Mechanical Circulatory Support in Paraganglioma Induced Cardiogenic Shock and Intestinal Ischemia: Lessons from a Complex Case and Narrative Review
Abstract
1. Introduction
2. Material and Methods
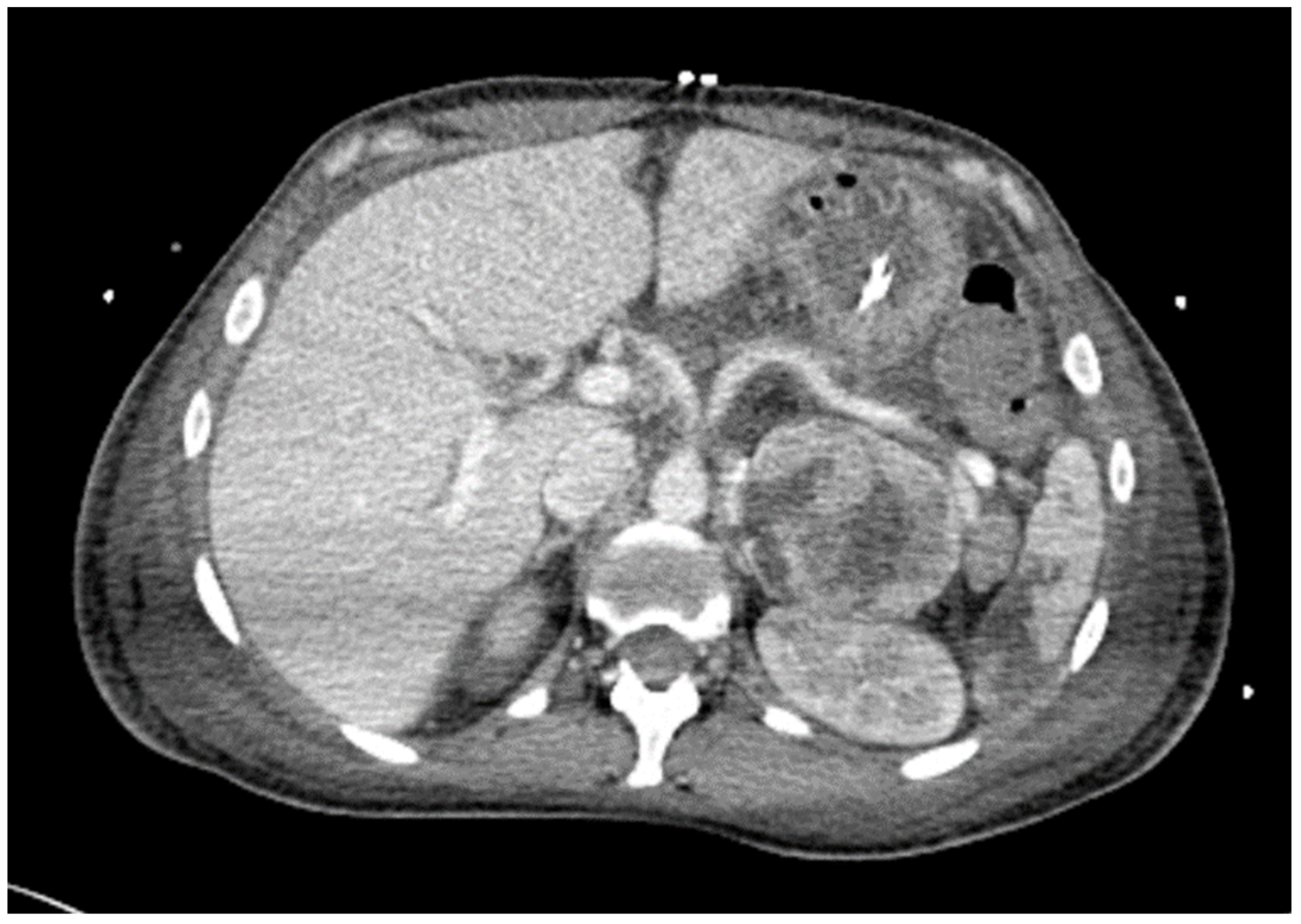
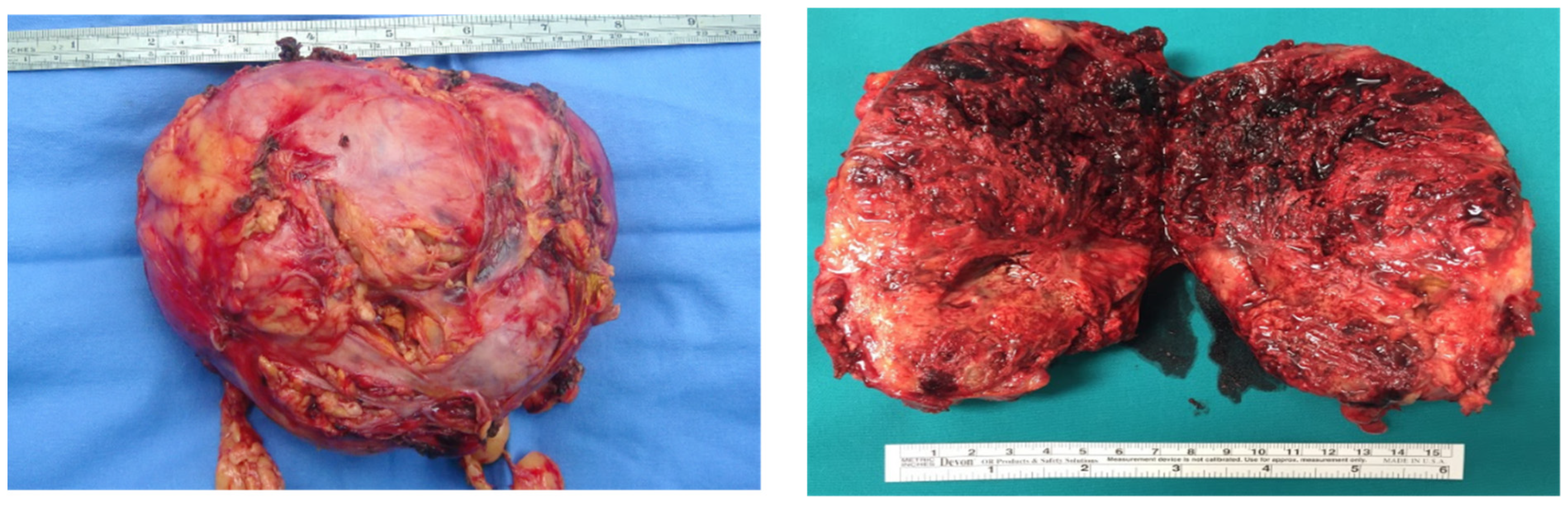
2.1. Case Presentation
2.2. Review Methods
2.3. Search Strategies
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopetschke, R.; Slisko, M.; Kilisli, A.; Tuschy, U.; Wallaschofski, H.; Fassnacht, M.; Ventz, M.; Beuschlein, F.; Reincke, M.; Reisch, N.; et al. Frequent incidental discovery of phaeochromocytoma: Data from a German cohort of 201 phaeochromocytoma. Eur. J. Endocrinol. 2009, 161, 355–361. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline incollaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef]
- Naranjo, J.; Dodd, S.; Martin, Y.N. Perioperative management of pheochromocytoma. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1427–1439. [Google Scholar] [CrossRef]
- Riester, A.; Weismann, D.; Quinkler, M.; Lichtenauer, U.; Sommerey, S.; Halbritter, R.; Penning, R.; Spitzweg, C.; Schopohl, J.; Beuschlein, F.; et al. Life-Threatening Events in Patients with Pheochromocytoma. Eur. J. Endocrinol. 2015, 173, 757–764. [Google Scholar] [CrossRef]
- Scholten, A.; Cisco, R.M.; Vriens, M.R.; Cohen, J.K.; Mitmaker, E.J.; Liu, C.; Tyrrell, J.B.; Shen, W.T.; Duh, Q.-Y. Pheochromocytoma Crisis Is Not a Surgical Emergency. J. Clin. Endocrinol. Metab. 2013, 98, 581–591. [Google Scholar] [CrossRef]
- Choudhary, M.; Chen, Y.; Friedman, O.; Cuk, N.; Ben-Shlomo, A. Pheochromocytoma Crisis Presenting with ARDS Successfully Treated with ECMO-Assisted Adrenalectomy. AACE Clin. Case Rep. 2021, 7, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Lentschener, C.; Gaujoux, S.; Tesniere, A.; Dousset, B. Point of Controversy: Perioperative Care of Patients Undergoing Pheochromocytoma RemovalTime for a Reappraisal? Eur. J. Endocrinol. 2011, 165, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Meijs, A.C.; Snel, M.; Corssmit, E.P.M. Pheochromocytoma/paraganglioma Crisis: Case Series from a Tertiary Referral Center for Pheochromocytomas and Paragangliomas. Hormones 2021, 20, 395–403. [Google Scholar] [CrossRef]
- Whitelaw, B.C.; Prague, J.K.; Mustafa, O.G.; Schulte, K.M.; Hopkins, P.A.; Gilbert, J.A. Phaeochromocytoma [Corrected] Crisis. Clin. Endocrinol. 2013, 80, 468, Erratum in Clin. Endocrinol. 2014, 80, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.T.; Challis, B.G.; Pitfield, D.; Mahroof, R.M.; Jamieson, N.; Bhagra, C.J.; Vuylsteke, A.; Pettit, S.J.; Chatterjee, K.C. Management of an acute catecholamine-induced cardiomyopathy and circulatory collapse: A multidisciplinary approach. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 17–0122. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sauneuf, B.; Chudeau, N.; Champigneulle, B.; Bouffard, C.; Antona, M.; Pichon, N.; Marrache, D.; Sonneville, R.; Marchalot, A.; Welsch, C.; et al. Pheochromocytoma Crisis in the ICU: A French Multicenter Cohort Study with Emphasis on Rescue Extracorporeal Membrane Oxygenation. Crit. Care Med. 2017, 45, e657–e665. [Google Scholar] [CrossRef]
- Kaese, S.; Schülke, C.; Fischer, D.; Lebiedz, P. Pheochromocytoma-induced takotsubo-like cardiomyopathy and global heart failure with need for extracorporal life support. Intensive Care Med. 2013, 39, 1473–1474. [Google Scholar] [CrossRef]
- Law, C.; Khaliq, A.; Guglin, M. Reversible cardiogenic shock due to catecholamine- induced cardiomyopathy: A variant of takotsubo? Am. J. Emerg. Med. 2013, 31, 1621.e1-3. [Google Scholar] [CrossRef]
- Shawa, H.; Bajaj, M.; Cunningham, G.R. Pheochromocytoma-induced atrialtachycardia leading to cardiogenic shock and cardiac arrest: Resolution with atrioventricular node ablation and pacemaker placement. Tex. Heart Inst. J. 2014, 41, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Wang, C.-H.; You, H.-C.; Chou, N.-K.; Yu, H.-Y.; Chi, N.-H.; Huang, S.-C.; Wu, I.-H.; Tseng, L.-J.; Lin, M.-H.; et al. Highlighting Indication of extracorporeal membrane oxygenation in endocrine emergencies. Sci. Rep. 2015, 5, 13361. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Su, L.; Long, Y.; Du, W.; Miao, Q.; Li, F.; Jin, Z.; Zeng, Z.; Luo, A.; et al. Pheochromocytoma crisis with severe cyclic blood pressure fluctuations in a cardiac pheochromocytoma patient successfully resuscitated by extracorporeal membrane oxygenation: A case report. Medicine 2015, 94, e790. [Google Scholar] [CrossRef] [PubMed]
- Flam, B.; Broomé, M.; Frenckner, B.; Bränström, R.; Bell, M. Pheochromocytomainduced inverted takotsubo-like cardiomyopathy leading to cardiogenic shock successfully treated with extracorporeal membrane oxygenation. J. Intensive Care Med. 2015, 30, 365–372. [Google Scholar] [CrossRef]
- Vagner, H.; Hey, T.M.; Elle, B.; Jensen, M.K. Embolisation of pheochromocytoma to stabilise and wean a patient in cardiogenic shock from emergency extracorporeal life support. BMJ Case Rep. 2015, 2015, bcr2014206069. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Agozzino, M.; Pellegrini, C.; Narula, N.; Pietrabissa, A.; Concardi, M.; Caspani, C.; Ghirotto, E.; Giordano, C.; Smirnova, A.; et al. Endomyocardial Biopsy in acute cardiogenic shock: Diagnosis of pheochromocytoma. Int. J. Cardiol. 2016, 202, 897–899. [Google Scholar] [CrossRef]
