Abstract
Background/Objectives: Aging leads to cognitive decline that may progress to dementia. Transcranial direct current stimulation (tDCS) has emerged as a strategy to improve cognitive functions in older adults with mild cognitive impairment (MCI). This study reviews the effectiveness of tDCS in these populations. Methods: A systematic review and meta-analysis was conducted following the PRISMA 2020 guidelines. Randomized controlled trials obtained from PubMed, Scopus, Cinahl, and Web of Science were included. Studies with tDCS intervention in older adults with MCI were selected, excluding those without a control group or that did not measure relevant cognitive variables. Methodological quality was analyzed with the PEDro scale and a meta-analysis was applied with random-effects models. Results: A total of 27 studies were included in this review, of which 13 were part of the meta-analysis. tDCS showed significant improvements in global cognitive function (p < 0.001) and selective attention (p = 0.044), but not in mental flexibility or visual attention. Positive effects on quality of life and depressive symptoms were also reported in some studies. Conclusions: tDCS may improve cognitive functions in older adults with MCI, but inconsistencies persist in its magnitude and duration. It is recommended to standardize protocols and conduct studies with greater methodological rigor and long-term follow-up.
1. Introduction
Population aging represents a growing challenge for health systems and society as a whole [1]. As life expectancy increases, so does the prevalence of age-related cognitive impairments [2], raising the need for effective strategies to preserve brain function and delay cognitive decline [3]. Among the main cognitive problems in the older population are difficulties in memory, processing speed, attention and executive functions, key aspects for the autonomy and quality of life of individuals [4]. Despite advances in the understanding of brain aging, the development of interventions that can mitigate or reverse these effects remains a priority area of research [5].
Within this context, transcranial direct current stimulation (tDCS) has emerged as a promising technique to modulate brain activity and improve cognitive performance in diverse populations [6], including elderly people and patients with mild cognitive impairment (MCI) [7]. tDCS is a noninvasive neuromodulation technique that employs a low-intensity electric current applied through the scalp to alter neuronal excitability in specific areas of the brain [8]. Its mechanism of action is based on the modification of the membrane potential of neurons, facilitating or inhibiting synaptic activity depending on the polarity of the stimulus [9].
The ability of tDCS to modulate cortical excitability has led to its exploration in a wide variety of contexts, including the enhancement of learning, working memory, attention, and executive control [10]. Previous studies have suggested that stimulation applied to regions such as the dorsolateral prefrontal cortex (DLPFC) may generate benefits in working memory and cognitive processing in older adults [11,12]. Furthermore, its application combined with other strategies, such as cognitive training or physical exercise, has shown enhancing effects on brain plasticity [13]. One of the main attractions of tDCS is its safety profile, as it is considered a well-tolerated technique with minimal adverse effects, which are usually limited to mild tingling or itching sensations at the application site [14]. However, its efficacy varies depending on multiple factors, including the intensity and duration of stimulation, the location of the electrode, and individual differences in response to treatment [15]. In addition to its safety and simplicity, an emerging area of interest is the feasibility of tDCS as a home-based therapy. Recent studies suggest that tDCS can be self-administered or applied with the assistance of a caregiver in home settings, with appropriate training and remote supervision protocols [16]. This approach could be especially valuable for older adults with reduced mobility or limited access to specialized care, offering a scalable and cost-effective intervention in cognitive rehabilitation. These findings support the broader applicability of tDCS beyond controlled clinical environments, aligning with the current need for accessible, patient-centered therapeutic options.
Mild cognitive impairment, considered an intermediate stage between normal aging and dementia, has been a key focus of tDCS research [17]. Individuals with MCI have been documented to have alterations in neural networks involved in episodic memory and executive functions, suggesting that modulating these areas through transcranial stimulation may offer significant benefits [18]. Clinical trials have reported improvements in memory tasks and verbal fluency following repeated tDCS sessions, although the magnitude and duration of these effects remain under debate [15,19]. Importantly, emerging evidence highlights that the earlier therapies such as tDCS are administered during the MCI stage, the greater their potential efficacy [20]. Therefore, early identification of suitable candidates becomes crucial. In this context, easily applicable tools such as neuropsychological screening instruments (as reviewed in the present study) and novel blood-based biomarkers—particularly phosphorylated Tau217 and Tau181—may offer valuable support for clinical decision making [21]. While the recent studies already cited [22,23] have indicated that pTau181 and pTau217 in plasma can distinguish amyloid-positive from amyloid-negative individuals, this evidence is significantly strengthened by quantitative syntheses of the literature. Notably, the meta-analysis by van Maurik et al. [24] reported that plasma pTau217 achieves an AUC above 0.90 for identifying amyloid positivity even in cognitively unimpaired individuals. Similarly, the large-scale systematic review by Hansson [25] confirmed the clinical reliability of these biomarkers across all stages of the Alzheimer’s disease spectrum. Additionally, multicenter studies such as Janelidze et al. [26] and Palmqvist et al. [27] found that pTau217 outperforms pTau181 in discriminative accuracy and performs comparably to CSF and PET measures. This body of evidence strongly supports the use of blood-based pTau biomarkers in early detection strategies for identifying patients who may benefit from neuromodulation interventions like tDCS.
Another emerging field in the use of tDCS is its combination with neuropsychological rehabilitation therapies [28]. Recent research has explored the impact of transcranial stimulation on the recovery of cognitive functions in patients with acquired brain damage or neurodegenerative diseases such as Alzheimer’s and Parkinson’s, so these results suggest that tDCS can enhance the effects of cognitive rehabilitation, favoring the consolidation of learning and the reorganization of impaired neural circuits [29]. However, despite the growing interest in tDCS as a tool for cognitive improvement, there are still important challenges in its clinical implementation, since it presents limitations such as the lack of standardized protocols, the heterogeneity in the results of the studies and the need for research with methodologically robust designs that allow evaluating its long-term effectiveness [30].
In view of these considerations, the present review aims to analyze the current evidence on the application of transcranial stimulation in cognitive-related capacities, with special emphasis on its impact on mild cognitive impairment and its potential as an intervention strategy in aging populations.
