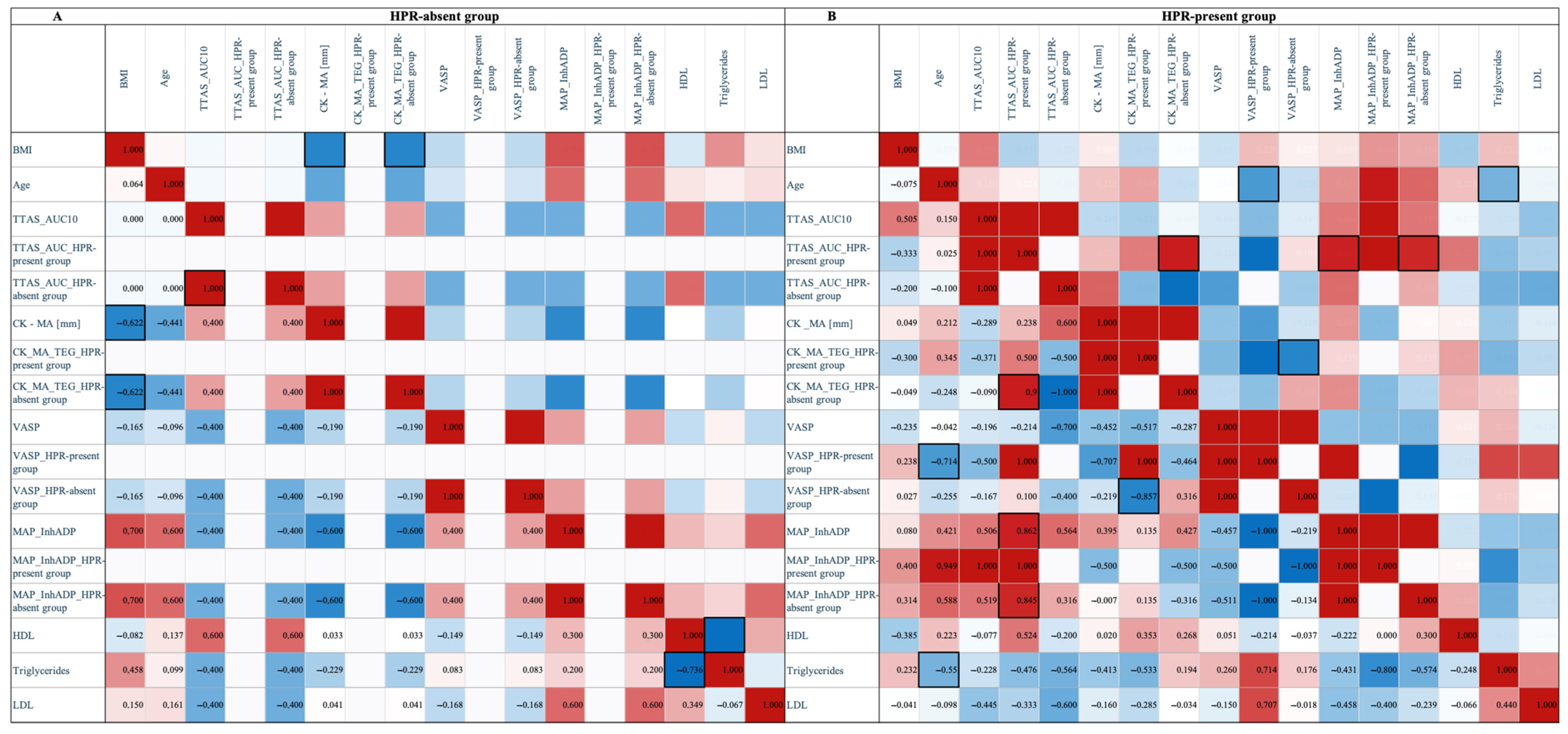
3.4. HPR-Present vs. HPR-Absent
3.5. Kaplan–Meier Survival Analysis
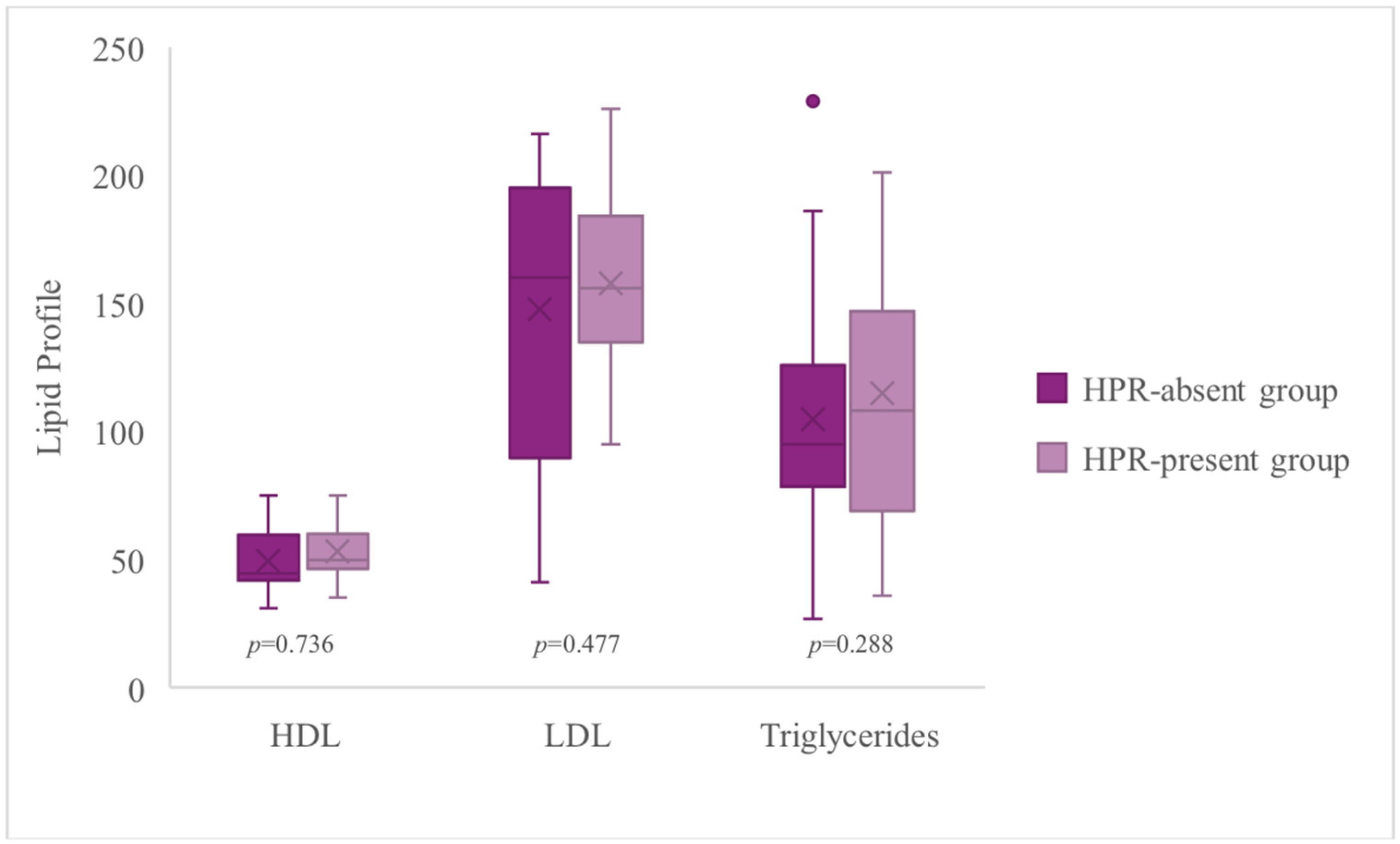
3.6. High On-Treatment Platelet Reactivity and Lipid Profile
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ribatti, D.; Crivellato, E. Giulio Bizzozero and the discovery of platelets. Leuk. Res. 2007, 31, 1339–1341. [Google Scholar] [CrossRef]
- Marcus, A.J.; Zucker, M.B. The Physiology of Blood Platelets; Gurne and Stratton, Inc.: New York, NY, USA, 1965. [Google Scholar]
- Hellem, A.J. The adhesiveness of human blood platelets in vitro. Scand. J. Clin. Lab. Investig. 1960, 12, 1–117. [Google Scholar] [CrossRef]
- Gaarder, A.; Jonsen, J.; Laland, S.; Hellem, A.; Owren, P.A. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature 1961, 192, 531–532. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Hiatt, B.L.; O’Connor, C.M. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003, 107, 2908–2913. [Google Scholar] [CrossRef]
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T.; et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933. [Google Scholar] [CrossRef]
- Bonello, L.; Berbis, J.; Laine, M.; Armero, S.; Bessereau, J.; Jacquin, L.; Bonello, C.; Camillieri, E.; Barragan, P.; Dignat-George, F.; et al. Biological efficacy of a 600 mg loading dose of clopidogrel in ST-elevation myocardial infarction. Thromb. Haemost. 2012, 108, 101–106. [Google Scholar] [CrossRef]
- Melnichnikova, O.S.; Nazarova, I.A.; Sirotkina, O.V.; Panov, A.V.; Abesadze, I.T.; Alugishvili, M.Z.; Lokhovinina, N.L.; Vavilova, T.V. Integral tests of the hemostasis system in assessing the efficiency of acetylsalicylic acid in patients with ischemic heart disease. Biomed. Khim. 2021, 67, 427–433. [Google Scholar] [CrossRef]
- Zheng, K.L.; Wallen, H.; Aradi, D.; Godschalk, T.C.; Hackeng, C.M.; Dahlen, J.R.; Ten Berg, J.M. The Total Thrombus Formation (T-TAS) platelet (PL) assay, a novel test that evaluates whole blood platelet thrombus formation under physiological conditions. Platelets 2022, 33, 273–277. [Google Scholar] [CrossRef]
- Wen, M.; Li, Y.; Qu, X.; Zhu, Y.; Tian, L.; Shen, Z.; Yang, X.; Shi, X. Comparison of platelet reactivity between prasugrel and ticagrelor in patients with acute coronary syndrome: A meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 430. [Google Scholar] [CrossRef]
