Effects of Dance-Based Aerobic Training on Functional Capacity and Risk of Falls in Older Adults with Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Sample Calculation
2.3. Randomization
2.4. Intervention
2.5. Outcomes
2.5.1. Muscle Strength
2.5.2. Flexibility
2.5.3. Balance
2.5.4. Gait Speed
2.6. Statistical Analysis
3. Results
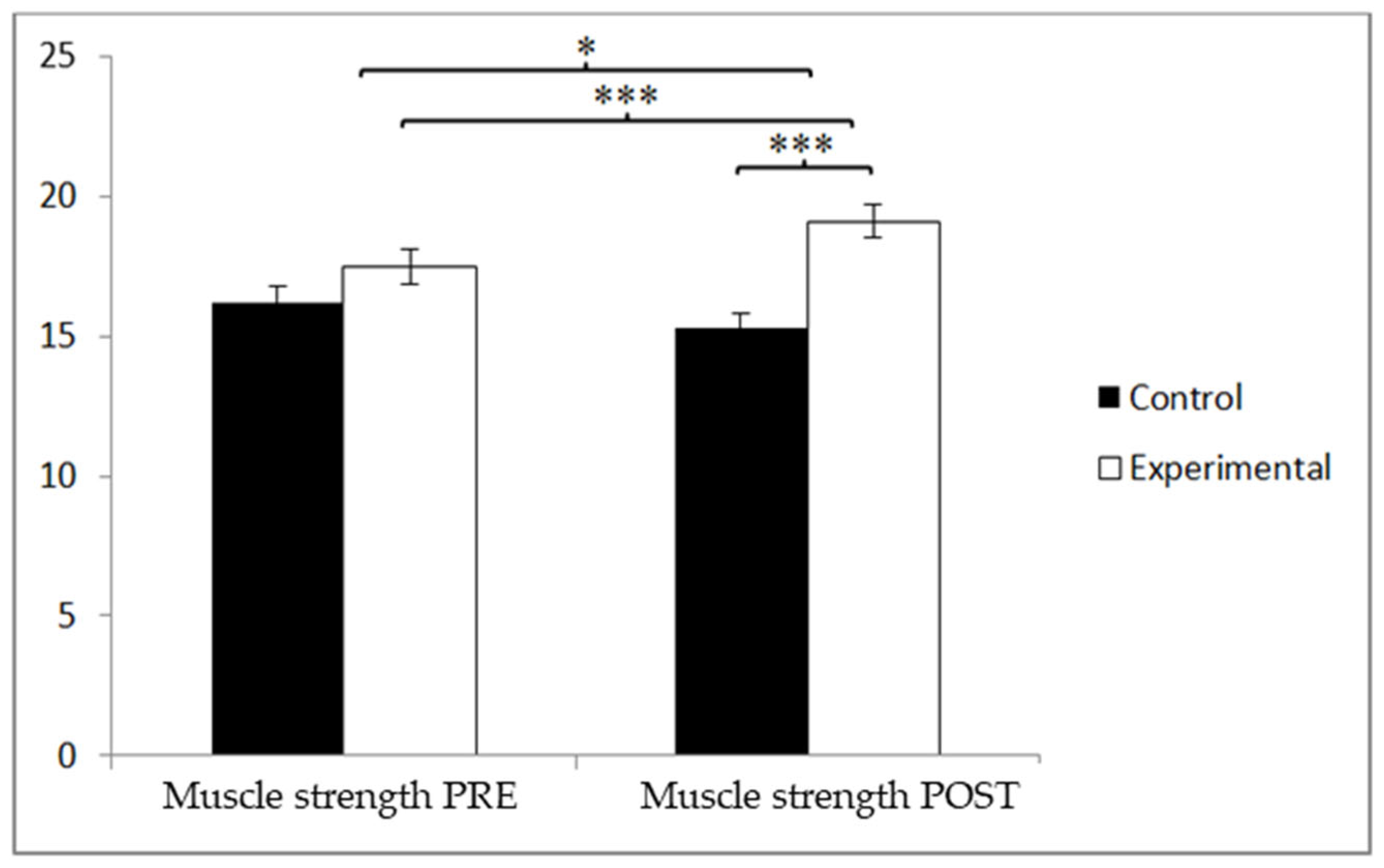
3.1. Muscle Strength
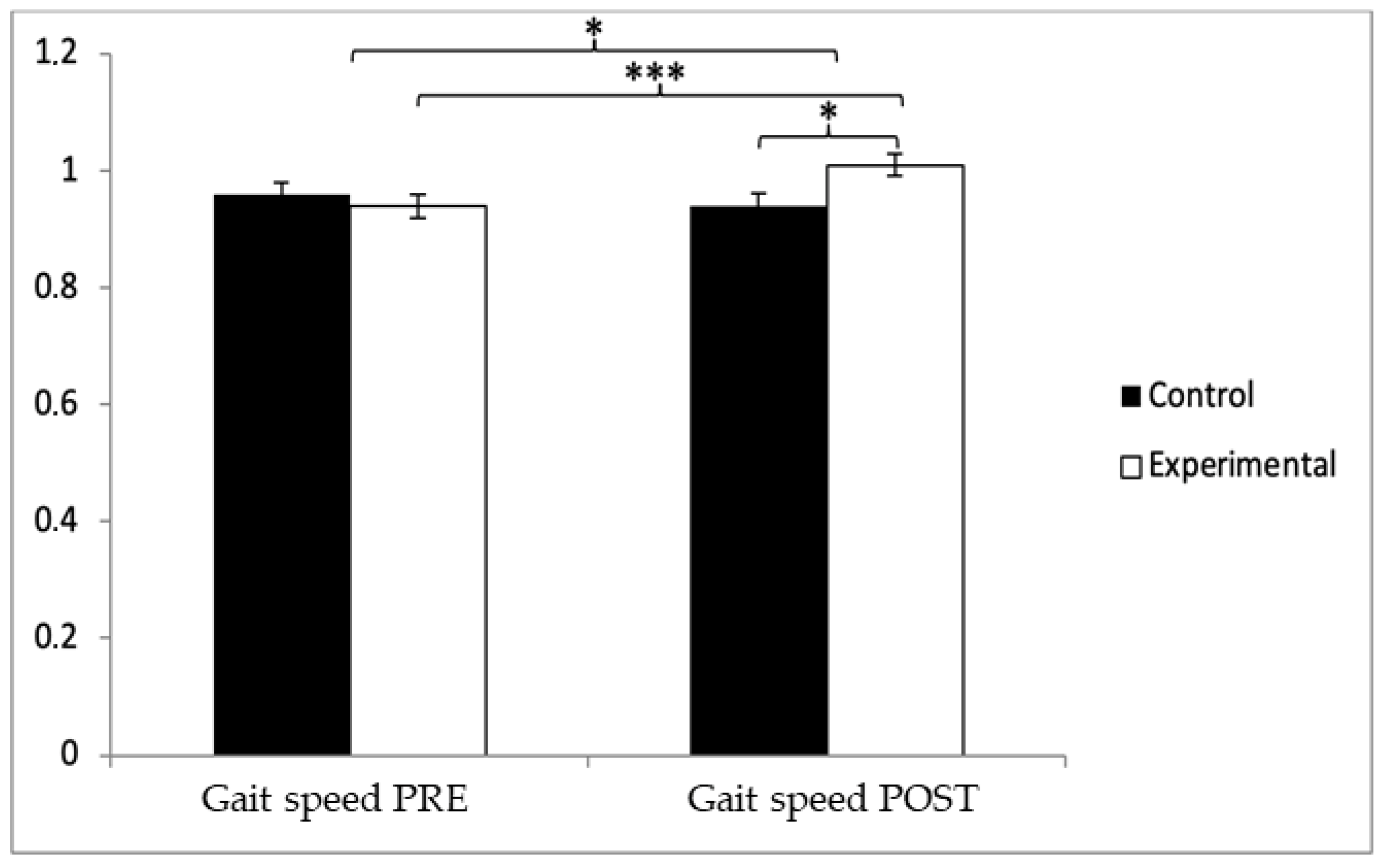
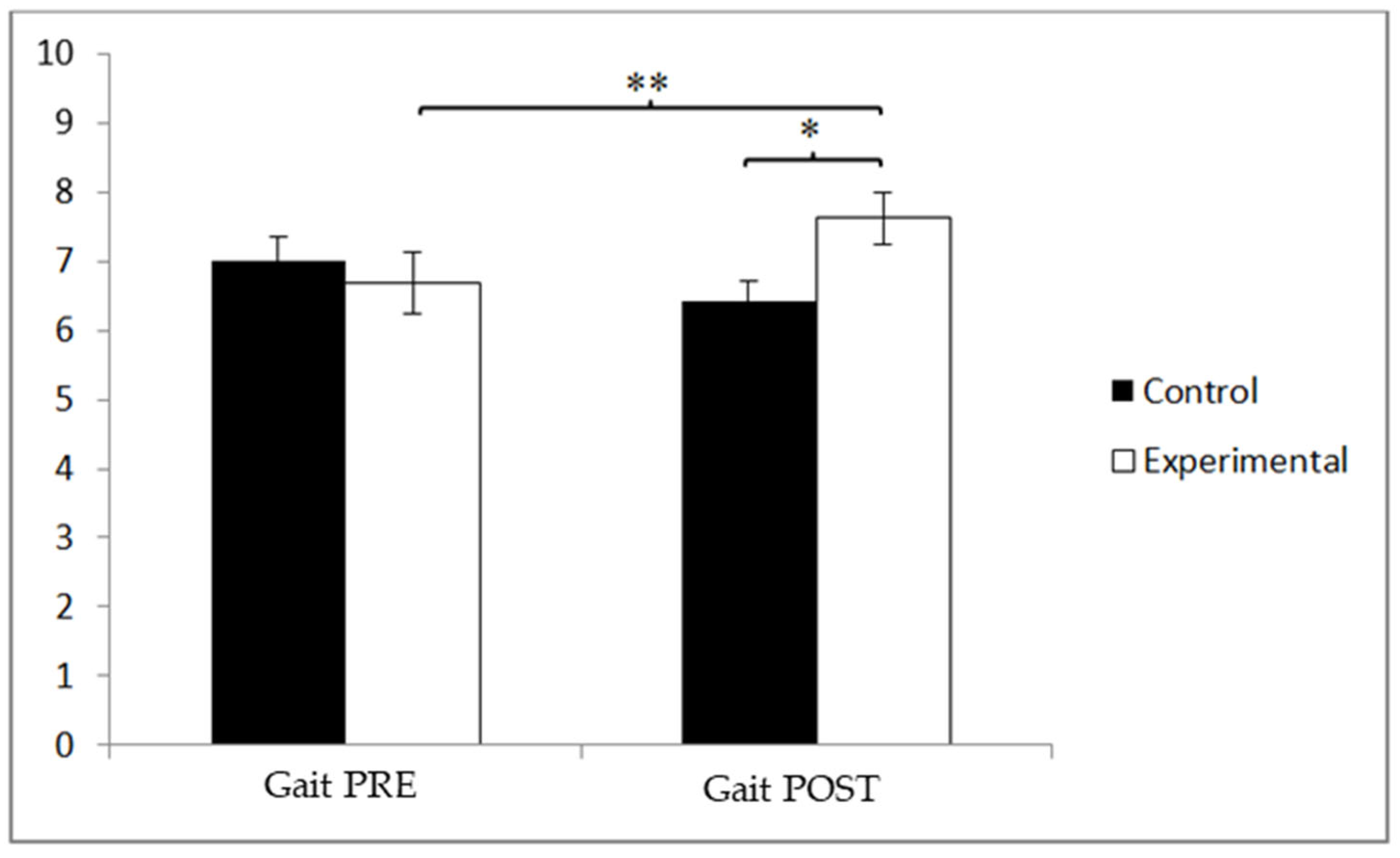
3.2. Gait Speed
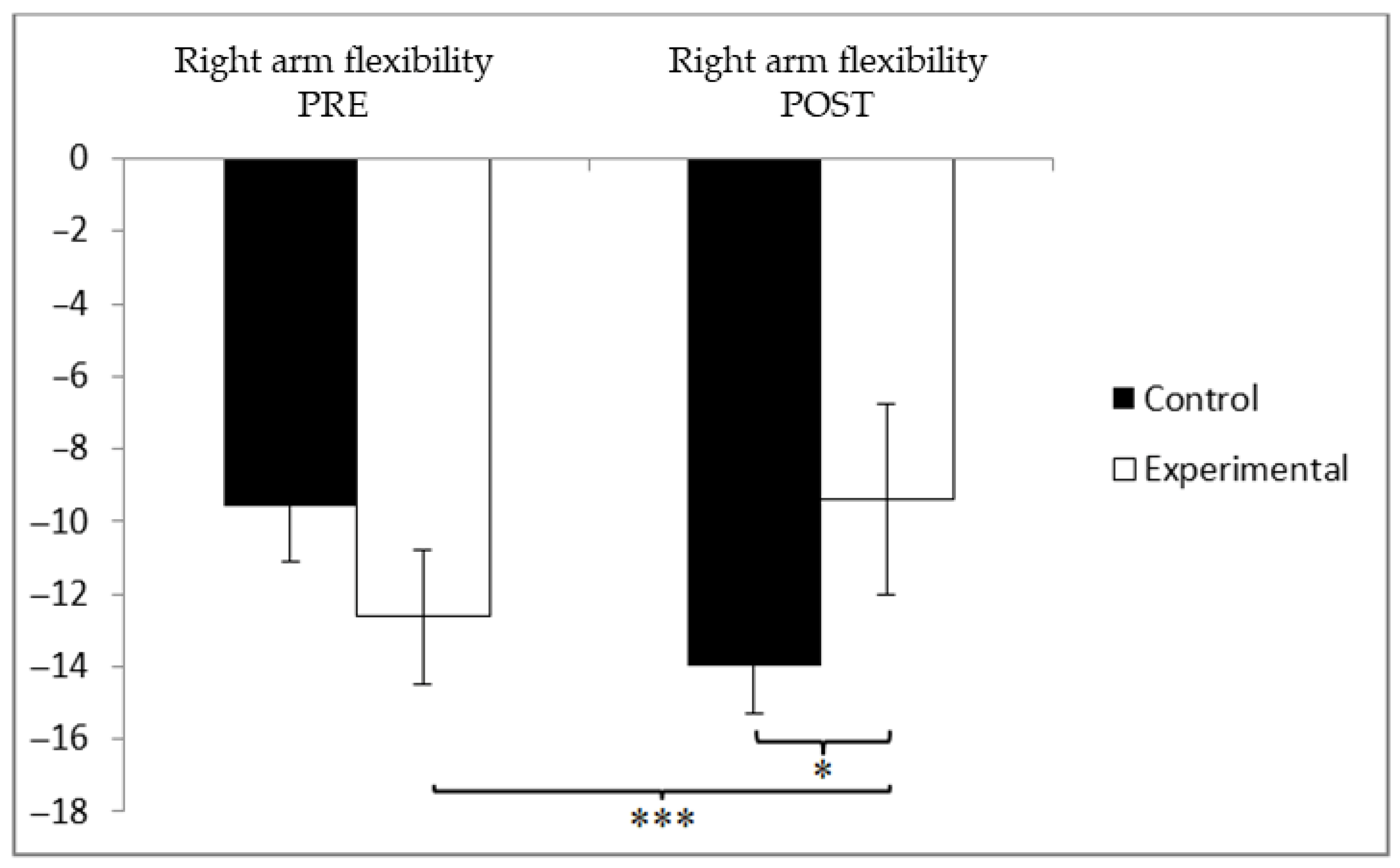
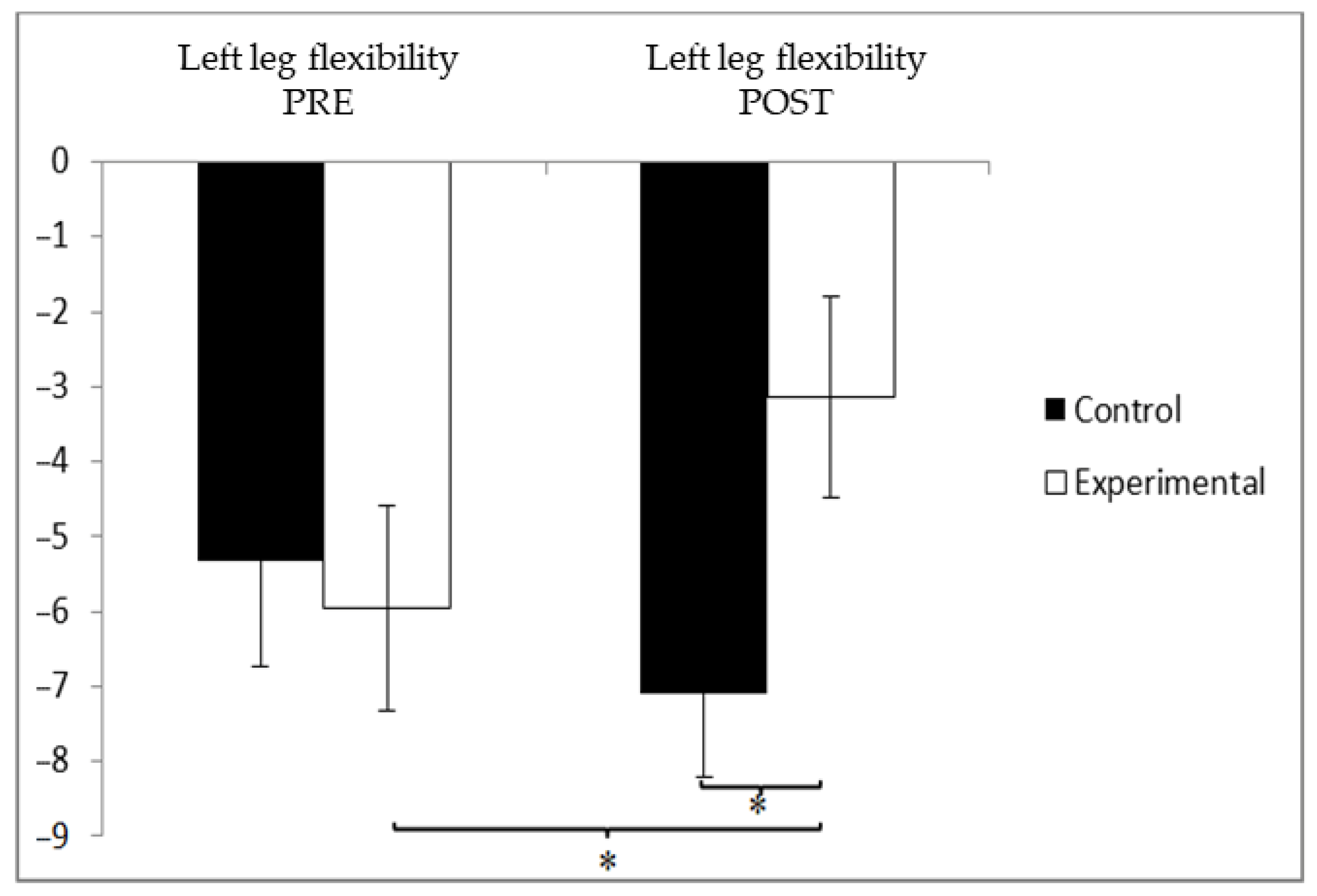
3.3. Flexibility