2. Materials and Methods
This review was carried out in accordance with the 2020 PRISMA statement guidelines [31] and the predefined protocol registered in PROSPERO (CRD420250655761). Additionally, the methodological approach followed the recommendations set forth in the “Cochrane Manual for Systematic Reviews of Interventions” [32].
2.1. Sources of Information
A literature search was conducted from January to February 2025 using the PubMed, Scopus, Cinahl, and Web of Science (WOS) databases.
2.2. Search Strategy
Various keywords were employed in the following search string: (“transcranial direct current stimulation” OR “transcranial current stimulation” OR “tDCS”) AND (“mild cognitive impairment” OR “mild neurocognitive impairment” OR “mci”) AND (“older adults” OR “elderly” OR “aging”).
2.3. Inclusion Criteria
The articles chosen had to fulfill these criteria: (i) the studies must be randomized controlled trials (RCTs); (ii) the intervention must involve tDCS as an important part of the treatment; (iii) participants must be from the older population (with an average age of over 60 years); and (iv) participants must have a confirmed diagnosis of MCI using validated diagnostic methods, such as the Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), Petersen’s criteria, criteria of the MCI Working Group of the European Consortium on Alzheimer’s Disease., diagnosis of aMCI and criteria of the National Institute on Aging-Alzheimer’s Association.
2.4. Exclusion Criteria
Articles were excluded if they met any of these criteria: (i) studies without a non-intervention reference group; (ii) studies that did not measure the relevant study variables (cognitive variables such as cognitive status, memory, executive function, and processing speed); (iii) the presence of other conditions such as cancer, stroke, cardiovascular disease, lung disease, and/or kidney disease; and (iv) participants who did not meet the minimum required attendance rate for intervention program sessions (less than two treatment sessions).
2.5. Study Selection Process
The study selection process began with the removal of duplicate entries and articles without available abstracts. Titles and abstracts were carefully reviewed to exclude those that did not meet the established eligibility criteria. Articles that passed this initial phase were then evaluated in full text to assess their suitability for inclusion in the meta-analysis. To ensure objectivity and minimize potential bias, two authors (J.C.-S. and J.M.M.-P.) independently carried out the selection process. Any disagreements regarding the eligibility of a study were resolved through consultation with a third author (M.d.C.C.-F.), who provided their judgment to reach a consensus. This thorough procedure ensured that all included studies were relevant and met the predefined criteria.
2.6. Data Extraction
The primary variable in this study was the cognitive performance of older patients with mild cognitive impairment. On the other hand, depression and quality of life were also included as secondary variables. Data extraction involved collecting details such as authorship, publication year, study location, population characteristics (sample size, age, and group allocation), study design, outcomes, measurement tools, intervention descriptions, measurement timelines, attrition rates, adverse effects, and key findings.
2.7. Assessment of Methodological Quality
Methodological quality was assessed using the PEDro scale [29], which features an 11-item checklist. The maximum score is 10 points, as the first item (“eligibility criteria”) is excluded from the final score. Each item is rated as either “Yes” (1 point) or “No” (0 points). Quality levels are classified as follows: scores between 0 and 3 indicate “Poor” quality, 4 and 5 represent “Fair” quality, 6 to 8 indicate “Good” quality, and scores above 9 are considered “Excellent” [33,34].
The Cochrane RoB-2 tool was also used to assess the risk of bias in each of the selected articles. It is a tool designed to assess the risk of bias in randomized studies, specifically in randomized clinical trials. Its purpose is to assess the risk of bias in randomized studies based on five key domains. The tool classifies the risk of bias of each study as low, high, or unclear [35].
2.8. Analytic Decisions for Meta-Analysis
The results are presented in a forest plot, detailing the lead author, year of publication, sample size, individual effects expressed using the Hedge index (g) and the combined effect with its 95% confidence interval, accompanied by the corresponding p value. The choice between a fixed-effects or random-effects model will depend on the heterogeneity and variability identified through the Cochrane Q and I2 indices. In the meta-analysis, only studies in which the control group received usual care or was assigned to a waiting list were included. For stratified or subgroup analyses, studies were grouped according to the type of intervention implemented, carrying out independent meta-analyses within each category. This strategy facilitated the assessment of variability and effect size in each subgroup, allowing a more detailed interpretation of the findings. Finally, the publication date was analyzed using a funnel plot.
3. Results
3.1. Study Selection Process
An initial search across multiple databases identified 385 articles. The search was then refined within the same databases by targeting specific document types (articles and randomized clinical trials) and filtering for keywords in titles and abstracts, while also removing duplicates. This process resulted in 84 unique articles. These articles were then screened based on their titles and abstracts, narrowing the selection to 55 potential candidates for qualitative evaluation. In the end, 17 articles [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] met the inclusion criteria and were included in the meta-analysis, while the remaining 38 were excluded. The selection process is detailed further in Figure 1.
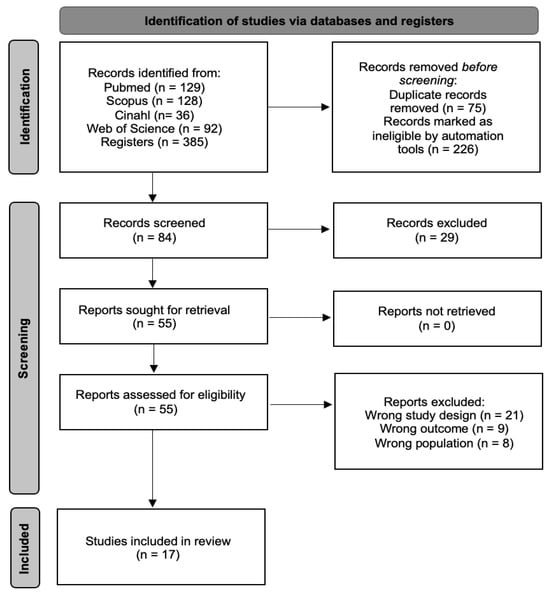
Figure 1.
Study selection process flow chart.
