Abstract
Background: The application of robotic adrenalectomy (RA) has been increasing. However, there is still controversy about whether RA is more feasible than laparoscopic adrenalectomy (LA) for pheochromocytoma (PHEO). Methods: We conducted a systematic review of published articles between 2013 and 2025 according to the PRISMA statement and the Cochrane Handbook for systematic reviews of interventions. The search was conducted in MEDLINE (PubMed, Scholar, and Cochrane databases). Results: Overall, seven studies including 879 patients (RA 358; LA 521) were included. RA might have larger tumor size (MD −0.66, 95% CI −1.18 to 0.13; p < 0.00001) but not for BMI patients (MD −0.24, 95% CI −1.44 to 0.96; p < 0.00001). There were no statistically significant differences in intraoperative complication, conversion to open surgery, postoperative complications, transfusion rate, and perioperative hemodynamic outcomes with the exception of a higher lowest systolic blood pressure in the LA group (MD −1.09, 95% CI −2.35 to 0.18; p < 0.00001). Moreover, estimated blood loss (MD 29.52, 95% CI 4.19 to 54.84; p < 0.00001), operative time (MD 3.85, 95% CI −16.11 to 23.80; p < 0.00001), and the length of hospital stay were in favor of RA (MD 0.42, 95% CI 0.09 to 0.74; p < 0.0001). Conclusions: Both LA and RA are safe and feasible approaches for adrenalectomy in the case of pheochromocytoma. RA seems to have better perioperative results, but further prospective randomized control studies are required to draw definitive conclusions.
1. Introduction
Pheochromocytoma (PHEO) is a neuroendocrine tumor arising from the chromaffin cells of the adrenal medulla [1]. These tumors often secrete catecholamines—epinephrine, norepinephrine, and dopamine—leading to clinical manifestations such as headache, palpitations, and excessive sweating [1,2,3,4]. Despite being historically described as a classical triad, these symptoms occur in only about 25% of cases [2,3]. Hypertension remains the most frequent presentation, reported in 80–90% of patients [2], and approximately 12% of patients may develop cardiovascular complications, particularly in those with larger tumors at diagnosis [5].
Surgical resection through adrenalectomy is considered the gold standard for treating PHEO [5,6]. Traditionally, open adrenalectomy was the preferred method, although it is often associated with significant morbidity due to the extensive incisions and tissue dissection required [6,7,8]. Since the 1990s, laparoscopic adrenalectomy (LA) has emerged as a less invasive alternative, offering comparable efficacy [6]. Meta-analyses have shown that LA results in reduced intraoperative blood loss, lower rates of hemodynamic instability, and better postoperative recovery compared to the open approach [7,8].
Selecting the most appropriate surgical technique is a key component in the management of PHEO. Various laparoscopic strategies have been described, with the anterior and lateral transperitoneal approaches, as well as the lateral and posterior retroperitoneal routes, being among the most frequently utilized [9]. The lateral transperitoneal approach is particularly favored for its enhanced visualization facilitated by gravitational organ retraction, its alignment with standard anatomical landmarks, and its suitability for resecting larger adrenal masses [4].
In patients with prior abdominal surgeries, the posterior retroperitoneal approach offers the advantage of avoiding intra-abdominal adhesions, enabling direct access to the adrenal gland without manipulation of other abdominal organs. This technique also allows for bilateral adrenalectomy without repositioning the patient [10].
Both transperitoneal and retroperitoneal techniques are considered safe and effective; however, subtle distinctions exist, and several factors—including the surgeon’s experience, the patient’s body habitus, comorbidities, and potential for complications—should guide the choice of approach.
Laparoscopy, despite its advantages, presents inherent technical limitations such as restricted instrument motion, two-dimensional imaging, and potential amplification of physiologic tremors, which can complicate procedures involving large PHEOs [11]. Robotic adrenalectomy (RA) has been developed to address these challenges, offering enhanced dexterity, tremor filtration, and three-dimensional visualization [12]. RA has increasingly been reported as a safe and feasible alternative for PHEO surgery [11,12,13], although some studies, such as that by Park et al., have not demonstrated significant clinical advantages over the laparoscopic technique [14].
There are concerns that the robotic approach may lead to increased cardiovascular risk, potentially due to the absence of haptic feedback, which could contribute to excessive catecholamine release during manipulation. Nonetheless, these findings are based on small sample sizes, limiting the strength of the conclusions [11,12]. Thus, the question of whether LA or RA offers superior outcomes in the treatment of PHEO remains unresolved.
In light of these considerations, we conducted a systematic review and meta-analysis to compare laparoscopic and robotic adrenalectomy for PHEO, focusing on intraoperative metrics and 30-day postoperative outcomes.