- Dang Van, S.; Hamy, A.; Hubert, N.; Fouquet, O. Cardiogenic shock induced by a voluminous phaeochromocytoma rescued by concomitant extracorporeal life support and open left adrenalectomy. Eur. J. Cardiothorac. Surg. 2016, 50, 782–783. [Google Scholar] [CrossRef]
- Hekimian, G.; Kharcha, F.; Bréchot, N.; Schmidt, M.; Ghander, C.; Lebreton, G.; Girerd, X.; Tresallet, C.; Trouillet, J.-L.; Leprince, P.; et al. Extracorporeal membrane oxygenation for pheochromocytoma-induced cardiogenic shock. Ann. Intensive Care 2016, 6, 117. [Google Scholar] [CrossRef]
- van Zwet, C.J.; Rist, A.; Haeussler, A.; Graves, K.; Zollinger, A.; Blumenthal, S. Extracorporeal Membrane Oxygenation for Treatment of Acute Inverted Takotsubo-Like Cardiomyopathy from Hemorrhagic Pheochromocytoma in Late Pregnancy. A A Case Rep. 2016, 7, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Mita, K.; Tsugita, K.; Yasuda, Y.; Matsuki, Y.; Obata, Y.; Matsuki, Y.; Kamisawa, S.; Shigemi, K. A successfully treated case of cardiac arrest after Caesarean section complicated by pheochromocytoma crisis and amniotic fluid embolism. J. Anesthesia 2017, 31, 140–143. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Bouchard, D.; Jolicoeur, E.M.; Chronopoulos, A.; Garneau, P.Y.; Lamarche, Y. Extracorporeal membrane oxygenation in pheochromocytoma-induced cardiogenic shock. Asian Cardiovasc. Thorac. Ann. 2018, 26, 314–316. [Google Scholar] [CrossRef]
- Kang, M.S.; Sandhu, C.S.; Singh, N.; Evans, T. Initiation of levothyroxine in a patient with hypothyroidism inducing adrenal crisis requiring VA ECMO: A tale of preventable disaster. BMJ Case Rep. 2019, 12, e230601. [Google Scholar] [CrossRef]
- Mierke, J.; Loehn, T.; Linke, A.; Ibrahim, K.; Savarese, G.; Xaplanteris, P.; D’AMario, D.; Cassar, M.P.; Green, P. Reverse takotsubo cardiomyopathy– life-threatening symptom of an incidental pheochromocytoma: A case report. Eur. Hear. J.-Case Rep. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Huang, D.; Tilton, S.; Cavarocchi, N.C.; Hirose, H. Cardiogenic Shock Requiring Extracorporeal Membrane Oxygenation Support in a Patient with Panhypopituitarism: A Case Report. Cureus 2019, 11, e4995. [Google Scholar] [CrossRef]
- Garla, V.V.; Gosi, S.; Kanduri, S.; Lien, L. A case of catecholamine-induced cardiomyopathy treated with extracorporeal membrane oxygenation. BMJ Case Rep. 2019, 12, e230196. [Google Scholar] [CrossRef] [PubMed]
