Pregnancy-Related Acute Kidney Injury: Causes and Its Impact on Perinatal Outcomes—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
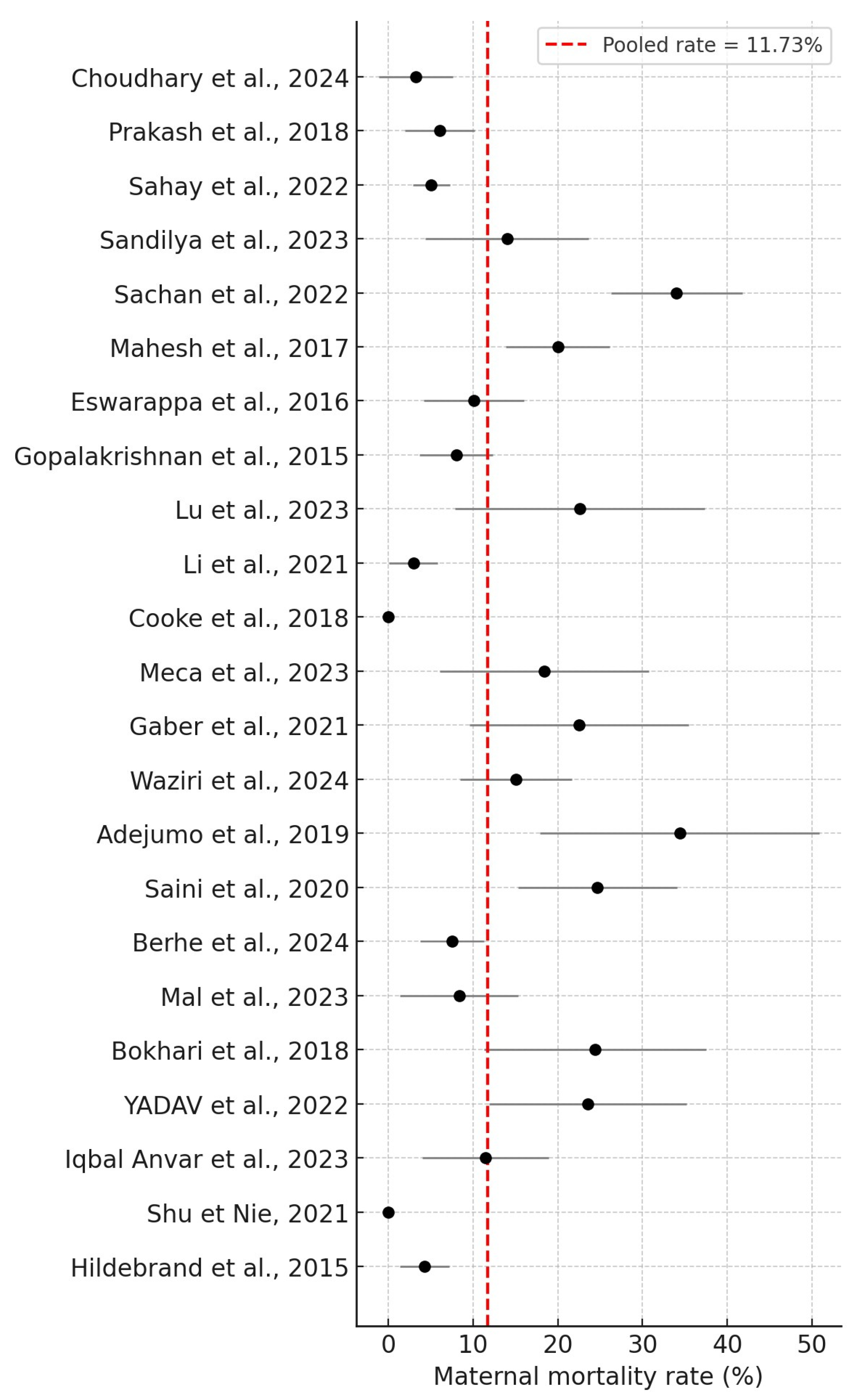
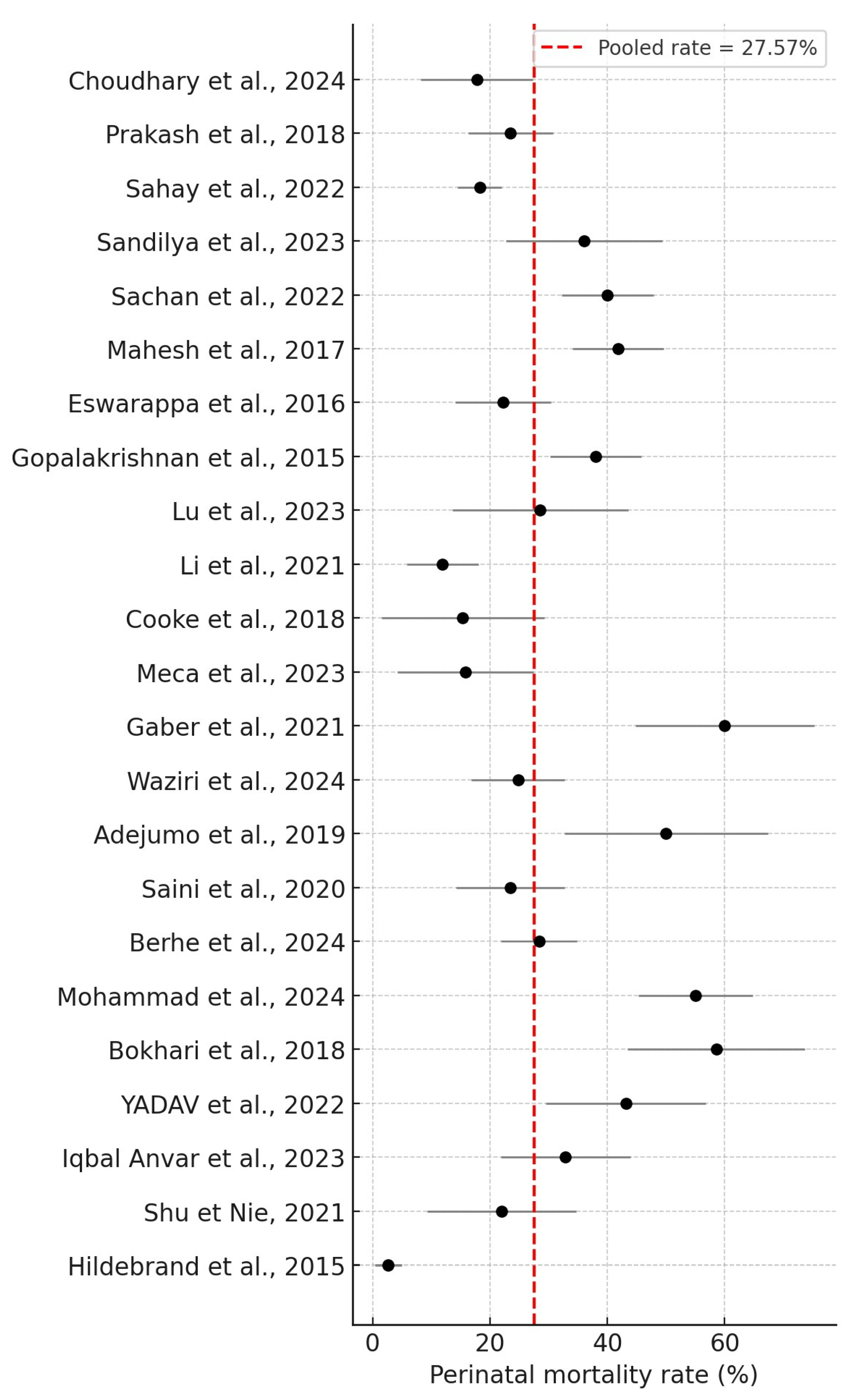
3. Results
4. Discussion
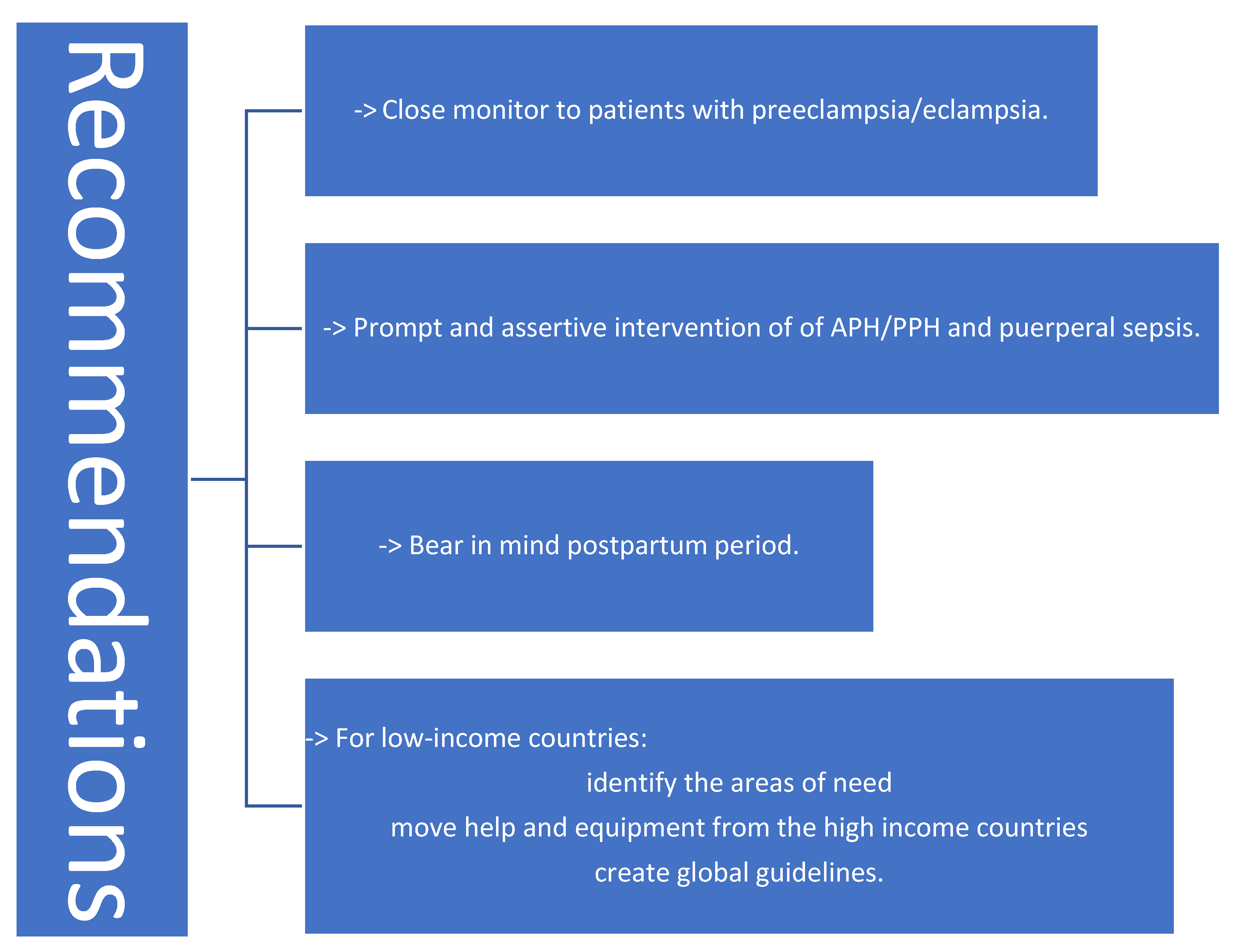
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRAKI | Pregnancy-Related Acute kidney injury |
| AKI | Acute Kidney Injury |
| HELLP syndrome | Hemolysis Elevated Liver enzymes and Low Platelet levels |
| DIC | Disseminated Intravascular Coagulation |
| HUS | Hemolytic Uremic Syndrome |
| RIFLE | Risk, Injury, Failure, Loss of function, and End-stage renal disease criteria |
| ICU | Intensive Care Unit |
| NICU | Neonatal Intensive Care Unit |
| KDIGO | Kidney Disease Guidelines established by Improving Global Outcomes |
| sCr | serum Creatinine |
| Cr | Creatinine |
| ISN | International Society of Nephrology |
| CKD | Chronic Kidney Dysfunction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| AFLP | Acute Fatty Liver of Pregnancy |
| APH | Antepartum Hemorrhage |
| PPH | Postpartum Hemorrhage |
| TMA | Thrombotic Microangiopathy |
| IUD | Intrauterine Death |
| PRBC | Packed Red Blood Cell |
| RRT | Renal Replacement Therapy |
| CRRT | Continuous Renal Replacement Therapy |
| AKI-RRT | AKI-renal replacement therapy |
| HDP | Hypertensive Disorders of Pregnancy |
| PIH | Pregnancy-Induced Hypertension |
| LBW | Low Birth Weight |
| IUGR | Intrauterine Growth Restriction |
| FGR | Fetal Growth Restriction |
| SLE | Systemic Lupus Erythematosus |
References
- Ricci, Z.; Cruz, D.N.; Ronco, C. Classification and staging of acute kidney injury: Beyond the RIFLE and AKIN criteria. Nat. Rev. Nephrol. 2011, 7, 201–208. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Liu, D.; He, W.; Li, Y.; Xiong, M.; Wang, L.; Huang, J.; Jia, L.; Yuan, S.; Nie, S. Epidemiology of acute kidney injury in hospitalized pregnant women in China. BMC Nephrol. 2019, 20, 67. [Google Scholar] [CrossRef]
