Quantum Cardiovascular Medicine: From Hype to Hope—A Critical Review of Real-World Applications
Abstract
1. Introduction
2. Methods
2.1. Strategy for Literature Search
2.1.1. Database Selection
2.1.2. Search Terms and Strategy
2.1.3. Search Parameters
2.2. Study Selection Process
2.2.1. Screening Procedures
2.2.2. Inclusion Criteria
2.2.3. Exclusion Criteria
2.2.4. Selection Results
2.3. Data Extraction and Quality Assessment
2.3.1. Data Extraction Framework
2.3.2. Quality Assessment
2.4. Data Synthesis and Analysis
Synthesis Approach
2.5. Limitations and Bias Mitigation
2.5.1. Recognized Limitations
2.5.2. Bias Reduction
2.5.3. PRISMA 2020 Caveats
3. Quantum Sensing Technologies in Cardiovascular Medicine
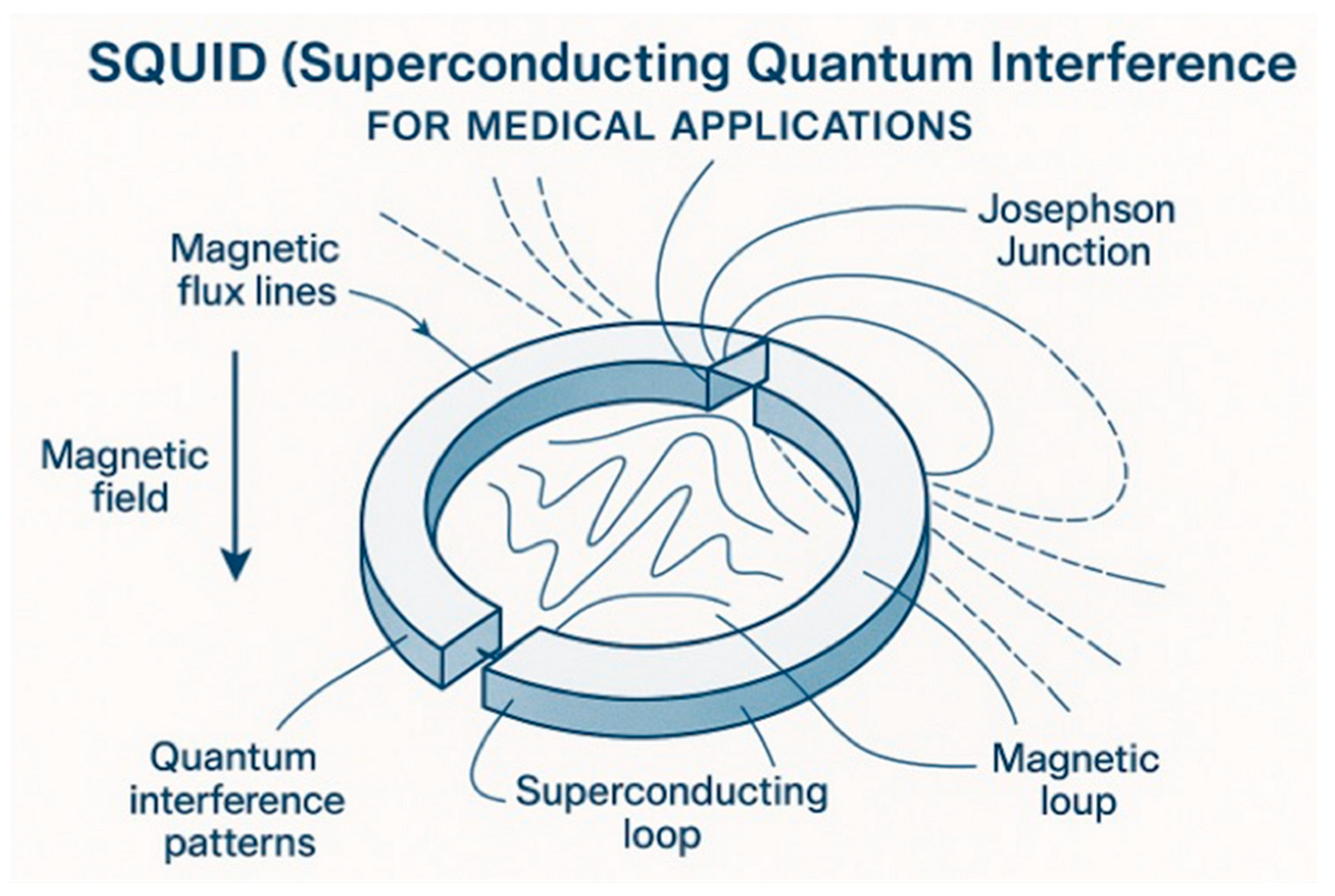
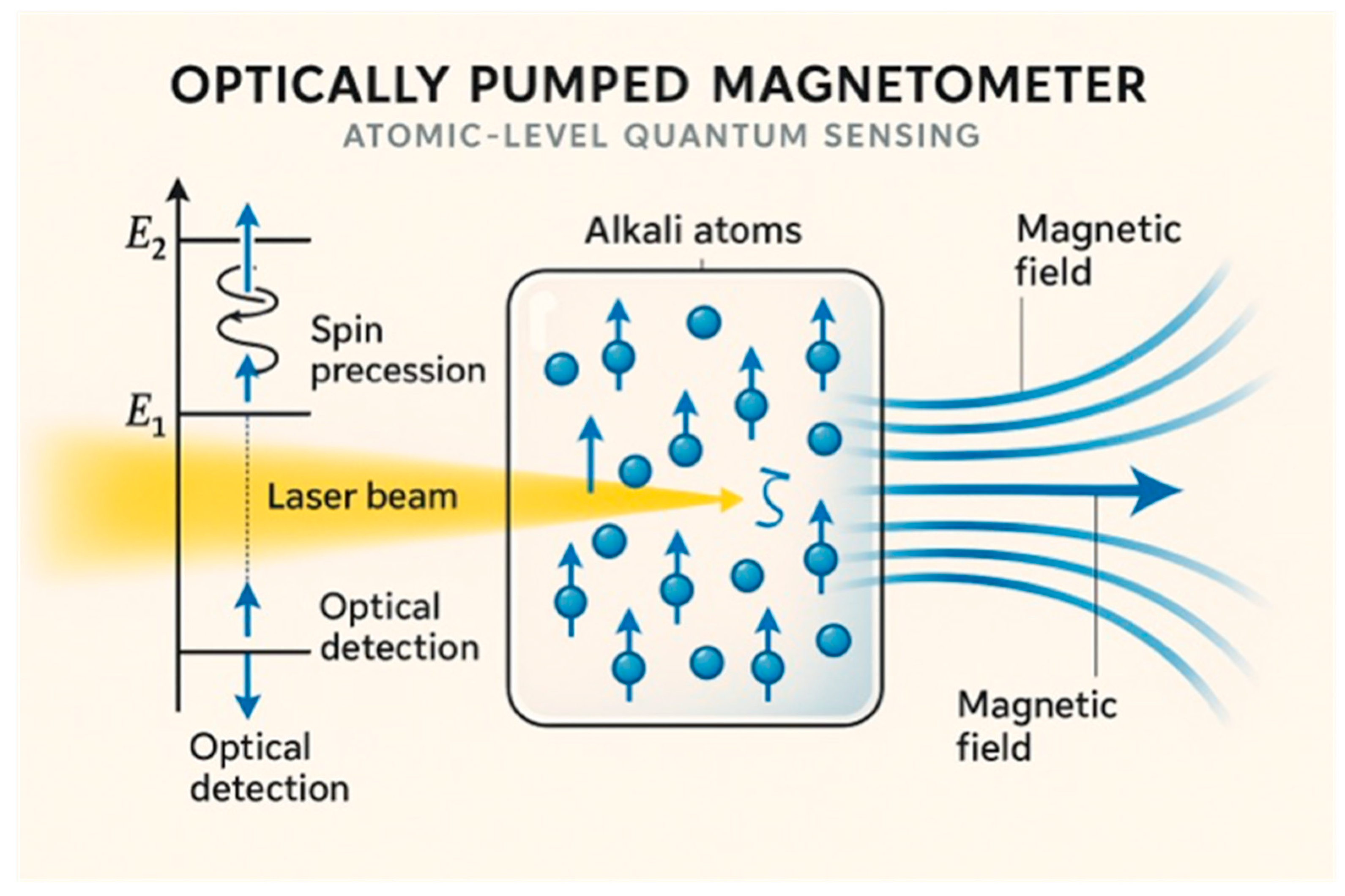
3.1. Fundamental Principles and Technological Platforms
3.2. Clinical Applications and Real-World Implementations
4. Quantum Machine Learning in Cardiovascular Risk Prediction and Diagnosis
4.1. Theoretical Foundations and Quantum Advantages
4.2. Clinical Applications and Performance Evaluation
4.3. Limitations and Challenges in Clinical Implementation
5. Quantum-Enhanced Drug Discovery and Therapeutic Applications
5.1. Quantum Computing in Cardiovascular Drug Development
5.2. Quantum Molecular Modeling and Protein–Drug Interactions
6. Current Challenges and Limitations
6.1. Technical and Hardware Limitations
6.2. Clinical Validation and Regulatory Challenges