- Min, D. Catastrophic catecholamine-induced cardiomyopathy rescued by extracorporeal membrane oxygenation in recurrent malignant pheochromocytoma. Yeungnam Univ. J. Med. 2019, 36, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kiamanesh, O.; Vu, E.N.; Webber, D.L.; Lau, E.; Kapeluto, J.E.; Stuart, H.; Wood, D.A.; Wong, G.C. Pheochromocytoma-Induced Takotsubo Syndrome Treated with Extracorporeal Membrane Oxygenation: Beware of the Apical Sparing Pattern. JACC Case Rep. 2019, 1, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Montalto, A.; Nicolò, F.; Polizzi, V.; Comisso, M.; Musumeci, F. Fast myocardial recovery ensured by the combined use of V-A ECMO and IMPELLA CP in cardiogenic shock related to a pheochromocytoma crisis. J. Card. Surg. 2020, 35, 2367–2369. [Google Scholar] [CrossRef]
- Faria, B.M.M.; Português, J.; Roncon-Albuquerque, R., Jr.; Pimentel, R. Inverted takotsubo syndrome complicated with cardiogenic shock requiring venoarterial extracorporeal membrane oxygenation in a patient with bilateral pheochromocytoma: A case report. Eur. Heart J.-Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Dominedò, C.; D’aVino, E.; Martinotti, A.; Cingolani, E.; Cameli, M.; Cankovic, M.Z.; Mukherjee, R.; Guella, E. A rare pheochromocytoma complicated by cardiogenic shock and posterior reversible encephalopathy syndrome: Case report. Eur. Heart J.-Case Rep. 2021, 5, ytaa513. [Google Scholar] [CrossRef]
- Attisani, M.; Pocar, M.; Brenna, D.; Marro, M.; Rinaldi, M.; Boffini, M. Extracorporeal Membrane Oxygenation with Ventricular Unloading Allows for Immediate Adrenergic Blockage in Pheochromocytoma-Induced Cardiogenic Shock. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 3039–3041. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhou, L.; Mo, X.; Huang, G.; Wu, P. Successful treatment of severe electrolyte imbalance-induced cardiac arrest caused by adrenal tuberculosis with ECMO in the ED. Int. J. Emerg. Med. 2021, 14, 55. [Google Scholar] [CrossRef]
- Nakayama, T.; Ito, K.; Inagaki, F.; Miyake, W.; Katagiri, D.; Mihara, F.; Takemura, N.; Kokudo, N. Pheochromocytoma Crisis Rescued by Veno-Arterial Extracorporeal Membrane Oxygenation and Continuous Renal Replacement Therapy. Am. Surg. 2023, 89, 2857–2860. [Google Scholar] [CrossRef]
- Chen, J.; Jin, G.; Zhu, Y.; Hu, W.; He, H.; Wang, C.; Cai, X. The importance of perioperative and complication management in the treatment of pheochromocytoma crisis with venoarterial extracorporeal membrane oxygenation (V-A ECMO): A case report and review of the literature. Perfusion 2021, 38, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.S.; DeNino, W.F.; Jara, C.B.; Schwartzman, A.D.; Butterly, A.D.; Sauer, W.J. Case Report of Fulminant Eosinophilic Myocarditis with Concomitant Pheochromocytoma. A A Pract. 2021, 15, e01348. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Krieg, K.; Massberg, S.; Orban, M.; Hassan, M.; Asher, E.; Cankovic, M.Z.; Simovic, S.; Ranganathan, D. Venoarterial extracorporeal membrane oxygenation as bridge to effective treatment in a 19-year-old woman with acute adrenal crisis: A case report. Eur. Heart J.-Case Rep. 2021, 5, ytab031. [Google Scholar] [CrossRef]