- Cheung, K.L.; Lafayette, R.A. Renal Physiology of Pregnancy. Adv. Chronic Kidney Dis. 2013, 20, 209–214. [Google Scholar] [CrossRef]
- Shalaby, A.S.; Shemies, R.S. Pregnancy-related acute kidney injury in the African continent: Where do we stand? A systematic review. J. Nephrol. 2022, 35, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Task Force on Hypertension in Pregnancy, Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Gupte, S.; Wagh, G. Preeclampsia-eclampsia. J. Obstet. Gynecol. India 2014, 64, 4–13. [Google Scholar] [CrossRef]
- Shah, S.; Verma, P. Pregnancy-Related Acute Kidney Injury—Do We Know What to Do? Nephron 2022, 147, 35. [Google Scholar] [CrossRef]
- Ahmed, D.M.; Mengistu, T.S.; Endalamaw, A.G. Incidence and factors associated with outcomes of uterine rupture among women delivered at Felegehiwot referral hospital, Bahir Dar, Ethiopia: Cross sectional study. BMC Pregnancy Childbirth 2018, 18, 447. [Google Scholar] [CrossRef]
- Acharya, A.; Santos, J.; Linde, B.; Anis, K. Acute kidney injury in pregnancy-current status. Adv. Chronic Kidney Dis. 2013, 20, 215–222. [Google Scholar] [CrossRef]
- Davidson, B.; Bajpai, D.; Shah, S.; Jones, E.; Okyere, P.; Wearne, N.; Gumber, R.; Saxena, N.; Osafo, C. Pregnancy-Associated Acute Kidney Injury in Low-Resource Settings: Progress Over the Last Decade. Semin. Nephrol. 2022, 42, 151317. [Google Scholar] [CrossRef]
- Jim, B.; Garovic, V.D. Acute Kidney Injury in Pregnancy. Semin. Nephrol. 2017, 37, 378. [Google Scholar] [CrossRef] [PubMed]
- Kazma, J.M.; van den Anker, J.; Allegaert, K.; Dallmann, A.; Ahmadzia, H.K. Anatomical and physiological alterations of pregnancy. J. Pharmacokinet. Pharmacodyn. 2020, 47, 271. [Google Scholar] [CrossRef] [PubMed]