6.3. Economic and Accessibility Considerations
7. Future Directions and Emerging Opportunities
7.1. Next-Generation Quantum Sensing Platforms
7.2. Quantum-Enhanced Personalized Medicine
7.3. Quantum Biology and Fundamental Understanding
8. Emerging Ideas/Controversies
8.1. Quantum Biology
8.2. Quantum Machine Learning at the Bedside
8.3. Future Sensing Platforms and Personalization
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CardiAQ | Cardiac Quantum (diagnostic platform) |
| CC BY | Creative Commons Attribution |
| CIED | Cardiac Implantable Electronic Device |
| CMR | Cardiac Magnetic Resonance |
| CNN | Convolutional Neural Network |
| CQS | Cardiac Quantum Spectrum |
| CT | Computed Tomography |
| CVD | Cardiovascular Disease |
| DL | Deep Learning |
| ECG | Electrocardiogram |
| fT/√Hz | Femtoteslas per square root hertz |
| GBD | Global Burden of Disease |
| GHz | Gigahertz |
| ICD | Implantable Cardioverter-Defibrillator |
| kT | Boltzmann constant times temperature |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LSTM | Long Short-Term Memory |
| MCG | Magnetocardiography |
| meV | Millielectron volt |
| MRI | Magnetic Resonance Imaging |
| ML | Machine Learning |
| NND | Number Needed to Diagnose |
| NNS | Number Needed to Screen |
| NISQ | Noisy Intermediate-Scale Quantum |
| NV | Nitrogen-Vacancy |
| OPM | Optically Pumped Magnetometer |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| QALY | Quality-Adjusted Life Year |