3.2. Methodological Quality
The methodological quality of the studies included was assessed using the PEDro scale, with scores obtained from the PEDro website. All twenty-seven were rated as “Good”. To maintain objectivity and consistency in the evaluation, two independent reviewers scored the studies based on the PEDro scale. In cases of disagreement, a conflict resolution procedure was followed. The reviewers discussed the differences, and if they could not reach a consensus, a third reviewer was involved. This approach ensured that study grading was as accurate and reliable as possible. A comprehensive assessment of the methodological quality can be found in Table 1. The risk of bias was also assessed using the Cochrane RoB-2 tool, allowing each article to be scored based on whether the risk of bias was low, unclear, or high. Of the 17 included articles, 5 present a low risk of bias, while the rest are considered to have a low or unclear risk. The full assessment of each section can be seen in Table 2.

Table 1.
Methodological Quality of the included articles.

Table 2.
RoB-2 to assess the risk of bias.
3.3. Characteristics of the Studies
All the studies included in this systematic review and meta-analysis were randomized controlled trials carried out in Italy [37,44,48], China [38,39,41,43], Germany [36], the United States [42,45,51], South Korea [40,52], Australia [46], Iran [47], Czech Republic [49] and Thailand [50]. A total of 650 participants participated in these studies, with 333 in the control group and 317 in the intervention group, which focused on transcranial stimulation. There is a higher representation of women among the total participants in the studies included in the systematic review. The average age of the participants was 71.5 years (Table 3).

Table 3.
Characteristics of the included studies.
3.4. Study Results
Of the 17 articles included in this systematic review, 9 were excluded from the meta-analysis. The article by Lengu et al. [42] was excluded because it focused on evaluating alterations in brain rest and activity, as well as changes in different neurotransmitters. A similar situation occurred with Turnbull et al. [51] and Yun et al. [52], who assessed specific neurological elements observed through imaging tests. Finally, the remaining articles [36,44,46,48,49,50] were excluded for not evaluating global cognitive function, visual attention, mental flexibility, or selective attention as main variables.
The main objective of this systematic review with meta-analysis was to evaluate cognitive abilities. Regarding this evaluation, six articles used the MoCA [38,39,41,42,45,47], while one study employed the MMSE to assess global cognitive function [37]. Four trials used the TMT to study visual attention and mental flexibility. On the other hand, three studies employed the Stroop test to analyze selective attention. Among the studies included in the meta-analysis that assessed global cognition, four reported significant improvements in favor of tDCS [37,39,45,47]. Two of them show results mainly obtained after the treatment, with a tendency to decrease during follow-up, except for the study by Manor et al. [45], in which the results are obtained after the treatment and persist for at least a short follow-up period (two weeks). He et al. [39] only provides data on the variables after treatment. Nevertheless, no article achieves significant results. after treatment or after follow-up for visual attention and mental flexibility, measured using TMT-A and TMT-B, respectively, while two articles [37,40] showed significant changes in selective attention in the post-treatment assessment, achieving a statistical value of p < 0.05.
Significant results in cognitive abilities were also observed in the studies excluded from the meta-analysis [44,46,48,50], based on their respective scales measuring specific cognitive aspects and executive function, favoring tDCS. The remaining article [49] did not reach statistical significance (p > 0.05) when measuring changes in cognitive abilities after tDCS treatment and after the follow-up. Additionally, neurotransmitter levels and changes observed through neuroimaging were studied, reporting significant effects in favor of tDCS [39,42]. In the following cases [44,46], the results were obtained after treatment and after one or three months of follow-up, respectively, maintaining improvement over time. However, Stonsaovapak et al. evaluate the results only after treatment. Finally, Sandrini et al. [48] reported that the results were observed after treatment and after one month of follow-up, although the positive effects were only sustained in the short term.
In addition, this systematic review also considered secondary variables such as depression and quality of life. Depression was studied in four trials [37,40,46], one of them used the Beck Depression Inventory (BDI) [37], while Kim et al. [40] and Martin et al. [46] employed the Epidemiologic Studies Depression Scale (CESD), Hamilton Rating Scale for Depression (HAMD), and Montgomery Asberg Depression Rating Scale (MADRS). All of them reported significant improvements in favor of tDCS. These results are achieved immediately after treatment [40,47], while Martin et al. [46] obtain them after treatment and during 3 months of follow-up.
Finally, quality of life was analyzed in two studies [46,47] using the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) and the Quality of Life in Alzheimer’s Disease (QoLAD). In this way, Martin et al. [46] did not obtain significant results; however, significant results were achieved by Rezakhani et al. [7]. These results are obtained immediately after treatment and during 3 months of follow-up.
3.5. Meta-Analysis
The studies included in this review provided statistical data for four meta-analyses (one for global cognition variables, one for visual attention, one for mental flexibility, and one for selective attention). The findings of each meta-analysis are summarized in Table 4.

Table 4.
Main findings in meta-analyses.
3.5.1. Subgroup Analysis
A subgroup analysis was performed considering the study variables. The findings showed a remarkable statistical significance, with moderate and inversely directional Hedge’s g effect sizes. Subgroup analyses based on this assessment tool showed uniform effect sizes in all cases. The consistency of these results indicates that the selection of the assessment tool had a minimal influence on the observed treatment effects.
3.5.2. Global Cognitive Function
The effectiveness of tDCS on global cognitive function was assessed across seven studies, including seven independent comparisons, with data from 214 participants. Of these studies, one assessed global cognition using the MMSE and six used the MoCA. Our meta-analysis found low-quality evidence of a large effect in favor of tDCS (SMD = 0.477; 95% CI: 0.200 to 0.754; p = 0.001) (Figure 2). There was moderate heterogeneity (I2 = 57.96%; Q = 14.27; df = 6; p = 0.027) (Table 5) and no evidence of publication bias (Egger’s test, p = 0.88). Sensitivity analyses revealed no variations when individual studies were removed. To assess the clinical relevance of the observed effects, the standardized mean differences (SMDs) were compared with the Minimum Clinically Important Difference (MCID) values reported in the individual studies. In most cases, the improvements in global cognitive function—particularly those measured by MoCA and MMSE—exceeded the MCID thresholds. For instance, studies by De Sousa et al. [36], Fileccia et al. [37], González et al. [38], Lau et al. [41], and Rezakhani et al. [47] reported effect sizes that surpassed the MCID for MoCA (ranging from 0.9 to 1.7 points). This suggests that the observed changes are not only statistically significant, but also clinically meaningful for older adults with MCI.