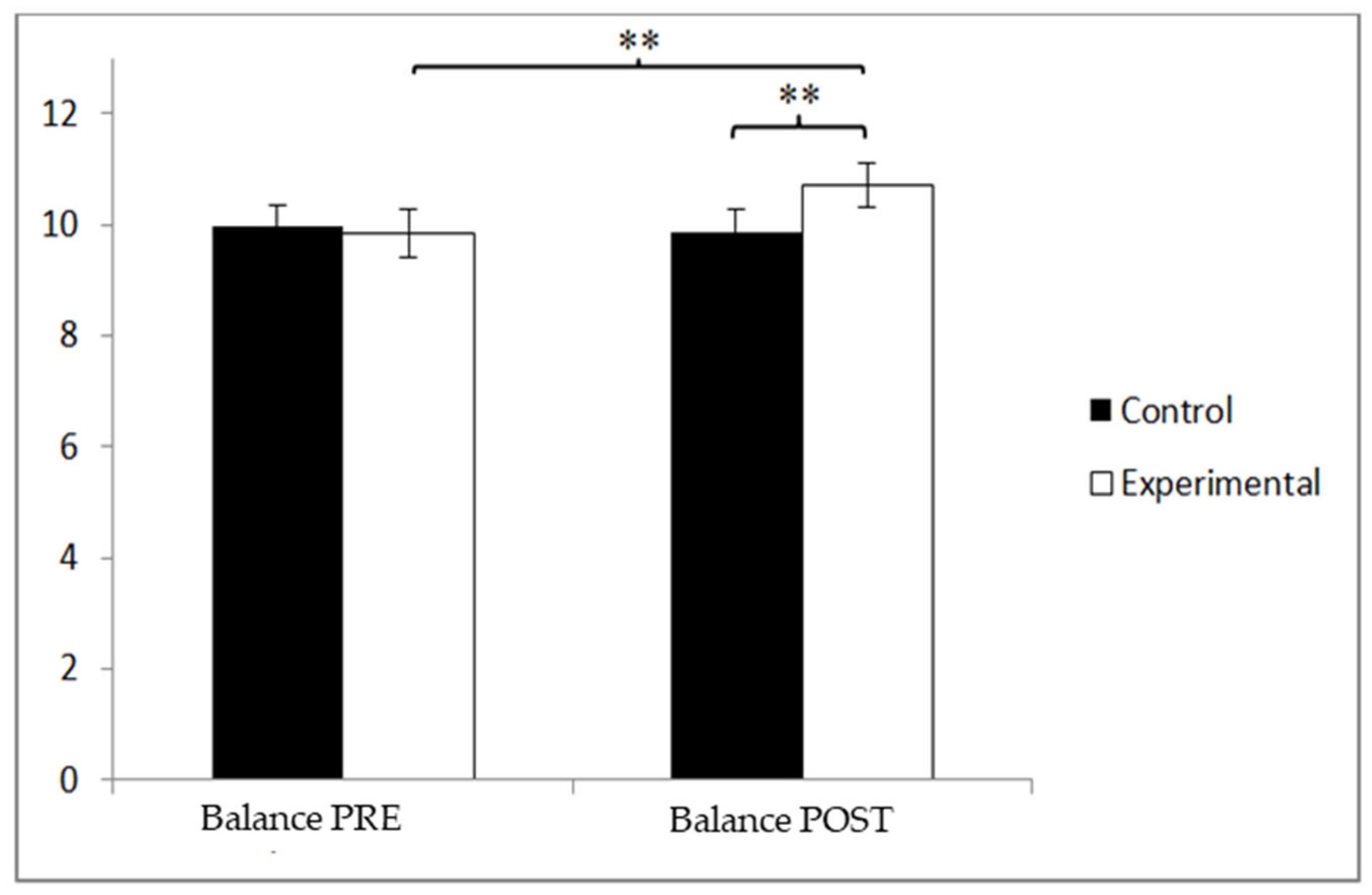
3.4. Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public Health 2021, 50, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 4 April 2025).
- National Research Council (US) Panel on a Research Agenda and New Data for an Aging World. The Health of Aging Populations. In Preparing for an Aging World: The Case for Cross-National Research; National Academies Press (US): Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK98373/ (accessed on 5 April 2025).
- Cai, Y.; Han, Z.; Cheng, H.; Li, H.; Wang, K.; Chen, J.; Liu, Z.X.; Xie, Y.; Lin, Y.; Zhou, S.; et al. The Impact of Ageing Mechanisms on Musculoskeletal System Diseases in the Elderly. Front. Immunol. 