| QCBM | Quantum Circuit Born Machine |
| QML | Quantum Machine Learning |
| QuEL | Quantum-Enhanced Learning |
| SQUID | Superconducting Quantum Interference Device |
| VQE | Variational Quantum Eigensolver |
References
- World Health Organization. Cardiovascular Diseases (CVDs); Internet; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 22 June 2025).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 25. [Google Scholar] [CrossRef] [PubMed]
- Biamonte, J.; Wittek, P.; Pancotti, N.; Rebentrost, P.; Wiebe, N.; Lloyd, S. Quantum machine learning. Nature 2017, 549, 195–202. [Google Scholar] [CrossRef]
- Wang, J.; He, F.; Sun, S. Construction of a new smooth support vector machine model and its application in heart disease diagnosis. PLoS ONE 2023, 18, e0280804. [Google Scholar] [CrossRef]
- Barry, J.F.; Schloss, J.M.; Bauch, E.; Turner, M.J.; Hart, C.A.; Pham, L.M.; Walsworth, R.L. Sensitivity Optimization for NV-Diamond Magnetometry. Rev. Mod. Phys. 2020, 92, 015004. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Newcastle-Ottawa Quality Assessment Form for Cohort Studies. n.d. Available online: https://www.google.com/url?sa=i&url=https%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fbooks%2FNBK115843%2Fbin%2Fappe-fm3.pdf&psig=AOvVaw13n6U7mXyU6kiP_y61cQc8&ust=1755783180184000&source=images&cd=vfe&opi=89978449&ved=0CAYQrpoMahcKEwiwtq6XwJmPAxUAAAAAHQAAAAAQBA (accessed on 2 June 2025).