2. Material and Methods
Institutional review board approval and informed consent from participants were unnecessary for the present study.
2.1. Search Strategy
We conducted a systematic review of published articles between 2013 and 2025 according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement [15] and according to the Cochrane Handbook for systematic reviews of interventions [16]. The review protocol was not registered. The search was conducted in MEDLINE (PubMed, Scholar, and Cochrane databases) [17] using the following string research: (Pheocromocitoma) AND (surgery), OR (adrenalectomy), OR (laparoscopy), OR (mini-invasive), OR (mininvasive), OR (robot).
Cross-referencing using articles initially identified was also performed to include additional articles that did not explicitly focus on the topic but reported the requested information in subgroup analyses. Two reviewers (C.B. and A.G.) evaluated the articles retrieved from searches, and disagreements on study selection or data extraction were resolved by consensus and discussion among reviewers.
The PICOS (Population, Intervention, Comparison, Outcomes, and Studies) question was generated from a discussion within the authors. The following PICOS question was adopted:
P: Patients with a pheochromocytoma.
I: Laparoscopic adrenalectomy.
C: Robotic adrenalectomy.
O: Intraoperative complications, blood loss, hemodynamic instability, conversion, operative time, postoperative complications, length of hospital stay (LOS), mortality.
S: Randomized controlled trials, non-randomized controlled trials (retrospective and prospective cohort studies)
2.2. Inclusion Criteria
Articles written in English, Italian, and Spanish were included. All articles included patients who underwent minimally invasive (laparoscopic or robotic) adrenalectomy for PHEO.
2.3. Exclusion Criteria
Articles were excluded if they included patients with other types of adrenal lesions; if they included a cohort of patients retrieved from an article already included; if it was not possible to isolate data only regarding adrenalectomy for PHEO; if they were comments, case reports, correspondence and letters to the editor, editorials, technical surgical notes, conference articles, imaging studies, and articles involving animals.
2.4. Risk of Bias Assessment of Included Articles
Three authors (C.B., A.G., A.B.) used the Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) tool and Cochrane Risk of Bias 2.0 (RoB 2.0) tool in non-randomized and randomized trials, respectively, to assess the risk of bias of the included studies [18,19].
2.5. Study Design
After screening the title and abstract, articles that fulfilled the inclusion criteria were identified, and their full text was reviewed. Data were extracted and stored in an Excel chart (Microsoft Corporation, Redmond, WA, USA).
The following data were extracted from each article: first author, year of publication, type of study, number of patients, sex, age, body mass index (BMI), lesion side (right, left, bilateral), lesion size, American Society of Anesthesiologists (ASA) grade, minimally invasive approach (laparoscopic or robotic), intraoperative events/complications, hemodynamic instability, estimated blood loss (EBL), intraoperative systolic blood pressure and heart rate, conversion to open surgery, operative time, postoperative complications, LOS, and mortality.
2.6. Statistical Analysis
Categorical data were reported as absolute numbers and percentages, while continuous variables were presented as means with corresponding standard deviations (SD). Variables were included in the pooled analysis only when reported by at least two independent studies. Statistical analyses were conducted using Review Manager software (RevMan version 5.4.1; The Nordic Cochrane Centre, Cochrane Collaboration, www.training.cochrane.org, accessed on 1 March 2025). Two independent reviewers (A.B. and A.G.) verified the data entries in the forest plot tables to ensure accuracy.
For dichotomous outcomes, relative risks (RRs) with 95% confidence intervals (CIs) were calculated, while mean differences (MDs) with 95% CIs were used for continuous variables. When studies reported continuous data as medians and ranges, the corresponding means and SDs were estimated using the method described by Hozo et al. [20]. An RR was considered statistically significant at a p value < 0.05 if the 95% CI did not include 1, and an MD was deemed significant under the same P threshold if the 95% CI did not include 0.
Statistical heterogeneity across studies was assessed using both the χ2 test and Higgins’ I2 statistic. A χ2 p value < 0.100 combined with an I2 value > 50% was interpreted as indicating substantial heterogeneity. In addition to statistical heterogeneity, clinical (e.g., differences in baseline patient characteristics, interventions, or outcome definitions) and methodological heterogeneity (e.g., variations in study design and risk of bias) were also taken into account when selecting the appropriate analytical model. Given the considerable heterogeneity observed, a random-effect model was applied to all meta-analyses [21].
2.7. Grading the Quality of Evidence
Three authors (C.B., A.G., A.B.) independently evaluated the quality of evidence for imprecision, inconsistency, indirectness, and publication bias according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach [22]. Certainty of evidence (CoE) was classified as very low, low, moderate, or high [22]. Subsequently, a summary table was created using the GRADE profiler software (version 3.6.1) [22].