- Myatt, T.; Barker, M. Pheochromocytoma Leading to Multiorgan Failure in a Pregnant Patient: A Case Report. Clin. Pract. Cases Emerg. Med. 2021, 5, 394–398. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xu, C.-Y.; Geng, C.-J.; Liu, L.; Zhang, M.-Z.; Wang, H.; Xiao, R.-T.; Liu, L.; Zhang, G.; Ni, C.; et al. Anesthesia and perioperative management for giant adrenal Ewing’s sarcoma with inferior vena cava and right atrium tumor thrombus: A case report. World J. Clin. Cases 2022, 10, 643–655. [Google Scholar] [CrossRef]
- Lyu, T.; Niu, J.; Liu, Z.; Li, T. Case Report: Early Resection of Pheochromocytoma in a Patient with Cardiogenic Shock Due to Pheochromocytoma-Induced Cardiomyopathy With Extracorporeal Life Support. Front. Cardiovasc. Med. 2022, 9, 788644. [Google Scholar] [CrossRef]
- Luo, S.; Cui, Q.; Wang, D. Case Report: Surgical Intervention Under Pheochromocytoma Multisystem Crisis: Timing and Approach. Front. Oncol. 2022, 12, 908039. [Google Scholar] [CrossRef]
- Fennell, D.; Miller, C.; Ludgate, S.; Conneely, J.; O’brien, S.; Conrick-Martin, I.; Hastings, J.; McQuaid, S.E. Two cases of cardiomyopathy associated with phaeochromocytoma successfully managed with veno-arterial extracorporeal membrane oxygenation (V-A ECMO). Endocrinol. Diabetes Metab. Case Rep. 2023, 2023, 22–0392. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, A.; Qi, M.; Xiong, B.; Zhang, S.; Zhou, J.; Cao, Y. Pheochromocytoma crisis with refractory Acute Respiratory Distress Syndrome (ARDS), Takotsubo syndrome, emergency adrenalectomy, and need for Extracorporeal Membrane Oxygenation (ECMO) in a previously undiagnosed and asymptomatic patient, due to the use of metoclopramide. BMC Endocr. Disord. 2023, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zhu, Y.; Wang, S.; Fang, H.; Chen, G. Continuous blood purification in patients with pheochromocytoma crisis: A case report. Clin. Case Rep. 2023, 11, e8036. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.Q.; Tran, H.T.N.; Tran, T.V.; Nguyen, T.T. An Undetected Pheochromocytoma Leading to Fulminant Adrenergic Myocarditis Complicated by Cardiogenic Shock. JCEM Case Rep. 2023, 1, luad142. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.; Lee, D.I.; Lee, J.-H.; Kim, S.; Lee, S.Y.; Bae, J.-W.; Hwang, K.-K.; Kim, D.-W.; Cho, M.-C.; et al. Successful extracorporeal membrane oxygenation treatment of catecholamine-induced cardiomyopathy-associated pheochromocytoma: A case report. Acute Crit. Care 2024, 39, 194–198. [Google Scholar] [CrossRef]