- Ling, S.J.; Moebs, W.; Sanny, J. Superconductors. In University Physics; OpenStax: Houston, TX, USA, 2016; Volume 2. [Google Scholar]
- Budker, D.; Romalis, M. Optical Magnetometry. Nat. Phys. 2006, 3, 227–234. [Google Scholar] [CrossRef]
- Aslam, N.; Zhou, H.; Urbach, E.K. Quantum sensors for biomedical applications. Nat. Rev. Phys. 2023, 5, 157–169. [Google Scholar] [CrossRef]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Sternickel, K.; Braginski, A.I. Biomagnetism using SQUIDs: Status and perspectives. Supercond. Sci. Technol. 2006, 19, S160. [Google Scholar] [CrossRef]
- Her, A.Y.; Dischl, D.; Kim, Y.H.; Kim, S.W.; Shin, E.S. Magnetocardiography for the detection of myocardial ischemia. Front. Cardiovasc. Med. 2023, 10, 1242215. [Google Scholar] [CrossRef]
- Pena, M.E.; Pearson, C.L.; Goulet, M.P.; Kazan, V.M.; DeRita, A.L.; Szpunar, S.M.; Dunne, R.B. A 90-second magnetocardiogram using a novel analysis system to assess for coronary artery stenosis in Emergency department observation unit chest pain patients. IJC Heart Vasc. 2020, 26, 100466. [Google Scholar] [CrossRef]
- Özkartal, T.; Demarchi, A.; Caputo, M.L.; Baldi, E.; Conte, G.; Auricchio, A. Perioperative Management of Patients with Cardiac Implantable Electronic Devices and Utility of Magnet Application. J. Clin. Med. 2022, 11, 691. [Google Scholar] [CrossRef]
- Suwalski, P.; Wilke, F.; Latinova, E.; Paci, G.; Satilmis, G.; Klingel, K.; Kelle, S.; Weiner, J., 3rd; Beule, D.; Lüscher, T.F.; et al. The MagMa Study: Quantum Magnetocardiography in Non-Ischemic Cardiomyopathy. medRxiv 2025. [Google Scholar] [CrossRef]
- Babu, S.V.; Ramya, P.; Gracewell, J. Revolutionizing heart disease prediction with quantum-enhanced machine learning. Sci. Rep. 2024, 14, 7453. [Google Scholar] [CrossRef]
- Smith, F.E.; Langley, P.; van Leeuwen, P.; Hailer, B.; Trahms, L.; Steinhoff, U.; Brouke, J.P.; Murray, A. Comparison of magnetocardiography and electrocardiography: A study of automatic measurement of dispersion of ventricular repolarization. Europace 2006, 8, 887–893. [Google Scholar] [CrossRef]
- New Medical Services and New Technologies; Centers for Medicare & Medicaid Services: Baltimore, MD, USA, 2025.
- Larner, A.J. Number Needed to Diagnose, Predict, or Misdiagnose: Useful Metrics for Non-Canonical Signs of Cognitive Status? Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Khan, M.S.; Mori, C.; Filippatos, G.S.; Ponikowski, P.; Comin-Colet, J.; Roubert, B.; Spertus, J.A.; Anker, S.D. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur. J. Heart Fail. 2020, 22, 999–1005. [Google Scholar] [CrossRef]