- Siribamrungwong, M.; Chinudomwong, P. Relation between acute kidney injury and pregnancy-related factors. J. Acute Dis. 2016, 5, 22–28. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Ahmad, A.; Kumari, A.; Prasad, D.; Kumar, N. Acute Kidney Injury in Pregnancy: A Prospective Study. Cureus 2024, 16, e58982. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Ganiger, V.C.; Prakash, S.; Iqbal, M.; Kar, D.P.; Singh, U.; Verma, A. Acute kidney injury in pregnancy with special reference to pregnancy-specific disorders: A hospital based study (2014-2016). J. Nephrol. 2018, 31, 79–85. [Google Scholar] [CrossRef]
- Sahay, M.; Priyashree; Dogra, L.; Ismal, K.; Vali, S. Pregnancy-related Acute Kidney Injury in Public Hospital in South India: Changing Trends. J. Assoc. Physicians India 2022, 70, 63–68. [Google Scholar] [CrossRef]
- Sandilya, S.; Rani, K.U.; Kumar, R. Risk factors and fetomaternal outcome in pregnancy-related acute kidney injury. J. Fam. Med. Prim. Care 2023, 12, 3346–3350. [Google Scholar] [CrossRef]
- Sachan, R.; Shukla, S.; Shyam, R.; Sachan, P.L.; Patel, M.L. Feto-maternal outcome of pregnancy related acute kidney injury in a North Indian population. J. Fam. Community Med. 2022, 29, 204–211. [Google Scholar] [CrossRef]
- Mahesh, E.; Puri, S.; Varma, V.; Madhyastha, P.R.; Bande, S.; Gurudev, K.C. Pregnancy-related acute kidney injury: An analysis of 165 cases. Indian. J. Nephrol. 2017, 27, 113–117. [Google Scholar] [CrossRef]
- Eswarappa, M.; Madhyastha, P.R.; Puri, S.; Varma, V.; Bhandari, A.; Chennabassappa, G. Postpartum acute kidney injury: A review of 99 cases. Ren. Fail. 2016, 38, 889–893. [Google Scholar] [CrossRef]
- Gopalakrishnan, N.; Dhanapriya, J.; Muthukumar, P.; Sakthirajan, R.; Dineshkumar, T.; Thirumurugan, S.; Balasubramaniyan, T. Acute kidney injury in pregnancy--a single center experience. Ren. Fail. 2015, 37, 1476–1480. [Google Scholar] [CrossRef]