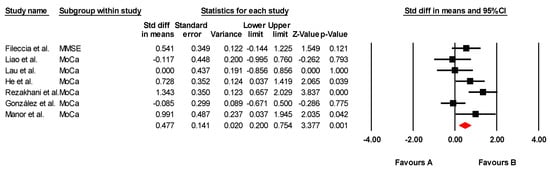
Figure 2.
Forest plot of the effectiveness of tDCS on global cognitive function [37,38,39,41,43,45,47].

Table 5.
Study characteristics and heterogeneity statistics for global cognitive function.
3.5.3. Visual Attention
The effectiveness of tDCS on visual attention was assessed across four studies, including four independent comparisons, with data from 123 participants. All studies measured visual attention using the Trail Making Test Part A (TMT-A). Our meta-analysis found low-quality evidence of a small, non-significant effect in favor of tDCS (SMD = −0.200; 95% CI: −0.559 to 0.158; p = 0.274) (Figure 3). There was no heterogeneity among studies (I2 = 0%; Q = 1.25; df = 3; p = 0.740) (Table 6) and no evidence of publication bias (Egger’s test, p = 0.15). Sensitivity analyses showed no variations when individual studies were removed. No variations were found by sensitivity analysis.
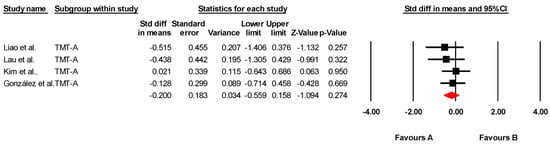
Figure 3.
Forest plot of the effectiveness of tDCS on visual attention [38,40,41,43].

Table 6.
Study characteristics and heterogeneity statistics for visual attention.
3.5.4. Mental Flexibility
The effectiveness of transcranial direct current stimulation (tDCS) on mental flexibility was assessed across four studies, including four independent comparisons, with data from 123 participants. All studies measured mental flexibility using the Trail Making Test Part B (TMT-B). Our meta-analysis found low-quality evidence of a very small, non-significant effect in favor of tDCS (SMD = 0.031; 95% CI: –0.326 to 0.389; p = 0.864) (Figure 4). There was no heterogeneity among studies (I2 = 0%; Q = 0.79; df = 3; p = 0.851) (Table 7) and no evidence of publication bias (Egger’s test, p = 0.14). Sensitivity analyses revealed no changes when individual studies were excluded.
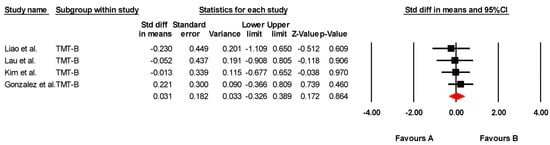
Figure 4.
Forest plot of the effectiveness of tDCS on mental flexibility [38,40,41,43].

Table 7.
Study characteristics and heterogeneity statistics for mental flexibility.
3.5.5. Selective Attention
The effectiveness of transcranial direct current stimulation (tDCS) on selective attention was assessed across six independent comparisons from three studies, including data from 305 participants. All studies measured selective attention using variations in the Stroop test. Our meta-analysis found low-quality evidence of a moderate and statistically significant effect in favor of tDCS (SMD = −0.589; 95% CI: −0.902 to −0.277; p < 0.001) (Figure 5). There was substantial heterogeneity among the studies (I2 = 81.5%; Q = 27.09; df = 5; p = 0.001) (Table 8) but no evidence of publication bias (Egger’s test, p = 0.573). Sensitivity analyses revealed no significant variations when individual studies were removed.
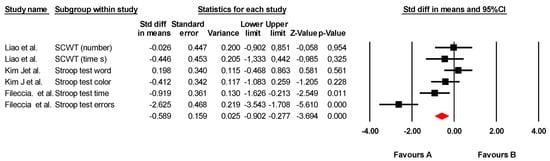
Figure 5.
Forest plot of the effectiveness of tDCS on selective attention [37,40,43].

Table 8.
Study characteristics and heterogeneity statistics for selective attention.
4. Discussion
The present study aimed to evaluate the efficacy of tDCS in improving cognitive abilities in older adults with MCI. The findings indicate significant improvements in global cognitive function, selective attention, and to a lesser extent, mental flexibility and visual attention. These results support the hypothesis that tDCS can modulate cortical activity and contribute to the preservation of cognitive function in at-risk populations, although discrepancies persist in the magnitude of the effects and their durability.
Global cognitive function refers to the brain’s ability to process, store, and retrieve information necessary for the execution of complex tasks, encompassing multiple cognitive domains such as memory, attention, executive function, language, and visuospatial skills [2]. Its assessment is essential in the early detection of cognitive decline and in the monitoring of interventions aimed at improving brain performance. In this study, global cognitive function was assessed using the MoCA and the MMSE, tests widely used in the detection of mild cognitive impairment and neurodegenerative diseases such as Alzheimer’s disease. Both tests allow estimating general cognitive ability and have been validated in various populations. The results obtained showed significant improvements in MoCA and MMSE scores after the application of tDCS, suggesting a positive effect on cortical activity in key regions such as the dorsolateral prefrontal cortex. Importantly, the clinical relevance of these cognitive improvements was supported by MCID analysis. Several included studies provided sufficient data to estimate whether the observed changes exceeded the minimum threshold to be perceived as beneficial by patients. In the case of MoCA, the majority of studies reported gains exceeding the MCID (typically 1.0–1.5 points), indicating that tDCS led to improvements that were not only statistically detectable but also likely to have a meaningful impact in real-world cognitive functioning. This reinforces the argument for the potential utility of tDCS as a therapeutic tool in clinical settings for older adults with MCI.