2024, 15, 1405621. [Google Scholar] [CrossRef]
- Geda, Y.E. Mild Cognitive Impairment in Older Adults. Curr. Psychiatry Rep. 2012, 14, 320–327. [Google Scholar] [CrossRef]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental Pollutants as Risk Factors for Neurodegenerative Disorders: Alzheimer and Parkinson Diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef]
- Doğancı, O.; Sertel, M. Determination of Balance, Fall Risk, and Kinesiophobia in Individuals with Alzheimer’s Dementia. Front. Psychol. 2025, 16, 1535440. [Google Scholar] [CrossRef]
- Smith, L.; Sánchez, G.F.L.; Shin, J.I.; Oh, H.; Kostev, K.; Tully, M.A.; Barnett, Y.; Butler, L.T.; Veronese, N.; Soysal, P.; et al. The Association between Physical Multimorbidity and Fall-Related Injury among Adults Aged > 50 Years from Low- and Middle-Income Countries. Eur. J. Ageing 2025, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Rondão, C.A.M.; Mota, M.P.; Oliveira, M.M.; Peixoto, F.; Esteves, D. Multicomponent Exercise Program Effects on Fitness and Cognitive Function of Elderlies with Mild Cognitive Impairment: Involvement of Oxidative Stress and BDNF. Front. Aging Neurosci. 2022, 14, 950937. [Google Scholar] [CrossRef]
- Kirk-Sanchez, N.; McGough, E. Physical Exercise and Cognitive Performance in the Elderly: Current Perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef]
- Committee on Fitness Measures and Health Outcomes in Youth; Food and Nutrition Board; Institute of Medicine; Pate, R.; Oria, M.; Pillsbury, L. (Eds.) Health-Related Fitness Measures for Youth: Flexibility. In Fitness Measures and Health Outcomes in Youth; National Academies Press (US): Washington, DC, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK241323/ (accessed on 6 April 2025).