- Spertus, J.A.; Jones, P.G.; Sandhu, A.T.; Arnold, S.V. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhanka, S.; Sharma, A.; Bansal, R.; Fahlevi, M.; Rabby, F.; Aljuaid, M. A hybrid framework for heart disease prediction using classical and quantum-inspired machine learning techniques. Sci. Rep. 2025, 15, 25040. [Google Scholar] [CrossRef]
- Gupta, R.S.; Wood, C.E.; Engstrom, T.; Pole, J.D.; Shrapnel, S. Quantum Machine Learning for Digital Health? A Systematic Review. npj Digit. Med. 2025, 8, 237. [Google Scholar] [CrossRef]
- Aaronson, S. The Limits of Quantum Computers. Sci. Am. Mag. 2008, 298. [Google Scholar] [CrossRef]
- Chow, J.C.L. Quantum Computing in Medicine. Med. Sci. 2024, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Romero, J.; Olson, J.P.; Degroote, M.; Johnson, P.D.; Kieferová, M.; Kivlichan, I.D.; Menke, T.; Peropadre, B.; Sawaya, N.P.; et al. Quantum Chemistry in the Age of Quantum Computing. Chem. Rev. 2019, 119, 10856–10915. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Vakili, M.; Gorgulla, C.; Snider, J.; Nigam, A.; Bezrukov, D.; Varoli, D.; Aliper, A.; Polykovsky, D.; Padmanabha Das, K.M.; Cox, H., III; et al. Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors. Nat. Biotechnol. 2025, 1–6. [Google Scholar] [CrossRef]
- McArdle, S.; Endo, S.; Aspuru-Guzik, A.; Benjamin, S.; Yuan, X. Quantum computational chemistry. Rev. Mod. Phys. 2020, 92, 015003. [Google Scholar] [CrossRef]
- Peruzzo, A.; McClean, J.; Shadbolt, P.; Yung, M.H.; Zhou, X.Q.; Love, P.J. A variational eigenvalue solver on a photonic quantum processor. Nat. Commun. 2014, 5, 4213. [Google Scholar] [CrossRef]
- Reiher, M.; Wiebe, N.; Svore, K.M.; Wecker, D.; Troyer, M. Elucidating reaction mechanisms on quantum computers. Proc. Natl. Acad. Sci. USA 2017, 114, 7555–7560. [Google Scholar] [CrossRef]
- Aspuru-Guzik, A.; Dutoi, A.D.; Love, P.J.; Head-Gordon, M. Chemistry: Simulated quantum computation of molecular energies. Science 2005, 309, 1704–1707. [Google Scholar] [CrossRef]
- Tuckerman, M.E. Ab initio molecular dynamics: Basic concepts, current trends and novel applications. J. Phys. Condens. Matter 2002, 14, R1297. [Google Scholar] [CrossRef]
- Benedetti, M.; García-Pintos, D.; Perdomo, O.; Leyton-Ortega, V.; Nam, Y.; Perdomo-Ortiz, A. A generative modeling approach for benchmarking and training shallow quantum circuits. npj Quantum Inf. 2019, 5, 45. [Google Scholar] [CrossRef]
- Preskill, J. Quantum Computing in the NISQ era and beyond. Quantum 2018, 2, 79. [Google Scholar] [CrossRef]
- Rembold, C.M. Number needed to screen: Development of a statistic for disease screening. Br. Med. J. 1998, 317, 307–312. [Google Scholar] [CrossRef]