- Laine, M.; Toesca, R.; Berbis, J.; Frere, C.; Barnay, P.; Pansieri, M.; Peyre, J.P.; Michelet, P.; Bessereau, J.; Camilleri, E.; et al. Platelet reactivity evaluated with the VASP assay following ticagrelor loading dose in acute coronary syndrome patients undergoing percutaneous coronary intervention. Thromb. Res. 2013, 132, e15–e18. [Google Scholar] [CrossRef]
- Schüpke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef]
- Urzgruber, P.; Niessner, A. Prasugrel vs. ticagrelor after acute coronary syndrome: A critical appraisal of the ISAR-REACT 5 trial. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 273–274. [Google Scholar]
- Kubica, J.; Adamski, P.; Ładny, J.R.; Kaźmierczak, J.; Fabiszak, T.; Filipiak, K.J.; Gajda, R.; Gąsior, M.; Gąsior, Z.; Gil, R.; et al. Pre-hospital treatment of patients with acute coronary syndrome: Recommendations for medical emergency teams. Expert position update 2022. Cardiol. J. 2022, 29, 540–552. [Google Scholar] [CrossRef]
- Mitsis, A.; Gragnano, F. Myocardial Infarction with and without ST-segment Elevation: A Contemporary Reappraisal of Similarities and Differences. Curr. Cardiol. Rev. 2021, 17, e230421189013. [Google Scholar] [CrossRef]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’Ascenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-year prognostic differences and management strategies between ST-elevation and non-ST-elevation myocardial infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs 2025, online ahead of print. [Google Scholar] [CrossRef]
- Koźlik, M.; Harpula, J.; Chuchra, P.J.; Nowak, M.; Wojakowski, W.; Gąsior, P. Drug-Eluting Stents: Technical and Clinical Progress. Biomimetics 2023, 8, 72. [Google Scholar] [CrossRef]
- Caracciolo, A.; Mazzone, P.; Laterra, G.; Garcia-Ruiz, V.; Polimeni, A.; Galasso, S.; Saporito, F.; Carerj, S.; D’Ascenzo, F.; Marquis-Gravel, G.; et al. Antithrombotic Therapy for Percutaneous Cardiovascular Interventions: From Coronary Artery Disease to Structural Heart Interventions. J. Clin. Med. 2019, 8, 2016. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Ostrowska, M.; Sikora, J.; Kubica, J.M.; Sroka, W.D.; Stankowska, K.; Buszko, K.; Navarese, E.P.; Jilma, B.; et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: The randomized, double-blind, placebo-controlled IMPRESSION trial. Eur. Heart J. 2016, 37, 245–252. [Google Scholar] [CrossRef]