- Institute of Medicine (US) Division of Health Promotion and Disease Prevention; Berg, R.L.; Cassells, J.S. (Eds.) Falls in Older Persons: Risk Factors and Prevention. In The Second Fifty Years: Promoting Health and Preventing Disability; National Academies Press (US): Washington, DC, USA, 1992. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235613/ (accessed on 6 April 2025).
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; A Michaleff, Z.; Howard, K.; Clemson, L.; Hopewell, S.; E Lamb, S. Exercise for Preventing Falls in Older People Living in the Community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; van der Velde, N.; Martin, F.C.; Petrovic, M.; Tan, M.P.; Ryg, J.; Aguilar-Navarro, S.; Alexander, N.B.; Becker, C.; Blain, H.; et al. World Guidelines for Falls Prevention and Management for Older Adults: A Global Initiative. Age Ageing 2022, 51, afac205. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. [Google Scholar] [CrossRef]
- Rondão, C.A.; Mota, M.P.; Esteves, D. Physical Activity Interventions in Older Adults with a Cognitive Impairment: A Critical Review of Reviews. Aging Med. 2023, 6, 290–306. [Google Scholar] [CrossRef]
- Fong Yan, A.; Nicholson, L.L.; Ward, R.E.; Hiller, C.E.; Dovey, K.; Parker, H.M.; Low, L.-F.; Moyle, G.; Chan, C. The Effectiveness of Dance Interventions on Psychological and Cognitive Health Outcomes Compared with Other Forms of Physical Activity: A Systematic Review with Meta-analysis. Sports Med. 2024, 54, 1179–1205. [Google Scholar] [CrossRef]
- Norouzi, E.; Hosseini, F.; Vaezmosavi, M.; Gerber, M.; Pühse, U.; Brand, S. Zumba Dancing and Aerobic Exercise Can Improve Working Memory, Motor Function, and Depressive Symptoms in Female Patients with Fibromyalgia. Eur. J. Sport Sci. 2020, 20, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Yhun-Lo, C.; Wolff, L.; Wang, J.; Olsen, K.N.; Forde-Thompson, W. Cognitive benefits of music in aerobic exercise: Evidence from a Bayesian network meta-analysis in adults with mild cognitive impairment. Arch. Gerontol. Geriatr. 2025, 134, 105848. [Google Scholar] [CrossRef]
- Rehfeld, K.; Lüders, A.; Hökelmann, A.; Lessmann, V.; Kaufmann, J.; Brigadski, T.; Müller, P.; Müller, N.G. Dance Training Is Superior to Repetitive Physical Exercise in Inducing Brain Plasticity in the Elderly. PLoS ONE 2018, 13, e0196636. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.; Ibrahim, A.; Hamide, A.; Tran, T.; Candreva, E.; Baltaji, J. Exercise-Induced Neuroplasticity: Adaptive Mechanisms and Preventive Potential in Neurodegenerative Disorders. Physiologia 2025, 5, 13. [Google Scholar] [CrossRef]
- Muratori, L.M.; Lamberg, E.M.; Quinn, L.; Duff, S.V. Applying Principles of Motor Learning and Control to Upper Extremity Rehabilitation. J. Hand Ther. 2013, 26, 94–103. [Google Scholar] [CrossRef]
- Liu, C.; Su, M.; Jiao, Y.; Ji, Y.; Zhu, S. Effects of Dance Interventions on Cognition, Psycho-Behavioral Symptoms, Motor Functions, and Quality of Life in Older Adult Patients With Mild Cognitive Impairment: A Meta-Analysis and Systematic Review. Front. Aging Neurosci. 2021, 13, 706609. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, I.; Parastatidis, T.; Tsolaki, A.; Gkioka, M.; Karakostas, A.; Douka, S.; Tsolaki, M. International Ballroom Dancing Against Neurodegeneration: A Randomized Controlled Trial in Greek Community-Dwelling Elders with Mild Cognitive Impairment. Am. J. Alzheimers Dis. Other Demen. 2017, 32, 489–499. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.; Jia, Y.; Liu, Y.; Shang, B.; Liu, P.; Bao, X.; Chen, L. Effects of Dance Intervention on Global Cognition, Executive Function and Memory of Older Adults: A Meta-Analysis and Systematic Review. Aging Clin. Exp. Res. 2020, 32, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Liu, L.; Yang, Y. The Effect of Dance-Based Interventions on Functional Mobility in Older Adults: A Meta-Analysis. Front. Psychol. 2022, 13, 966675. [Google Scholar] [CrossRef]
- Teixeira-Machado, L.; Azevedo-Santos, I.F.; DeSantana, J.M. Dance and Neuroplasticity: A Systematic Review. Neurosci. Biobehav. Rev. 2019, 96, 232–240. [Google Scholar] [CrossRef]
- Hwang, P.W.; Braun, K.L. The Effectiveness of Dance Interventions to Improve Older Adults’ Health: A Systematic Literature Review. Altern. Ther. Health Med. 2015, 21, 64–70. [Google Scholar] [PubMed]