- Chaudhury, A.R.; Saini, S.; Divyaveer, S.; Maurya, P.; Sircar, D.; Dasgupta, S.; Sen, D.; Bandyopadhyay, S.; Pandey, R. The changing face of pregnancy-related acute kidney injury from eastern part of India: A hospital-based, prospective, observational study. Saudi J. Kidney Dis. Transpl. 2020, 31, 493–502. [Google Scholar] [CrossRef]
- Yadav, S.; Chauhan, M.; Jain, D.; Aggarwal, H.K.; Yadav, R.K. Renal Outcomes of Pregnancy-Related Acute Kidney Injury: A Single Centre Experience in India. Maedica 2022, 17, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Anvar, M.I.; Talwar, S.; Mallapur, S. A Retrospective Study on Clinical Outcomes of Pregnancy-Related Acute Kidney Injury Patients at a South Indian Tertiary Care Hospital. Cureus 2023, 15, e49610. [Google Scholar] [CrossRef]
- Lu, W.; Hu, M.J.; Zhu, D.D.; Lin, F.J.; Huang, H.D. Clinical characteristics and prognosis of pregnancy-related acute kidney injury: A case series study. Int. Urol. Nephrol. 2023, 55, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Zhang, M.; Xu, L.; Li, G.; Wen, Y.; Wang, W. Pregnancy-related acute kidney injury at high altitude: A retrospective observational study in a single center. BMC Nephrol. 2021, 22, 215. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Nie, F. Clinical characteristics and prognosis of postpartum acute kidney injury. J. Int. Med Res. 2021, 49, 1–9. [Google Scholar] [CrossRef]
- Mohammad, N.; Qazi, Q.; Liaqat, N. Frequency of adverse perinatal outcomes in patients with pregnancy related acute renal (kidney) injury in a tertiary care hospital. Pak. J. Med. Sci. 2024, 40, 2267–2270. [Google Scholar] [CrossRef]
- Mal, P.; Ahsan, M.N.; Kumar, M.; Gurbukshani, S.; Fatima, A.; Khanzada, I. Acute Kidney Injury Due to Obstetric Complications. J. Coll. Physicians Surg. Pak. 2023, 33, 535–538. [Google Scholar] [CrossRef]