- Holm, M.; Tornvall, P.; Beck, O.; Fux, T.; van der Linden, J. Impact of morphine dose on ticagrelor uptake and platelet inhibition in patients with ST-segment elevation myocardial infarction—A substudy from the prospective randomized MOVEMENT trial. Thromb. Update 2021, 5, 100071. [Google Scholar] [CrossRef]
- Silvain, J.; Storey, R.F.; Cayla, G.; Esteve, J.B.; Dillinger, J.G.; Rousseau, H.; Tsatsaris, A.; Baradat, C.; Salhi, N.; Hamm, C.W.; et al. P2Y12 receptor inhibition and effect of morphine in patients undergoing primary PCI for ST-segment elevation myocardial infarction. The PRIVATE-ATLANTIC study. Thromb. Haemost. 2016, 116, 369–378. [Google Scholar]
- Parodi, G.; Valenti, R.; Bellandi, B.; Migliorini, A.; Marcucci, R.; Comito, V.; Carrabba, N.; Santini, A.; Gensini, G.F.; Abbate, R.; et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J. Am. Coll. Cardiol. 2013, 61, 1601–1606. [Google Scholar] [CrossRef]
- Franchi, F.; Rollini, F.; Cho, J.R.; Bhatti, M.; DeGroat, C.; Ferrante, E.; Dunn, E.C.; Nanavati, A.; Carraway, E.; Suryadevara, S.; et al. Impact of Escalating Loading Dose Regimens of Ticagrelor in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Results of a Prospective Randomized Pharmacokinetic and Pharmacodynamic Investigation. JACC Cardiovasc. Interv. 2015, 8, 1457–1467. [Google Scholar]
- Hagihara, K.; Kazui, M.; Kurihara, A.; Yoshiike, M.; Honda, K.; Okazaki, O.; Farid, N.A.; Ikeda, T. A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Metab. Dispos. 2009, 37, 2145–2152. [Google Scholar] [CrossRef]
- Hu, X.; Wang, P.; Zeng, D.; Hu, G.X. The effect of gene polymorphism on ticagrelor metabolism: An in vitro study of 22 CYP3A4 variants in Chinese Han population. PeerJ 2024, 12, e18109. [Google Scholar] [CrossRef]
- Liu, S.; Shi, X.; Tian, X.; Zhang, X.; Sun, Z.; Miao, L. Effect of CYP3A4*1G and CYP3A5*3 Polymorphisms on Pharmacokinetics and Pharmacodynamics of Ticagrelor in Healthy Chinese Subjects. Front. Pharmacol. 2017, 8, 176. [Google Scholar] [CrossRef]