This area plays an essential role in regulating working memory, decision making, and cognitive control, so its modulation by tDCS could contribute to the optimization of cognitive performance [7,53] These findings are consistent with previous studies that have reported improvements in general cognitive performance in patients with mild cognitive impairment (MCI) and in healthy older adults after multiple sessions of tDCS [54,55]. In particular, tDCS has been shown to enhance synaptic plasticity, favoring memory consolidation processes and optimizing information processing. A recent meta-analysis also pointed out that tDCS improves working memory and processing speed in older adults, reinforcing the evidence of its therapeutic potential in cognitive aging [56].
Visual attention is the ability to select and process relevant information in dynamic environments, allowing for efficient interaction with the environment. This process involves multiple neural networks, including those related to orientation, alertness, and executive control. In this study, visual attention was assessed using the TMT-A, a widely used test in neuropsychology that measures visual processing speed and sustained attention [3]. This test consists of connecting a series of numbers in sequential order as quickly as possible, allowing for the assessment of cognitive efficiency in tasks that require visual exploration and visuomotor coordination. The results of this study indicated improvements in execution times after the application of tDCS, suggesting a possible beneficial effect on processing speed and attentional efficiency. However, these differences did not reach statistical significance, which could be due to interindividual variability in response to stimulation or the need for longer or more intensive protocols. Although previous research has suggested that stimulation of the parietal cortex can influence attentional networks, the current evidence remains inconclusive [8]. Some studies have shown that tDCS can induce changes in functional connectivity between regions involved in perception and decision making, facilitating the selection of relevant information in complex visual tasks. These findings are relevant given that aging entails a decline in attentional functions, which can affect autonomy and performance in daily activities. However, other studies have found that the effects of tDCS may be short-lived if repeated sessions are not conducted to reinforce changes in cortical activity and consolidate long-term benefits. Therefore, future studies should explore longer stimulation protocols or combinations with other interventions to optimize the effects on visual attention and processing speed [57,58].
Mental flexibility is the ability to switch between different tasks or cognitive rules efficiently, allowing individuals to adapt to new situations, solve problems effectively, and modify strategies according to environmental demands [59]. This ability is a key component of executive functions and is closely related to decision making, planning, and cognitive control. Its assessment was performed using the TMT-B, a widely used neuropsychological test that measures the ability to switch between different stimulus sets, thereby assessing processing speed, working memory, and divided attention. The results of this study indicated only a minimal effect in participants who received tDCS, and this was not statistically significant. Variability across studies suggests that this effect is not uniform and may depend on multiple factors, such as electrode location, current intensity, and individual differences in brain plasticity. Previous studies have found that stimulation of the DLPFC can improve cognitive flexibility by increasing functional connectivity in executive networks, favoring the regulation of attention and the inhibition of automatic responses [60]. However, other studies have not replicated these findings, suggesting that mental flexibility may be less sensitive to tDCS stimulation than other cognitive functions, such as working memory or response inhibition [61]. This has led researchers to explore possible moderating variables, such as the duration of the intervention or the combination with other cognitive training strategies. Recent research has indicated that combining tDCS with structured cognitive training programs can significantly enhance the benefits in mental flexibility, maximizing the transfer of improvements to daily life and performance in complex tasks [62]. These findings reinforce the need for multimodal approaches, integrating brain stimulation with behavioral interventions and personalized strategies to optimize their effects on cognition.
Selective attention refers to the ability to focus on relevant information while inhibiting interference from distractors, playing a key role in executive control [10]. This ability is essential for efficient information processing in environments with multiple stimuli, and its alteration can significantly affect cognitive performance and decision making. In this study, it was assessed using the Stroop test, a task widely used in cognitive neuroscience to measure the ability to inhibit automatic and impulsive responses. Significant improvements in response times and accuracy were observed after the application of tDCS, suggesting a positive effect in the modulation of neural circuits associated with cognitive control. These findings are consistent with recent studies reporting that tDCS applied to the prefrontal cortex improves performance in Stroop tasks. However, the size of the effect varies according to factors such as age, cognitive status of the participant, and specific stimulation parameters [63]. Some studies suggest that tDCS may be particularly beneficial in people with more advanced cognitive impairment, possibly by facilitating synaptic plasticity and functional connectivity in affected neural networks [64]. Likewise, research has indicated that stimulation can influence the release of neurotransmitters such as dopamine, contributing to better regulation of executive control and cognitive flexibility. However, some studies have not found significant differences between stimulated and control groups, highlighting the need for clinical trials with more rigorous methodological designs, considering variables such as stimulation duration, current intensity, and individual differences in response to tDCS [56]. These findings underline the importance of continuing to explore the underlying mechanisms of tDCS and its potential application in clinical and neuroscientific settings.
Despite the positive findings, this study has several limitations. The heterogeneity in stimulation protocols makes it difficult to directly compare results across studies, highlighting the need to standardize tDCS application parameters. Furthermore, most of the studies included in this review have relatively small sample sizes, limiting the generalizability of the results. The durability of the effects also remains uncertain, as few trials have assessed the long-term impact of tDCS on cognitive function. Furthermore, due to the limited number of studies included in some subgroup analyses, funnel plots were not generated when fewer than 10 studies were available, in accordance with standard recommendations. Even when 11 studies are included, the funnel plot’s ability to detect publication bias has been shown to be limited, so the results should be interpreted with caution. It is also worth noting that mild cognitive impairment (MCI) does not inevitably progress to dementia in all individuals. Some cases may remain stable or even improve spontaneously over time. Therefore, while the improvements in cognitive function observed in this review are encouraging, they should be considered surrogate indicators of potential clinical benefit. Further research is needed to determine whether these effects translate into reduced rates of progression to dementia in the long term. Finally, although tDCS is a safe and well-tolerated technique, further studies are required to explore its effect in different patient subgroups and to analyze possible interactions with other cognitive intervention strategies. Clinical trials with more robust methodologies and long-term follow-ups are recommended to more accurately assess the efficacy and safety of tDCS in the treatment of mild cognitive impairment.