- Chen, M.; Yan, W.; Yang, Z.; Hong, T.; Jin, L.; Cao, D.; Gu, Y. Surgical anesthesia in a patient with a pheochromocytoma crisis supported by ECMO: A case report. J. Cardiothorac. Surg. 2024, 19, 674. [Google Scholar] [CrossRef]
- Nazari, M.A.; Hasan, R.; Haigney, M.; Maghsoudi, A.; Lenders, J.W.; Carey, R.M.; Pacak, K. Catecholamine-induced hypertensive crises: Current insights and management. Lancet Diabetes Endocrinol. 2023, 11, 942–954. [Google Scholar] [CrossRef]
- Alkaissi, H.; Nazari, M.A.; Vanova, K.H.; Uher, O.; Gordon, C.M.; Talvacchio, S.; Diachenko, N.; Mukvich, O.; Wang, H.; Glod, J.; et al. Rapid Cardiovascular Response to Belzutifan in HIF2A -Mediated Paraganglioma. N. Engl. J. Med. 2024, 391, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Kaneshima, H.; Miura, N.; Tsuchiya, A.; Morita, S.; Nakagawa, Y. Atomoxetine-Induced Pheochromocytoma and Paraganglioma Crisis Managed with Veno-Arterial Extracorporeal Membrane Oxygenation. Cureus 2024, 16, e73582. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef]
- Giordano, A.; Balla, A.; Prosperi, P.; Morales-Conde, S.; Bergamini, C. Robotic vs. Laparoscopic Adrenalectomy for Pheochromocytoma—A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3806. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Alemanno, G.; Bergamini, C.; Valeri, A.; Prosperi, P. Laparoscopic adrenalectomy for giant adrenal tumours: Technical considerations and surgical outcome. J. Minimal Access Surg. 2021, 17, 76–80. [Google Scholar] [CrossRef]
- Coccolini, F.; Roberts, D.; Ansaloni, L.; Ivatury, R.; Gamberini, E.; Kluger, Y.; Moore, E.E.; Coimbra, R.; Kirkpatrick, A.W.; Pereira, B.M.; et al. Share The open abdomen in trauma and non-trauma patients: WSES guidelines. World. J. Emerg. Surg. 2018, 13, 7. [Google Scholar] [CrossRef]

| First Author | Year | Patients, n | Mean Age (Years) | Sex | Adrenal Lesion | Initial LVEF (%) | Mechanical Circulatory Support; Duration (Days) | ICU-Admission -to-Surgery Interval (Days) | Hospital Mortality, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kaese [13] | 2013 | 1 | 43 | M | Right adrenal PHEO | NR | ECMO; 9 | NR, after ECMO weaning | 0 (0%) |
| Law [14] | 2013 | 1 | 23 | F | Right adrenal PHEO | 5 | ECMO; 1 | NR, after ECMO weaning | 0 (0%) |
| Shawa [15] | 2014 | 1 | 38 | F | Left adrenal PHEO | 15 | Tandem Heart; 1 | 26 | 0 (0%) |
| Riester [4] | 2015 | 2 | 18 | F | Right adrenal PHEO | NR | Impella; 2 | No surgery | 2 (100%) |