- Leeflang, M.M.G.; Bossuyt, P.M.M.; Irwig, L. Diagnostic test accuracy may vary with prevalence: Implications for evidence-based diagnosis. J. Clin. Epidemiol. 2009, 62, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, D.; Fenici, P.; Fenici, R. Clinical magnetocardiography: The unshielded bet—Past, present, and future. Front. Cardiovasc. Med. 2023, 10, 1232882. [Google Scholar] [CrossRef]
- Strand, S.; Lutter, W.; Strasburger, J.F.; Shah, V.; Baffa, O.; Wakai, R.T. Low-Cost Fetal Magnetocardiography: A Comparison of Superconducting Quantum Interference Device and Optically Pumped Magnetometers. J. Am. Heart Assoc. 2019, 8, 116833. [Google Scholar] [CrossRef]
- Barratt, A.; Irwig, L.; Glasziou, P.; Cumming, R.G.; Raffle, A.; Hicks, N.; Gray, J.M.; Guyatt, G.H. Users’ Guides to the Medical Literature XVII. How to Use Guidelines and Recommendations About Screening. JAMA 1999, 281, 2029–2034. [Google Scholar] [CrossRef]
- Mushlin, A.I.; Ruchlin, H.S.; Callahan, M.A. Cost-effectiveness of diagnostic tests. Lancet 2001, 358, 1353–1355. [Google Scholar] [CrossRef]
- Schuld, M.; Sinayskiy, I.; Petruccione, F. The quest for a Quantum Neural Network. Quantum Inf. Process. 2014, 13, 2567–2586. [Google Scholar] [CrossRef]
- Eze-Nliam, C.M.; Zhang, Z.; Weiss, S.A.; Weintraub, W.S. Cost-effectiveness assessment of cardiac interventions: Determining a socially acceptable cost threshold. Interv. Cardiol. 2014, 6, 45–55. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef]
- Chen, J.H.; Asch, S.M. Machine Learning and Prediction in Medicine—Beyond the Peak of Inflated Expectations. N. Engl. J. Med. 2017, 376, 2507–2509. [Google Scholar] [CrossRef] [PubMed]
- Awschalom, D.D.; Hanson, R.; Wrachtrup, J.; Zhou, B.B. Quantum technologies with optically interfaced solid-state spins. Nat. Photonics 2018, 12, 516–527. [Google Scholar] [CrossRef]
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef]
- Ismail, M.I.; Qaswal, A.B.; Ali, M.A.B.; Hamdan, A.; Alghrabli, A.; Harb, M.; Ibrahim, D.; Al-Jbour, M.N.; Almobaiden, I.; Alrowwad, K.; et al. Quantum mechanical aspects of cardiac arrhythmias: A mathematical model and pathophysiological implications. AIMS Biophys. 2023, 10, 401–439. [Google Scholar] [CrossRef]
- Ababneh, O.; Qaswal, A.B.; Alelaumi, A.; Khreesha, L.; Almomani, M.; Khrais, M.; Khrais, O.; Suleihat, A.; Mutleq, S.; Al-olaimat, Y.; et al. Proton Quantum Tunneling: Influence and Relevance to Acidosis-Induced Cardiac Arrhythmias/Cardiac Arrest. Pathophysiology 2021, 28, 400–436. [Google Scholar] [CrossRef]