5. Conclusions
The results of this review and meta-analysis indicate that tDCS can improve global cognitive function and selective attention in older adults with MCI. These findings suggest that tDCS has the potential to modulate brain activity and promote cognitive performance in at-risk populations. Although the efficacy of tDCS varies depending on the cognitive function assessed, the results support its use as a potential therapeutic tool. Standardization of application protocols and combination with other intervention strategies could optimize its benefits. Therefore, tDCS is presented as a promising alternative for the treatment of cognitive impairment, with the need to continue researching its implementation and long-term effects.
Author Contributions
Conceptualization, J.M.M.-P., M.C.-O. and Y.C.-C.; methodology, J.C.-S., M.S.-A. and P.V.-R.; formal analysis, M.C.-O. and M.d.C.C.-F.; writing—original draft preparation, J.M.M.-P. and M.S.-A.; writing—review and editing, J.C.-S. and P.V.-R.; supervision, Y.C.-C. and M.d.C.C.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| tDCS | transcranial direct current stimulation |
| MCI | mild cognitive impairment |
| WOS | Web of Science |
| RCTs | randomized controlled trials |
| MoCA | Montreal Cognitive Assessment |
| MMSE | Mini-Mental State Examination |
| CT | cognitive training |
| OLM | associative Object-Location Memory |
| atDCS | anodal transcranial direct current stimulation |
| HD-tDCS | high-definition transcranial direct current stimulation |
| fALFF | fractional Amplitude of Low-Frequency Fluctuation |
| ReHo | Regional Homogenity |
| CCT | Cognitive Control Training |
| ICCT | Interactive Computerized Cognitive Training |
| MRS | Magnetic Resonance Spectroscopy |
| GABA | Glutamate and Gamma-aminobutyric Acid |
| TUG | Timed Up-and-Go test |
| TMT-A | Trail Making Test-A |
| DLPFC | dorsolateral prefrontal cortex |
| DATL | Dominal Anterior Temporal Lobe |
| PFC-tDCS | prefrontal cortex transcranial direct current stimulation |
| tDCS-cog | transcranial direct current stimulation combined with cognitive training |
| VOMT | Visual Object Matching Task |
| CANTAB | Cambridge Neuropsychological Test Automated Battery |
| VSA | Visual Sustained Attention |
| SWM | Spatial Working Memory |
| VM | Visual Memory |
| SCWT | Stroop Color Word Test |
| rsFCNPS | functional connectivity Neuropsychiatric Symptoms |
| F | frequency |
| #S | number of sessions |
| D | duration |
References
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public Health 2021, 50, 2451–2460. [Google Scholar] [PubMed]
- Langa, K.M. Cognitive Aging, Dementia, and the Future of an Aging Population. In National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Committee on Population; Majmundar, M.K., Hayward, M.D., Eds.; Future Directions for the Demography of Aging: Proceedings of a Workshop; National Academies Press (US): Washington, DC, USA, 2018; Volume 9. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513075/ (accessed on 15 January 2025).
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar]
- Lee, P.L.; Huang, C.K.; Chen, Y.Y.; Chang, H.H.; Cheng, C.H.; Lin, Y.C.; Lin, C.-L. Enhancing Cognitive Function in Older Adults through Processing Speed Training: Implications for Cognitive Health Awareness. Healthcare 2024, 12, 532. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Preventing Dementia and Cognitive Impairment. Preventing Cognitive Decline and Dementia: A Way Forward; Downey, A., Stroud, C., Landis, S., Leshner, A.I., Eds.; National Academies Press (US): Washington, DC, USA, 2017. [Google Scholar] [PubMed]
- Xu, Y.; Huang, H.; Wu, M.; Zhuang, Z.; Liu, H.; Hou, M.; Chen, C. Transcranial Direct Current Stimulation for Cognitive Impairment Rehabilitation: A Bibliometric Analysis. Arch. Med. Res. 2025, 56, 103086. [Google Scholar] [PubMed]
- Chen, J.; Wang, Z.; Chen, Q.; Fu, Y.; Zheng, K. Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.; Cohen Kadosh, R. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar]
- Grider, M.H.; Jessu, R.; Kabir, R. Physiology, Action Potential. [Updated 2023 May 8]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538143/ (accessed on 16 January 2025).