- Liedes, H.; Pajula, J.; Vuorinen, A.L.; De Pretis, F.; van Gils, M.; Harno, K.; Lehto, M.; Niemi, M.; Lähteenmäki, J. CYP3A4*22 may increase bleeding risk in ticagrelor users. Basic Clin. Pharmacol. Toxicol. 2023, 133, 202–207. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Tantry, U.S.; Gesheff, T.; Wei, C.; Teng, R.; Antonino, M.J.; Patil, S.B.; Karunakaran, A.; et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation 2009, 120, 2577–2585. [Google Scholar] [CrossRef]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef]
- Lecchi, A.; La Marca, S.; Padovan, L.; Boscarino, M.; Peyvandi, F.; Tripodi, A. Flow-chamber device (T-TAS) to diagnose patients suspected of platelet function defects. Blood Transfus. 2024, 22, 55–64. [Google Scholar]
- Koziński, M.; Ostrowska, M.; Adamski, P.; Sikora, J.; Sikora, A.; Karczmarska-Wódzka, A.; Marszałł, M.P.; Boinska, J.; Laskowska, E.; Obońska, E.; et al. Which platelet function test best reflects the in vivo plasma concentrations of ticagrelor and its active metabolite? The HARMONIC study. Thromb. Haemost. 2016, 116, 1140–1149. [Google Scholar]
- Kang, A.M.; Fisher, E.S. Thromboelastography with Platelet Studies (TEG® with PlateletMapping®) After Rattlesnake Envenomation in the Southwestern United States Demonstrates Inhibition of ADP-Induced Platelet Activation as well as Clot Lysis. J. Med. Toxicol. 2020, 16, 24–32. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Fernandez-Ortiz, A.; Bernardo, E.; Ramírez, C.; Barrera-Ramirez, C.; Sabaté, M.; Hernández, R.; Moreno, R.; Escaned, J.; Alfonso, F.; et al. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb. Res. 2005, 115, 101–108. [Google Scholar] [CrossRef]
- Parodi, G.; Marcucci, R.; Valenti, R.; Gori, A.M.; Migliorini, A.; Giusti, B.; Buonamici, P.; Gensini, G.F.; Abbate, R.; Antoniucci, D. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011, 306, 1215–1223. [Google Scholar] [CrossRef]
- Angiolillo, D.J. Antiplatelet therapy in diabetes: Efficacy and limitations of current treatment strategies and future directions. Diabetes Care 2009, 32, 531–540. [Google Scholar] [CrossRef]
- Capranzano, P.; Ferreiro, J.L.; Angiolillo, D.J. Prasugrel in acute coronary syndrome patients undergoing percutaneous coronary intervention. Expert Rev. Cardiovasc. Ther. 2009, 7, 361–369. [Google Scholar] [CrossRef]