- Mattle, M.; Chocano-Bedoya, P.O.; Fischbacher, M.; Meyer, U.; Abderhalden, L.A.; Lang, W.; Mansky, R.; Kressig, R.W.; Steurer, J.; Orav, E.J.; et al. Association of Dance-Based Mind-Motor Activities With Falls and Physical Function Among Healthy Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2017688. [Google Scholar] [CrossRef]
- Laurentani, F.; Russo, C.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Lorio, A.; Corsi, A.A.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletalmuscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual; Human Kinetics: Champaign, IL, USA, 2001. [Google Scholar]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Salvá, A.; Bolibar, I.; Lucas, R.; Rojano-Luque, X. Utilización del POMA en nuestro medio para la valoración del equilibrio y la marcha en una población de personas mayores residentes en la comunidad. Rev. Esp. Geriatr. Gerontol. 2005, 40 (Suppl. 2), 36–44. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Zhong, Y.-J.; Meng, Q.; Su, C.-H. Mechanism-Driven Strategies for Reducing Fall Risk in the Elderly: A Multidisciplinary Review of Exercise Interventions. Healthcare 2024, 12, 2394. [Google Scholar] [CrossRef]
- Chuang, L.L.; Sung, W.H.; Chang, H.A.; Wang, R.Y. Effect of aerobic exercise on cognition and physical function in older adults with mild cognitive impairment: A systematic review and meta-analysis. Aging Res. Rev. 2022, 74, 101544. [Google Scholar] [CrossRef]
- Labban, J.D.; Etnier, J.L. Benefits of resistance training on functional mobility and cognition in older adults: A systematic review. J. Aging Phys. Act. 2021, 29, 276–286. [Google Scholar] [CrossRef]
- Tsang, T.W.; Kohn, M.; Chow, C.M.; Singh, M.F. Effects of Tai-Chi on muscle strength, balance, and mobility in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2013, 61, 641–649. [Google Scholar] [CrossRef]
- Barker, A.L.; Talevski, J.; Morello, R.T.; Brand, C.A.; Rahmann, A.E.; Urquhart, D.M. Effectiveness of Pilates exercise in treating people with chronic low back pain: A systematic review of systematic reviews. BMC Med. Res. Methodol. 2016, 16, 26. [Google Scholar]
- Liu, M.A.; DuMontier, C.; Murillo, A.; Hshieh, T.T.; Bean, J.F.; Soiffer, R.J.; Stone, R.M.; Abel, G.A.; Driver, J.A. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019, 134, 374–382. [Google Scholar] [CrossRef]
- de Souza, A.F.; de Oliveira, D.C.; Ramírez, P.C.; de Oliveira Máximo, R.; Luiz, M.M.; Delinocente, M.L.B.; Steptoe, A.; de Oliveira, C.; da Silva Alexandre, T. Low gait speed is better than frailty and sarcopenia at identifying the risk of disability in older adults. Age Ageing 2025, 54, afaf104. [Google Scholar] [CrossRef]
- Trombetti, A.; Hars, M.; Herrmann, F.R.; Kressig, R.W.; Ferrari, S.; Rizzoli, R. Effect of Music-Based Multitask Training on Gait, Balance, and Fall Risk in Elderly People: A Randomized Controlled Trial. Arch. Intern. Med. 2011, 171, 525–533. [Google Scholar] [CrossRef]
- Rehfeld, K.; Müller, P.; Aye, N.; Schmicker, M.; Dordevic, M.; Kaufmann, J.; Hökelmann, J.; Müller, N.G. Dancing or Fitness Sport? The Effects of Two Training Programs on Hippocampal Plasticity and Balance Abilities in Healthy Seniors. Front. Hum. Neurosci. 2018, 12, 305. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.; Wallbank, G.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community: An abridged Cochrane systematic review. Br. J. Sports Med. 2019, 53, 905–911. [Google Scholar] [CrossRef]
- Stathokostas, L.; McDonald, M.W.; Little, R.M.; Paterson, D.H. Flexibility of older adults aged 55-86 years and the influence of physical activity. J. Aging Res. 2013, 2013, 743843. [Google Scholar] [CrossRef]
- Federici, A.; Bellagamba, S.; Rocchi, M.B.L. Does dance-based training improve balance in adult and young old subjects? A pilot randomized controlled trial. Aging Clin. Exp. Res. 2017, 29, 215–221. [Google Scholar] [CrossRef]
- Cruz-Ferreira, A.; Fernandes, J.; Laranjo, L.; Bernardo, L.M.; Silva, A. Creative dance improves physical fitness and life satisfaction in older women. Res. Aging 2015, 37, 837–855. [Google Scholar] [CrossRef]
- Gyllensten, A.L.; Ekdahl, C.; Roos, E.M. The effect of a 10-week Hatha yoga program on joint mobility, balance and quality of life in older women. J. Bodyw. Mov. Ther. 2010, 14, 42–47. [Google Scholar] [CrossRef]