- Bokhari, S.R.A.; Inayat, F.; Jabeen, M.; Sardar, Z.; Saeed, S.; Malik, A.M.; Nasir, S.; Zareen, A.; Ahmad, H.I. Characteristics and Outcome of Obstetric Acute Kidney Injury in Pakistan: A Single-center Prospective Observational Study. Cureus 2018, 10, e3362. [Google Scholar] [CrossRef] [PubMed]
- Cooke, W.R.; Hemmilä, U.K.; Craik, A.L.; Mandula, C.J.; Mvula, P.; Msusa, A.; Dreyer, G.; Evans, R. Incidence, aetiology and outcomes of obstetric-related acute kidney injury in Malawi: A prospective observational study. BMC Nephrol. 2018, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.Z.; Shemies, R.S.; Baiomy, A.A.; Aladle, D.A.; Mosbah, A.; Abdel-Hady, E.S.; Sayed-Ahmed, N.; Sobh, M. Acute kidney injury during pregnancy and puerperium: An Egyptian hospital-based study. J. Nephrol. 2021, 34, 1611–1619. [Google Scholar] [CrossRef]
- Waziri, B.; Umar, I.A.; Magaji, A.; Umelo, C.C.; Nalado, A.M.; Wester, C.W.; Aliyu, M.H. Risk factors and outcomes associated with pregnancy-related acute kidney injury in a high-risk cohort of women in Nigeria. J. Nephrol. 2024, 37, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, O.A.; Akinbodewa, A.A.; Enikuomehin, O.C.; Lawal, O.M.; Abolarin, O.S.; Alli, O.E. Pregnancy-related acute kidney injury: Etiologies and short-term outcomes in a tertiary hospital in Southwest Nigeria. Saudi J. Kidney Dis. Transpl. 2019, 30, 1423–1430. [Google Scholar] [CrossRef]
- Orhewere, E.P.; Okoye, O.C.; Adejumo, O.A. Incidence of Pregnancy-Related Acute Kidney Injury in a Low Resource Setting: A Prospective Study. Niger. Med. J. 2023, 64, 627–636. [Google Scholar] [CrossRef]
- Berhe, E.; Teka, H.; Abraha, H.E.; Abera, B.T.; Gebru, M.A.; Gebremariam, T.; Yahya, M.; Amare, B.; Tadesse, H.; Gidey, H.; et al. Characteristics and outcome of pregnancy-related acute kidney injury in a teaching hospital in a low-resource setting: A five-year retrospective review. BMC Nephrol. 2024, 25, 182. [Google Scholar] [CrossRef]