| Chao [16] | 2015 | 4 | 25 | 1 F, 3 M | 3 Left adrenal PHEO, 1 Right adrenal PHEO | NR | ECMO, NR | NR, after ECMO Weaning (1 refused surgery) | 4 (100%) |
| Zhou [17] | 2015 | 1 | 35 | M | PARA | 20 | ECMO and IABP; 16 | NR, after ECMO Weaning | 0 (0%) |
| Flam [18] | 2015 | 1 | 46 | F | Left adrenal PHEO | 15 | ECMO; 15 | 66 | 0 (0%) |
| Vagner [19] | 2015 | 1 | 55 | F | Left adrenal PHEO | 15 | Impella then ECLS; 2 | 56 | 0 (0%) |
| Kodama [20] | 2016 | 1 | 37 | F | Right adrenal PHEO | 10 | ECMO; NR | NR, before ECMO weaning | 0 (0%) |
| Dang Van [21] | 2016 | 1 | 57 | M | PARA | 10 | ECMO;7 | NR, concomitant from the ECMO implantation | 0 (0%) |
| Hekimian [22] | 2016 | 9 | 43 | 7 F, 2 M | 4 Right adrenal PHEO, 5 Left adrenal PHEO | 16 (median) | ECMO; 15 (median) | 35 (median) | 3 (33.3%) |
| van Zwet [23] | 2016 | 1 | 27 | F (Pregnant) | Left adrenal PHEO | NR | ECMO; 7 | 22 | 0 (0%) |
| Mita [24] | 2016 | 1 | 29 | F, (Pregnant) | Left adrenal PHEO | Nr | IABP, 4; ECMO, 6 | 60 | 0 (0%) |
| Bouabdallaoui [25] | 2017 | 1 | 40 | F | Left adrenal PHEO | 15 | ECMO; 5 | 28 | 0 (0%) |
| Sauneuf [12] | 2017 | 14 | 43 | 7 F, 7 M | Nr | Nr | ECMO; 4 (median) | 10 (median) | 3 (21.4%) |
| Kang [26] | 2019 | 1 | 31 | M | Right adrenal PHEO | 10 | ECMO; 3 | NR, after ECMO Weaning | 0 (0%) |
| Mierke [27] | 2019 | 1 | 47 | M | Left adrenal PHEO | NR | Impella + ECMO; 3 | 33 | 0 (0%) |
| Huang [28] | 2019 | 1 | 58 | F | PARA | NR | ECMO; 5 | NR, after ECMO Weaning | 0 (0%) |
| Garla [29] | 2019 | 1 | 55 | F | Right adrenal PHEO | 11 | IABP + ECMO; 5 | 21 | 0 (0%) |
| Min [30] | 2019 | 1 | 31 | F | Right adrenal PHEO | 6 | ECMO; NR | 28 | 0 (0%) |
| Kiamanesh [31] | 2019 | 1 | 45 | F | Right adrenal PHEO | NR | ECMO; 3 | NR, after ECMO Weaning | 0 (0%) |
| Montalto [32] | 2020 | 1 | 28 | F | Left adrenal PHEO | 7 | ECMO, 4 + Impella, 6 | 21 | 0 (0%) |
| Dominedò [33] | 2020 | 1 | 26 | F | Bilateral Adrenal Pheo (MEN 1) | NR | ECMO; 14 | 35 | 0 (0%) |
| Dominedò [34] | 2020 | 1 | 28 | F | Left adrenal PHEO | 11 | ECMO, 4 + Impella, 5 | 18 | 0 (0%) |
| Attisani [35] | 2021 | 2 | 43 | 1 F, 1 M | 1 Left adrenal PHEO, 1 Right adrenal PHEO | NR | ECMO; 6 | 13 (median) | 0 (0%) |
| Yang [36] | 2021 | 1 | 40 | M | Bilateral Adrenal Pheo | NR | ECMO; 8 | NR, after ECMO Weaning | 0 (0%) |
| Nakayama [37] | 2021 | 1 | 59 | F | Left adrenal PHEO | 12 | IABP, 4 + ECMO, 3 | 39 | 0 (0%) |
| Chen [38] | 2021 | 1 | 55 | M | PARA | 8 | ECMO; 6 | 23 | 0 (0%) |
| Levin [39] | 2021 | 1 | 33 | F | PARA | NR | ECMO; 4 | 42 | 0 (0%) |
| Lüsebrink [40] | 2021 | 1 | 19 | F | PARA | 15 | ECMO; NR | NR, after ECMO Weaning | 0 (0%) |