- Bennabi, D.; Pedron, S.; Haffen, E.; Monnin, J.; Peterschmitt, Y.; Van Waes, V. Transcranial direct current stimulation for memory enhancement: From clinical research to animal models. Front. Syst. Neurosci. 2014, 8, 159. [Google Scholar]
- Au, J.; Katz, B.; Moon, A.; Talati, S.; Abagis, T.R.; Jonides, J.; Jaeggi, S.M. Post-training stimulation of the right dorsolateral prefrontal cortex impairs working memory training performance. J. Neurosci. Res. 2021, 99, 2351–2363. [Google Scholar]
- Goldthorpe, R.A.; Rapley, J.M.; Violante, I.R. A Systematic Review of Non-invasive Brain Stimulation Applications to Memory in Healthy Aging. Front. Neurol. 2020, 11, 575075. [Google Scholar] [CrossRef]
- Šimko, P.; Pupíková, M.; Gajdoš, M.; Rektorová, I. Cognitive Aftereffects of Acute tDCS Coupled with Cognitive Training: An fMRI Study in Healthy Seniors. Neural Plast. 2021, 2021, 6664479. [Google Scholar]
- Moshfeghinia, R.; Shekouh, D.; Mostafavi, S.; Hosseinzadeh, M.; Bahadori, A.R.; Abdollahifard, S.; Razmkon, A. The effects of transcranial direct-current stimulation (tDCS) on pain intensity of patients with fibromyalgia: A systematic review and meta-analysis. BMC Neurol. 2023, 23, 395. [Google Scholar] [CrossRef] [PubMed]
- Prathum, T.; Chantanachai, T.; Vimolratana, O.; Laksanaphuk, C.; Apiworajirawit, I.; Aneksan, B.; Latthirun, K.; Yang, C.-T.; Klomjai, W. A systematic review and meta-analysis of the impact of transcranial direct current stimulation on cognitive function in older adults with cognitive impairments: The influence of dosage parameters. Alzheimer’s Res. Ther. 2025, 17, 37. [Google Scholar]
- Antonioni, A.; Baroni, A.; Fregna, G.; Ahmed, I.; Straudi, S. The effectiveness of home-based transcranial direct current stimulation on chronic pain: A systematic review and meta-analysis. Digit. Health 2024, 10, 20552076241292677. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Cho, Y.; Lee, J.H. Transcranial Direct Current Stimulation for Global Cognition in Mild Cognitive Impairment. Chonnam Med. J. 2025, 61, 1–8. [Google Scholar] [CrossRef]
- Phipps, C.J.; Murman, D.L.; Warren, D.E. Stimulating Memory: Reviewing Interventions Using Repetitive Transcranial Magnetic Stimulation to Enhance or Restore Memory Abilities. Brain Sci. 2021, 11, 1283. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Sanderson-Cimino, M.; Elman, J.A.; Tu, X.M.; Gross, A.L.; Panizzon, M.S.; Gustavson, D.E.; Bondi, M.W.; Edmonds, E.C.; Eglit, G.M.L.; Eppig, J.S.; et al. Cognitive practice effects delay diagnosis of MCI: Implications for clinical trials. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12228. [Google Scholar]
- Xiao, Z.; Wu, W.; Ma, X.; Wu, J.; Liang, X.; Zhou, X.; Cao, Y.; Zhao, Q.; Ding, D. Plasma p-tau217, p-tau181, and NfL as early indicators of dementia risk in a community cohort: The Shanghai Aging Study. Alzheimers Dement. 2023, 15, e12514. [Google Scholar] [CrossRef]
- Kang, J.H.; Korecka, M.; Lee, E.B.; Cousins, K.A.Q.; Tropea, T.F.; Chen-Plotkin, A.A.; Irwin, D.J.; Wolk, D.; Brylska, M.; Wan, Y.; et al. Alzheimer Disease Biomarkers: Moving from CSF to Plasma for Reliable Detection of Amyloid and tau Pathology. Clin. Chem. 2023, 69, 1247–1259. [Google Scholar] [CrossRef]
- Dubois, B.; von Arnim, C.A.F.; Burnie, N.; Bozeat, S.; Cummings, J. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res. Ther. 2023, 15, 175. [Google Scholar] [CrossRef]
- Leuzy, A.; Mattsson-Carlgren, N.; Palmqvist, S.; Janelidze, S.; Dage, J.L.; Hansson, O. Blood-based biomarkers for Alzheimer’s disease EMBO Mol Med. 2022, 14, e14408.
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2023, 29, 761–773. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.; Proctor, N.; Chai, X.; Shcherbinin, S.; Triana-Baltzer, G.; et al. Plasma P-tau217 performs better than P-tau181 as a biomarker of Alzheimer’s disease Nat Commun. 2020, 11, 1683.
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Albishi, A.M. How does combining physical therapy with transcranial direct stimulation improve upper-limb motor functions in patients with stroke? A theory perspective. Ann. Med. Surg. 2024, 86, 4601–4607. [Google Scholar]
- Sanches, C.; Stengel, C.; Godard, J.; Mertz, J.; Teichmann, M.; Migliaccio, R.; Valero-Cabré, A. Past, Present, and Future of Non-invasive Brain Stimulation Approaches to Treat Cognitive Impairment in Neurodegenerative Diseases: Time for a Comprehensive Critical Review. Front. Aging Neurosci. 2021, 12, 578339. [Google Scholar]
- Fregni, F.; Nitsche, M.A.; Loo, C.K.; Brunoni, A.R.; Marangolo, P.; Leite, J.; Carvalho, S.; Bolognini, N.; Caumo, W.; Paik, N.J.; et al. Regulatory Considerations for the Clinical and Research Use of Transcranial Direct Current Stimulation (tDCS): Review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2015, 32, 22–35. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023). Cochrane. 2023. Available online: www.training.cochrane.org/handbook (accessed on 22 November 2023).
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy evidence database (pedro) scale. J. Physiother. 2020, 66, 59. [Google Scholar]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar]
- Higgins, J.P.T.; Savović, J.; Page, M.; Elbers, R.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley-Blackwell: Chichester, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- de Sousa, A.V.C.; Grittner, U.; Rujescu, D.; Külzow, N.; Flöel, A. Impact of 3-Day Combined Anodal Transcranial Direct Current Stimulation-Visuospatial Training on Object-Location Memory in Healthy Older Adults and Patients with Mild Cognitive Impairment. J. Alzheimers Dis. 2020, 75, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Fileccia, E.; Di Stasi, V.; Poda, R.; Rizzo, G.; Stanzani-Maserati, M.; Oppi, F.; Avoni, P.; Capellari, S.; Liguori, R. Effects on cognition of 20-day anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex in patients affected by mild cognitive impairment: A case-control study. Neurol. Sci. 2019, 40, 1865–1872. [Google Scholar] [CrossRef]