- Meca, D.C.; Varlas, V.N.; Mehedințu, C.; Cîrstoiu, M.M. Correlations between Maternal and Fetal Outcomes in Pregnant Women with Kidney Failure. J. Clin. Med. 2023, 12, 832. [Google Scholar] [CrossRef]
- Hildebrand, A.M.; Liu, K.; Shariff, S.Z.; Ray, J.G.; Sontrop, J.M.; Clark, W.F.; Hladunewich, M.A.; Garg, A.X. Characteristics and Outcomes of AKI Treated with Dialysis during Pregnancy and the Postpartum Period. J. Am. Soc. Nephrol. 2015, 26, 3085–3091. [Google Scholar] [CrossRef]
- Pandey, A.; Azim, A.; Gautam, M.; Saran, S.; Ahmed, A.; Mishra, P.; Saxena, S. Etiology of Pregnancy-related Acute Kidney Injury among Obstetric Patients in India: A Systematic Review. Indian J. Crit. Care Med. 2022, 26, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Prakash, S.; Ganiger, V.C. Changing epidemiology of acute kidney injury in pregnancy: A journey of four decades from a developing country. Saudi J. Kidney Dis. Transpl. 2019, 30, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.R.; Conti-Ramsden, F. Acute kidney injury in pregnancy including renal disease diagnosed in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2019, 57, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Gupta, R.D.; Islam, N.; Das, A.; Shaha, A.K.; Khan, M.A.I.; Rahman, M.M. Pregnancy Related Acute Renal Failure in a Tertiary Care Hospital in Bangladesh. J. Med. 2012, 13, 129–132. [Google Scholar] [CrossRef]
- Trakarnvanich, T.; Ngamvichchukorn, T.; Susantitaphong, P. Incidence of acute kidney injury during pregnancy and its prognostic value for adverse clinical outcomes: A systematic review and meta-analysis. Medicine 2022, 101, E29563. [Google Scholar] [CrossRef]
- Pregnancy-Related Acute Renal Failure: A Ten-Year Experience—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21422644/ (accessed on 16 March 2025).