- Bansilal, S.; Castellano, J.M.; Garrido, E.; Wei, H.G.; Freeman, A.; Spettell, C.; Garcia-Alonso, F.; Lizano, I.; Arnold, R.J.; Rajda, J.; et al. Assessing the Impact of Medication Adherence on Long-Term Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2016, 68, 789–801. [Google Scholar] [CrossRef]
- Kubica, A.; Kosobucka, A.; Fabiszak, T.; Gorog, D.A.; Siller-Matula, J.M. Assessment of adherence to medication in patients after myocardial infarction treated with percutaneous coronary intervention. Is there a place for newself-reported questionnaires? Curr. Med. Res. Opin. 2019, 35, 341–349. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Bergmark, B.A.; Bhatt, D.L.; Steg, P.G.; Budaj, A.; Storey, R.F.; Gurmu, Y.; Kuder, J.F.; Im, K.; Magnani, G.; Oude Ophuis, T.; et al. Long-Term Ticagrelor in Patients With Prior Coronary Stenting in the PEGASUS-TIMI 54 Trial. J. Am. Heart Assoc. 2021, 10, e020446. [Google Scholar] [CrossRef]
- Marcucci, R.; Valente, S.; Gori, A.M.; Chiostri, M.; Paniccia, R.; Giusti, B.; Cau, V.; Lazzeri, C.; Gensini, G.F.; Abbate, R. Global platelet hyperreactivity and elevated C-reactive protein levels predict long term mortality in STEMI patients. Thromb. Res. 2014, 134, 884–888. [Google Scholar] [CrossRef]
- Xie, D.; Hu, D.; Zhang, Q.; Sun, Y.; Li, J.; Zhang, Y. Increased high-sensitivity C-reactive protein, erythrocyte sedimentation rate and lactic acid in stroke patients with internal carotid artery occlusion. Arch. Med. Sci. 2016, 12, 546–551. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Moroni, F.; Corna, G.; Del Buono, M.G.; Golino, M.; Talasaz, A.H.; Decotto, S.; Markley, R.; Trankle, C.; Biondi-Zoccai, G.; Carbone, S.; et al. Impact of C-reactive protein levels and role of anakinra in patients with ST-elevation myocardial infarction. Int. J. Cardiol. 2024, 398, 131610. [Google Scholar] [CrossRef]
- Gomaraschi, M.; Sinagra, G.; Serdoz, L.V.; Pitzorno, C.; Fonda, M.; Cattin, L.; Calabresi, L.; Franceschini, G. The plasma concentration of Lpa-I:A-II particles as a predictor of the inflammatory response in patients with ST-elevation myocardial infarction. Atherosclerosis 2009, 202, 304–311. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. Circulation 2011, 124, e574–e651. [Google Scholar]