| Myatt [41] | 2021 | 1 | 36 | F (pregnant) | Left adrenal PHEO | 13 | ECMO; NR | NR, after ECMO Weaning | 0 (0%) |
| Choudhary [6] | 2021 | 1 | 30 | M | Right adrenal PHEO | 9 | ECMO; 4 | 37 | 0 (0%) |
| Wang [42] | 2022 | 1 | 20 | F | Right adrenal Ewing’s Sarcoma | NR | ECMO; 11 | NR | 0 (0%) |
| Lyu [43] | 2022 | 1 | 54 | F | Right adrenal PHEO | 20 | ECMO; 6 | NR, after ECMO Weaning | 0 (0%) |
| Luo [44] | 2022 | 1 | 50 | F | Left adrenal PHEO | NR | ECMO; 6 | 28 | 0 (0%) |
| Nakayama [37] | 2023 | 1 | 59 | F | Left adrenal PHEO | NR | ECMO; NR | 30 | 0 (0%) |
| Fennell [45] | 2023 | 2 | 42 | 2 F | 2 Right adrenal PHEO (1 case NF1) | 15 (median) | ECMO; 6 | 41 | 0(%) |
| Xie [46] | 2023 | 1 | 46 | F | Left adrenal PHEO | NR | ECMO; 7 | 11 | 0 (0%) |
| Zhong [47] | 2023 | 1 | 32 | M | Right adrenal PHEO | 8 | ECMO; NR | NR, after ECMO Weaning | 0 (0%) |
| Tran [48] | 2023 | 1 | 54 | M | Right adrenal PHEO | 25 | ECMO; 7 | NR, after ECMO Weaning | 0 (0%) |
| Park [49] | 2024 | 1 | 29 | F (pregnant) | Left adrenal PHEO | NR | ECMO; 8 | NR, after ECMO Weaning | 0 (0%) |
| Chen [50] | 2024 | 1 | 64 | F | Left adrenal PHEO | 20 | ECMO; NR | NR, after ECMO Weaning | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, A.; Canu, L.; Mastronardi, M.; Petrone, L.; Sparano, C.; Marzano, M.; Bergamini, C.; Prosperi, P. Mechanical Circulatory Support in Paraganglioma Induced Cardiogenic Shock and Intestinal Ischemia: Lessons from a Complex Case and Narrative Review. J. Clin. Med. 2025, 14, 5882. https://doi.org/10.3390/jcm14165882
Giordano A, Canu L, Mastronardi M, Petrone L, Sparano C, Marzano M, Bergamini C, Prosperi P. Mechanical Circulatory Support in Paraganglioma Induced Cardiogenic Shock and Intestinal Ischemia: Lessons from a Complex Case and Narrative Review. Journal of Clinical Medicine. 2025; 14(16):5882. https://doi.org/10.3390/jcm14165882
Chicago/Turabian StyleGiordano, Alessio, Letizia Canu, Manuela Mastronardi, Luisa Petrone, Clotilde Sparano, Mauro Marzano, Carlo Bergamini, and Paolo Prosperi. 2025. "Mechanical Circulatory Support in Paraganglioma Induced Cardiogenic Shock and Intestinal Ischemia: Lessons from a Complex Case and Narrative Review" Journal of Clinical Medicine 14, no. 16: 5882. https://doi.org/10.3390/jcm14165882
APA StyleGiordano, A., Canu, L., Mastronardi, M., Petrone, L., Sparano, C., Marzano, M., Bergamini, C., & Prosperi, P. (2025). Mechanical Circulatory Support in Paraganglioma Induced Cardiogenic Shock and Intestinal Ischemia: Lessons from a Complex Case and Narrative Review. Journal of Clinical Medicine, 14(16), 5882. https://doi.org/10.3390/jcm14165882