- da Silva, G.B.; De Francesco Daher, E.; Mota, R.M.S.; Menezes, F.A. Risk factors for death among critically ill patients with acute renal failure. Sao Paulo Med. J. 2006, 124, 257–263. [Google Scholar] [CrossRef]

| Newcastle–Ottawa Assessment Scale | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Compatibility | Outcome | Total | ||||||
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Start of Study | Assessment of Outcome | Adequacy of Duration of Follow-Up | Adequacy of Completeness of Follow-Up | |||
| Choudhary et al., 2024 [16] | √ | - | √ | √ | - | √ | √ | √ | 6/9 |
| Prakash et al., 2018 [17] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Sahay et al., 2022 [18] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Sandilya et al., 2023 [19] | √ | - | √ | √ | - | √ | √ | √ | 6/9 |
| Sachan et al., 2022 [20] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Mahesh et al., 2017 [21] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Eswarappa et al., 2016 [22] | √ | - | √ | √ | - | √ | √ | √ | 6/9 |
| Gopalakrishnan et al., 2015 [23] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Saini et al., 2020 [24] | √ | √ | √ | √ | √√ | √ | √ | √ | 9/9 |
| YADAV et al., 2022 [25] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Iqbal Anvar et al., 2023 [26] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Lu et al., 2023 [27] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Li et al., 2021 [28] | √ | √ | √ | √ | √√ | √ | √ | √ | 9/9 |
| Shu & Nie, 2021 [29] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Mohammad et al., 2024 [30] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Mal et al., 2023 [31] | √ | √ | √ | √ | - | √ | √ | √ | 7/9 |
| Bokhari et al., 2018 [32] | √ | - | √ | √ | √ | √ | - | √ | 6/9 |
| Cooke et al., 2018 [33] | √ | √ | √ | √ | √ | √ | √ | √ | 8/9 |
| Gaber et al., 2021 [34] | √ | - | √ | √ | √ | √ | √ | √ | 7/9 |
| Waziri et al., 2024 [35] | √ | √ | √ | √ | √√ | √ | √ | √ | 9/9 |
| Adejumo et al., 2019 [36] | √ | - | √ | √ | √ | √ | - | √ | 6/9 |
| Orhewere et al., 2023 [37] | √ | √ | √ | √ | √√ | √ | - | √ | 8/9 |
| Berhe et al., 2024 [38] | √ | √ | √ | √ | √√ | √ | - | √ | 8/9 |
| Meca et al., 2023 [39] | √ | - | √ | √ | √√ | - | √ | √ | 7/9 |
| Hildebrand et al., 2015 [40] | √ | √ | √ | √ | √√ | √ | √ | √ | 9/9 |
| Studies | Study Duration | Country | Design | No of Patients | Hemodialysis | Mean Age (Years) | Common Cause |
|---|---|---|---|---|---|---|---|
| (Choudhary et al., 2024) [16] | October/2021–September/2022 | India | Prospective | 62 | 39 | 25.08 ±4.25 |
|
| (Prakash et al., 2018) [17] | November/2014–July/2016 | India | Prospective | 132 | 62 | 26.8 ±4.8 |
|
| (Sahay et al., 2022) [18] | 10-year | India | Observational | 395 | 290 | 27 ±3 |
|
| (Sandilya et al., 2023) [19] | 2021–2022 | India | Prospective | 50 | 27 | 25.18 ±3.8 |
|
| (Sachan et al., 2022) [20] | June/2019–October/2020 | India | Prospective | 144 | 98 | 26.65 ±3.18 |
|
| (Mahesh et al., 2017) [21] | 2005–2014 | India | Prospective | 165 | 49 | 25 |
|
| (Eswarappa et al., 2016) [22] | 2005–2014 | India | Retrospective | 99 | 39 | 23 |
|
| (Gopalakrishnan et al., 2015) [23] | January/2010–December/2014 | India | Prospective | 150 | 96 | 25.4 ±4.73 |
|