| TTAS_AUC10 | CK_MA_TEG | VASP_PCI | MAP_InhADP | |
|---|---|---|---|---|
| TTAS_AUC10 | 42.11 | 15.79 | 10.53 | 10.53 |
| CK_MA_TEG | 15.79 | 30.56 | 5.56 | 0.00 |
| VASP_PCI | 10.53 | 5.56 | 22.22 | 2.78 |
| MAP_InhADP | 10.53 | 0.00 | 2.78 | 11.11 |
| Variable | Overall Study Population (n = 36) | HPR-Present Group (n = 22) | HPR-Absent Group (n = 14) | p-Value |
|---|---|---|---|---|
| Age [years] | 62.00 (7) | 62.00 (4) | 63.50 (28) | 0.435 |
| Male * | 66.7 (24) | 54.5 (12) | 85.7 (12) | 0.053 |
| BMI | 27.64 (3.62) | 27.84 (4.69) | 26.75 (2.77) | 0.935 |
| Hyperlipidemia * | 52.2 (12) | 43.8 (7) | 71.4 (5) | 0.221 |
| Current smoker * | 76.9 (20) | 83.3 (15) | 62.5 (5) | 0.245 |
| LDL [mg/dL] | 167.00 (58) | 165.50 (53) | 176.00 (93) | 0.736 |
| Triglycerides [mg/dL] | 92.50 (78) | 118.50 (101) | 73.50 (54) | 0.477 |
| HDL [mg/dL] | 49.50 (17) | 48.50 (15) | 59.50 (27) | 0.288 |
| T-TAS AUC10 | 29.70 (45.7) | 38.30 (59.2) | 25.10 (21.3) | 0.193 |
| CK_MA | 72.40 (3.5) | 72.50 (3.3) | 69.70 (4) | <0.001 |
| VASP (PRI) | 15.78 (8.98) | 17.13 (15.92) | 12.71 (9.59) | 0.063 |
| MAP_InhADP | 93.750 (25.9) | 93.75 (28.30) | 88.85 (23.9) | 0.638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczmarska-Wódzka, A.; Wszelaki, P.; Szymoniuk, S.; Pstrągowski, K.; Sikora, J. High On-Treatment Platelet Reactivity as a Tool for Risk Stratification in STEMI Patients. J. Clin. Med. 2025, 14, 6026. https://doi.org/10.3390/jcm14176026
Karczmarska-Wódzka A, Wszelaki P, Szymoniuk S, Pstrągowski K, Sikora J. High On-Treatment Platelet Reactivity as a Tool for Risk Stratification in STEMI Patients. Journal of Clinical Medicine. 2025; 14(17):6026. https://doi.org/10.3390/jcm14176026
Chicago/Turabian StyleKarczmarska-Wódzka, Aleksandra, Patrycja Wszelaki, Szymon Szymoniuk, Krzysztof Pstrągowski, and Joanna Sikora. 2025. "High On-Treatment Platelet Reactivity as a Tool for Risk Stratification in STEMI Patients" Journal of Clinical Medicine 14, no. 17: 6026. https://doi.org/10.3390/jcm14176026
APA StyleKarczmarska-Wódzka, A., Wszelaki, P., Szymoniuk, S., Pstrągowski, K., & Sikora, J. (2025). High On-Treatment Platelet Reactivity as a Tool for Risk Stratification in STEMI Patients. Journal of Clinical Medicine, 14(17), 6026. https://doi.org/10.3390/jcm14176026