- Hille, B. Ionic Channels in Excitable Membranes Current Problems and Biophysical Approaches. Biophys. J. 1978, 22, 283–294. [Google Scholar] [CrossRef]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef]
- Engel, G.S.; Calhoun, T.R.; Read, E.L.; Ahn, T.K.; Mančal, T.; Cheng, Y.C.; Blankenship, R.E.; Fleming, G.R. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 2007, 446, 782–786. [Google Scholar] [CrossRef]
- Ritz, T.; Adem, S.; Schulten, K. A Model for Photoreceptor-Based Magnetoreception in Birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef] [PubMed]
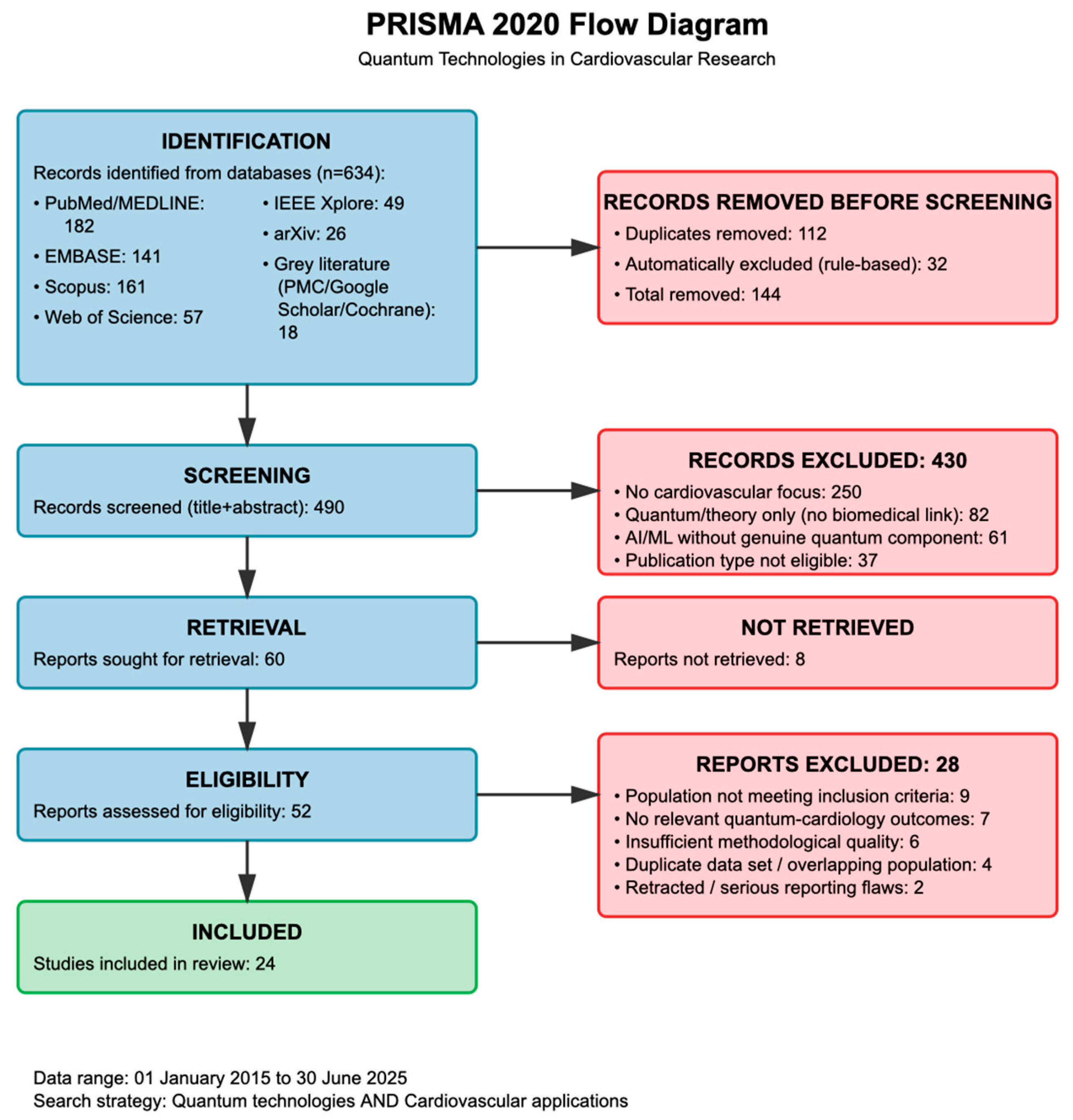

| Inclusion Criteria | Exclusion Criteria | Frequent Reasons for Exclusion |
|---|---|---|
|
|
|
| Domain | Quantum Technology Example | Classical/AI Equivalent | Quantum Advantage | Current Limitations/ Stage |
|---|---|---|---|---|
| Sensing | SQUID-based MCG 1; OPM 2; NV centers 3 | ECG, MRI, CT | Ultra-high sensitivity (fT range) 4; non-contact measurement, direct cardiac magnetic field detection | High cost (USD 100 k–1 M), specialized infrastructure, limited clinical validation studies |
| Machine Learning | Quantum–classical hybrid models for risk prediction | Classical ML/DL (Random Forests, CNNs, Transformers) | Marginal accuracy gains (typically ≤ 1%; occasionally higher in lab settings); faster training | Minimal clinical benefit; training speed does not translate to patient outcomes; NISQ and hardware constraints |
| Molecular Simulation | Variational Quantum Eigensolver (VQE), QCBM | Classical molecular dynamics, density functional theory | Potential for accurate simulation of complex molecular interactions; theoretical advantages for drug-target modeling | Experimental phase; no validated clinical applications; exceeds current quantum hardware capabilities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomala, M.; Kłaczyński, M. Quantum Cardiovascular Medicine: From Hype to Hope—A Critical Review of Real-World Applications. J. Clin. Med. 2025, 14, 6029. https://doi.org/10.3390/jcm14176029
Tomala M, Kłaczyński M. Quantum Cardiovascular Medicine: From Hype to Hope—A Critical Review of Real-World Applications. Journal of Clinical Medicine. 2025; 14(17):6029. https://doi.org/10.3390/jcm14176029
Chicago/Turabian StyleTomala, Marek, and Maciej Kłaczyński. 2025. "Quantum Cardiovascular Medicine: From Hype to Hope—A Critical Review of Real-World Applications" Journal of Clinical Medicine 14, no. 17: 6029. https://doi.org/10.3390/jcm14176029
APA StyleTomala, M., & Kłaczyński, M. (2025). Quantum Cardiovascular Medicine: From Hype to Hope—A Critical Review of Real-World Applications. Journal of Clinical Medicine, 14(17), 6029. https://doi.org/10.3390/jcm14176029