- Eyigor, S.; Karapolat, H.; Durmaz, B. Effects of a Pilates exercise program on flexibility, strength, balance, and quality of life in older women. Aging Clin. Exp. Res. 2007, 19, 235–240. [Google Scholar] [CrossRef]
- Deng, J.; Lei, T.; Du, X. Effects of sensory integration training on balance function and executive function in children with autism spectrum disorder: Evidence from Footscan and fNIRS. Front. Psychol. 2023, 14, 1269462. [Google Scholar] [CrossRef] [PubMed]
- Fastame, M.C.; Mulas, I.; Putzu, V.; Asoni, G.; Viale, D.; Mameli, I.; Pau, M. Executive and Motor Functions in Older Individuals with Cognitive Impairment. Behav. Sci. 2022, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Kattenstroth, J.C.; Kalisch, T.; Holt, S.; Tegenthoff, M.; Dinse, H.R. Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Front. Aging Neurosci. 2013, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Hackney, M.E.; Earhart, G.M. Effects of dance on movement control in Parkinson’s disease: A comparison of Argentine tango and American ballroom. J. Rehabil. Med. 2009, 41, 475–481. [Google Scholar] [CrossRef]
- Minta, K.; Colombo, G.; Taylor, W.R.; Schinazi, V.R. Differences in fall-related characteristics across cognitive disorders. Front. Aging Neurosci. 2023, 15, 1171306. [Google Scholar] [CrossRef] [PubMed]
- Merom, D.; Grunseit, A.; Eramudugolla, R.; Jefferis, B.; Anstey, K.J. Cognitive benefits of social dancing and walking in old age: The Dancing Mind randomized controlled trial. Front. Aging Neurosci. 2016, 8, 26. [Google Scholar] [CrossRef] [PubMed]




| Total (n = 92) | Experimental (n = 47) | Control (n = 45) | Value p | ||
|---|---|---|---|---|---|
| Age | 71.83 ± 2.96 | 71.43 ± 2.97 | 72.24 ± 2.92 | 0.783 | |
| Sex | Male | 34 (23.10) | 18 (52.90) | 16 (47.10) | 0.672 |
| Female | 58 (39.50) | 29 (50.00) | 29 (50.00) | ||
| Occupational Status | Retired | 67 (45.60) | 35 (52.20) | 32 (47.80) | 0.586 |
| Working | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Unemployed | 25 (17.00) | 12 (48.00) | 13 (52.00) | ||
| Marital Status | Single | 13 (8.80) | 7 (53.80) | 6 (46.20) | 0.710 |
| Married | 52 (35.40) | 26 (50.00) | 26 (50.00) | ||
| Divorced/Separated/Widowed | 27 (18.40) | 14 (51.90) | 13 (48.10) | ||
| Educational Status | No Education | 14 (9.50) | 8 (57.10) | 6 (42.90) | 0.090 |
| Primary Education | 54 (36.70) | 31 (57.40) | 23 (42.60) | ||
| Secondary Education | 16 (10.90) | 5 (31.20) | 11 (68.80) | ||
| University Education | 8 (5.40) | 3 (37.50) | 5 (62.50) | ||
| Height | 1.66 ± 0.13 | 1.66 ± 0.13 | 1.65 ± 0.12 | 0.965 | |
| Weight | 73.36 ± 11.95 | 72.57 ± 11.42 | 74.18 ± 12.56 | 0.984 | |
| BMI | 26.99 ± 5.06 | 26.42 ± 4.79 | 27.60 ± 5.32 | 0.758 | |
| Waist Circumference | 95.79 ± 9.00 | 93.94 ± 8.90 | 95.68 ± 9.13 | 0.798 | |
| Hip Circumference | 107.27 ± 7.41 | 106.62 ± 7.17 | 107.95 ± 7.68 | 0.606 | |
| Waist–hip Ratio | 0.89 ± 0.63 | 0.88 ± 0.68 | 0.89 ± 0.55 | 0.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Alcalá, M.; Carcelén-Fraile, M.d.C.; Vico-Rodríguez, P.; Cano-Orihuela, M.; Carcelén-Fraile, M.d.M. Effects of Dance-Based Aerobic Training on Functional Capacity and Risk of Falls in Older Adults with Mild Cognitive Impairment. J. Clin. Med. 2025, 14, 5900. https://doi.org/10.3390/jcm14165900
Sánchez-Alcalá M, Carcelén-Fraile MdC, Vico-Rodríguez P, Cano-Orihuela M, Carcelén-Fraile MdM. Effects of Dance-Based Aerobic Training on Functional Capacity and Risk of Falls in Older Adults with Mild Cognitive Impairment. Journal of Clinical Medicine. 2025; 14(16):5900. https://doi.org/10.3390/jcm14165900
Chicago/Turabian StyleSánchez-Alcalá, Marcelina, María del Carmen Carcelén-Fraile, Paulino Vico-Rodríguez, Marta Cano-Orihuela, and María del Mar Carcelén-Fraile. 2025. "Effects of Dance-Based Aerobic Training on Functional Capacity and Risk of Falls in Older Adults with Mild Cognitive Impairment" Journal of Clinical Medicine 14, no. 16: 5900. https://doi.org/10.3390/jcm14165900
APA StyleSánchez-Alcalá, M., Carcelén-Fraile, M. d. C., Vico-Rodríguez, P., Cano-Orihuela, M., & Carcelén-Fraile, M. d. M. (2025). Effects of Dance-Based Aerobic Training on Functional Capacity and Risk of Falls in Older Adults with Mild Cognitive Impairment. Journal of Clinical Medicine, 14(16), 5900. https://doi.org/10.3390/jcm14165900