| (Saini et al., 2020) [24] | January/2015–December/2016 | India | Prospective | 81 | 68 | Ν.A. |
|
| (YADAV et al., 2022) [25] | July/2015–August/2016 | India | Prospective | 51 | 14 | 29.5 |
|
| (Iqbal Anvar et al., 2023) [26] | May/2016- August/2020 | India | Retrospective | 70 | 34 | 24.56 ±4.2 |
|
| (Lu et al., 2023) [27] | January/2010–December/2020 | China | Retrospective | 31 | 13 | 29.16 ±4.97 |
|
| (Li et al., 2021) [28] | January/2015–December/2018 | China | Observational | 136 | N.A. | 27.7 ±5.6 |
|
| (Shu & Nie, 2021) [29] | January/2013–December/2017 | China | Retrospective | 37 | 20 | 29.65 ±6.70 |
|
| (Mohammad et al., 2024) [30] | August/2021–July/2022 | Pakistan | Prospective | 100 | 78 | 29.29 ±6.45 |
|
| (Mal et al., 2023) [31] | April-October/2023 | Pakistan | Observational | 60 | 51 | 28.67 ±5.41 |
|
| (Bokhari et al., 2018) [32] | 2018 | Pakistan | Prospective | 41 | 28 | 26 ±6 |
|
| (Cooke et al., 2018) [33] | September-December/2015 | Malawi | Prospective | 26 | 0 | 27 |
|
| (Gaber et al., 2021) [34] | December/2017–December/2019 | Egypt | Prospective | 40 | 15 | 28.7 ±5.9 |
|
| (Waziri et al., 2024) [35] | September/2019–July/2022 | Nigeria | Prospective | 113 | 14 | 28 ±6 |
|
| (Adejumo et al., 2019) [36] | 4-year period | Nigeria | Retrospective | 32 | 24 | 31.09 ±7.50 |
|
| (Orhewere et al., 2023) [37] | March-April/2020 | Nigeria | Prospective | 36 | 30 ±1.3 |
| |
| (Berhe et al., 2024) [38] | January/2017–December/2021 | Nigeria | Retrospective | 187 | 16 | 27 |
|
| (Meca et al., 2023) [39] | January/2019–December/2021 | Romania | Retrospective | 38 | 14 | 30.87 ±6.9 |
|
| (Hildebrand et al., 2015) [40] | 2015 | Canada | Retrospective | 188 | 188 | 25–35 |
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontomanolis, E.N.; Prokopakis, I.; Koutras, A.; Andreou, E.; Metaxas, D.; Boulieris, G.; Zachariou, E.; Sapantzoglou, I.; Papageorgiou, D.; Palios, V.-C.; et al. Pregnancy-Related Acute Kidney Injury: Causes and Its Impact on Perinatal Outcomes—A Systematic Review. J. Clin. Med. 2025, 14, 6031. https://doi.org/10.3390/jcm14176031
Kontomanolis EN, Prokopakis I, Koutras A, Andreou E, Metaxas D, Boulieris G, Zachariou E, Sapantzoglou I, Papageorgiou D, Palios V-C, et al. Pregnancy-Related Acute Kidney Injury: Causes and Its Impact on Perinatal Outcomes—A Systematic Review. Journal of Clinical Medicine. 2025; 14(17):6031. https://doi.org/10.3390/jcm14176031
Chicago/Turabian StyleKontomanolis, Emmanuel N., Ioannis Prokopakis, Antonios Koutras, Emmanouil Andreou, Dionysios Metaxas, Gerasimos Boulieris, Eleftherios Zachariou, Ioakeim Sapantzoglou, Dimitrios Papageorgiou, Vasileios-Chrysovalantis Palios, and et al. 2025. "Pregnancy-Related Acute Kidney Injury: Causes and Its Impact on Perinatal Outcomes—A Systematic Review" Journal of Clinical Medicine 14, no. 17: 6031. https://doi.org/10.3390/jcm14176031
APA StyleKontomanolis, E. N., Prokopakis, I., Koutras, A., Andreou, E., Metaxas, D., Boulieris, G., Zachariou, E., Sapantzoglou, I., Papageorgiou, D., Palios, V.-C., Karachalios, C., Papadimitriou, A., Daglas, K., Chionis, A., Lagadas, A., & Perros, P. (2025). Pregnancy-Related Acute Kidney Injury: Causes and Its Impact on Perinatal Outcomes—A Systematic Review. Journal of Clinical Medicine, 14(17), 6031. https://doi.org/10.3390/jcm14176031