- Gonzalez, P.C.; Fong, K.N.K.; Brown, T. Transcranial direct current stimulation as an adjunct to cognitive training for older adults with mild cognitive impairment: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2021, 64, 101536. [Google Scholar]
- He, F.; Li, Y.; Li, C.; Fan, L.; Liu, T.; Wang, J. Repeated anodal high-definition transcranial direct current stimulation over the left dorsolateral prefrontal cortex in mild cognitive impairment patients increased regional homogeneity in multiple brain regions. PLoS ONE 2021, 16, e0256100. [Google Scholar]
- Kim, J.; Park, S.; Kim, H.; Roh, D.; Kim, D.H. Home-based, Remotely Supervised, 6-Week tDCS in Patients With Both MCI and Depression: A Randomized Double-Blind Placebo-Controlled Trial. Clin. EEG Neurosci. 2024, 55, 531–542. [Google Scholar] [PubMed]
- Lau, C.I.; Liu, M.N.; Cheng, F.Y.; Wang, H.C.; Walsh, V.; Liao, Y.Y. Can transcranial direct current stimulation combined with interactive computerized cognitive training boost cognition and gait performance in older adults with mild cognitive impairment? a randomized controlled trial. J. Neuroeng. Rehabil. 2024, 21, 26. [Google Scholar] [CrossRef]
- Lengu, K.; Ryan, S.; Peltier, S.J.; Tyszkowski, T.; Kairys, A.; Giordani, B.; Hampstead, B.M. Effects of High Definition-Transcranial Direct Current Stimulation on Local GABA and Glutamate Levels Among Older Adults with and without Mild Cognitive Impairment: An Exploratory Study. J. Alzheimers Dis. 2021, 84, 1091–1102. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Liu, M.N.; Wang, H.C.; Walsh, V.; Lau, C.I. Combining Transcranial Direct Current Stimulation With Tai Chi to Improve Dual-Task Gait Performance in Older Adults With Mild Cognitive Impairment: A Randomized Controlled Trial. Front. Aging Neurosci. 2021, 13, 766649. [Google Scholar]
- Manenti, R.; Sandrini, M.; Gobbi, E.; Binetti, G.; Cotelli, M. Effects of Transcranial Direct Current Stimulation on Episodic Memory in Amnestic Mild Cognitive Impairment: A Pilot Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 1403–1413. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Harrison, R.; Lo, O.Y.; Travison, T.G.; Hausdorff, J.M.; Pascual-Leone, A.; Lipsitz, L. Transcranial Direct Current Stimulation May Improve Cognitive-Motor Function in Functionally Limited Older Adults. Neurorehabil. Neural Repair. 2018, 32, 788–798. [Google Scholar] [CrossRef]
- Martin, D.M.; Mohan, A.; Alonzo, A.; Gates, N.; Gbadeyan, O.; Meinzer, M.; Sachdev, P.; Brodaty, H.; Loo, C. A Pilot Double-Blind Randomized Controlled Trial of Cognitive Training Combined with Transcranial Direct Current Stimulation for Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. 2019, 71, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, S.; Amiri, M.; Hassani, A.; Esmaeilpour, K.; Sheibani, V. Anodal HD-tDCS on the dominant anterior temporal lobe and dorsolateral prefrontal cortex: Clinical results in patients with mild cognitive impairment. Alzheimers Res. Ther. 2024, 16, 27. [Google Scholar] [CrossRef]
- Sandrini, M.; Manenti, R.; Brambilla, M.; Cobelli, C.; Cohen, L.G.; Cotelli, M. Older adults get episodic memory boosting from noninvasive stimulation of prefrontal cortex during learning. Neurobiol. Aging 2016, 39, 210–216. [Google Scholar] [CrossRef]
- Šimko, P.; Pupíková, M.; Gajdoš, M.; Klobušiaková, P.; Vávra, V.; Šimo, A.; Rektorová, I. Exploring the impact of intensified multiple session tDCS over the left DLPFC on brain function in MCI: A randomized control trial. Sci. Rep. 2024, 14, 1512. [Google Scholar] [CrossRef] [PubMed]
- Stonsaovapak, C.; Hemrungroj, S.; Terachinda, P.; Piravej, K. Effect of Anodal Transcranial Direct Current Stimulation at the Right Dorsolateral Prefrontal Cortex on the Cognitive Function in Patients with Mild Cognitive Impairment: A Randomized Double-Blind Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.; Anthony, M.; Tadin, D.; Porsteinsson, A.P.; Heffner, K.; Lin, F.V. Effect of online tDCS to left somatomotor cortex on neuropsychiatric symptoms among older adults at risk for dementia. Cortex 2023, 159, 131–141. [Google Scholar] [CrossRef]
- Yun, K.; Song, I.U.; Chung, Y.A. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res. Ther. 2016, 8, 49. [Google Scholar] [CrossRef]
- Shin, Y.I.; Foerster, Á.; Nitsche, M.A. Transcranial direct current stimulation (tDCS)—Application in neuropsychology. Neuropsychologia 2015, 69, 154–175. [Google Scholar] [CrossRef]
- Meinzer, M.; Lindenberg, R.; Antonenko, D.; Flaisch, T.; Flöel, A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013, 33, 12470–12478. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar] [CrossRef]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [PubMed]
- Pope, P.A.; Brenton, J.W.; Miall, R.C. Task-Specific Facilitation of Cognition by Anodal Transcranial Direct Current Stimulation of the Prefrontal Cortex. Cereb. Cortex 2015, 25, 4551–4558. [Google Scholar]
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [PubMed]
- Manenti, R.; Sandrini, M.; Gobbi, E.; Cobelli, C.; Brambilla, M.; Binetti, G.; Cotelli, M. Strengthening of Existing Episodic Memories Through Non-invasive Stimulation of Prefrontal Cortex in Older Adults with Subjective Memory Complaints. Front. Aging Neurosci. 2017, 9, 401. [Google Scholar]
- Smith, J.Q.; Jones, M.R.; Brown, C.D. The Future of Work: Implications for Managerial Innovation and Resource Management. Bus. Horiz. 2018, 61, 1–12. [Google Scholar]
- Antonenko, D.; Fromm, A.E.; Thams, F.; Kuzmina, A.; Backhaus, M.; Knochenhauer, E.; Li, S.C.; Grittner, U.; Flöel, A. Cognitive training and brain stimulation in patients with cognitive impairment: A randomized controlled trial. Alzheimer’s Res. Ther. 2024, 16, 6. [Google Scholar] [CrossRef]
- Talsma, L.J.; Kroese, H.A.; Slagter, H.A. Boosting Cognition: Effects of Multiple-Session Transcranial Direct Current Stimulation on Working Memory. J. Cogn. Neurosci. 2017, 29, 755–768. [Google Scholar]
- Lindenberger, U.; Lövdén, M. Brain Plasticity in Human Lifespan Development: The Exploration-Selection-Refinement Model. Annu. Rev. Dev. Psychol. 2019, 1, 197–222. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).