3. Results
Following the inclusion and exclusion criteria, seven articles published between December 2013 and March 2025 were included [23,24,25,26,27,28,29], as shown in the PRISMA flow diagram (Figure 1) [15]. The assessment of the risk of bias based on the ROBINS-I and RoB 2.0 of the articles is shown in Table 1. Six articles included were retrospective analyses [23,25,26,27,28,29], and one article was a randomized control trial [24]. Six were single-center studies [23,24,25,27,28,29], and one was a multicenter study [26].
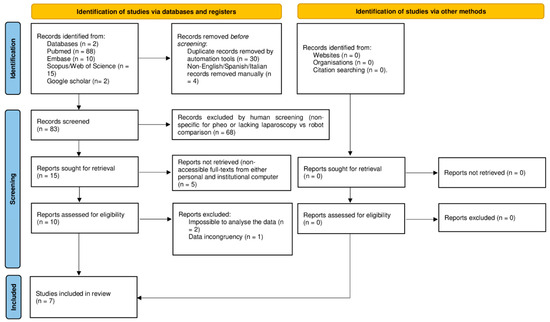
Figure 1.
Preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram [15].

Table 1.
Assessment of risk of bias of included articles based on Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) [18] and based on Cochrane Risk of Bias 2.0 (RoB 2.0) tool for randomized trials [19].
Table 2 shows preoperative patients’ characteristics. Overall, 879 patients were included in the analysis, of which 521 underwent LA (250 men, 48%, and 271 women, 52%), and 358 underwent RA (155 men, 43.8%, and 203 women, 56.7%). In the LA group, 265 (50.9%), 246 (47.2%), and 10 (1.9%) patients underwent right, left, and bilateral adrenalectomy, respectively. In the RA group, 179 (50%), 178 (49.7%), and 1 (0.3%) patients underwent right, left, and bilateral adrenalectomy, respectively. In the LA group, mean lesion size ranged between 4 and 7.6 cm, while in the RA group, it ranged between 4.6 and 8 cm. ASA grade was I–II and III–IV in 128 (39.8% out of 322 patients) and 194 (60.2% out of 322 patients) patients of the LA group, respectively, and 90 (43.9% out of 205 patients) and 115 (56.1% out of 205 patients) in the RA group, respectively [21,22,23,24,25,26,27].

Table 2.
Patients’ preoperative characteristics.
Table 3 reports perioperative results. In the LA group, 175 (40.3%) and 259 (59.7%) adrenalectomies out of 434 were performed by transperitoneal and retroperitoneal approaches, respectively. Meanwhile, in the RA group, 165 (51.9%) and 153 (48.1%) adrenalectomies out of 318 were performed by transperitoneal and retroperitoneal approaches, respectively [21,22,23,24,25,26,27].

Table 3.
Perioperative results.
Intraoperatively, in the LA group, 147 events (28.2%) occurred, including cardiac arrhythmia (1, 0.2%), transfusions (25, 4.8%), hemodynamic instability (113, 21.7%), and hypertensive crisis (8, 1.5%), and 6 complications (1.2%), including spleen bleeding requiring open splenectomy (1, 0.2%), organ injury requiring suture (2, 0.4%), cava vein injury requiring suture (1, 0.2%), bleeding (1, 0.2%), and suspecting spleen rupture requiring open exploration (1, 0.2%). In the RA group, 56 events (15.6%) occurred, including transfusions (19, 5.3%), hemodynamic instability (34, 9.5%), ventricular fibrillation (1, 0.3%), and hypertensive crisis (2, 0.6%), and 3 complications (0.8%) including organ injury requiring suture (1, 0.3%), bleeding (1, 0.3%), and diaphragmatic rupture (1, 0.3%) [23,24,25,26,27,28,29].
Conversion to open surgery occurred in 13 (2.5%) and 2 (0.6%) patients in the LA and RA groups, respectively. The reasons for conversion to open surgery in the LA group were as follows: tumor bleeding (1, 0.2%), accessory renal vein bleeding (1, 0.2%), difficult dissection (4, 0.8%), inadequate surgical field exposure (2, 0.4%), spleen bleeding (1, 0.2%), and four not reported. Meanwhile, in the RA group, the reasons for conversion were tumor adhesions (1, 0.3%) and inadequate surgical field exposure (1, 0.3%) [23,24,25,26,27,28,29].
Postoperative complications and transfusions occurred in 88 (16.9%) and 42 patients (11.7%) and 16 (3.1%) and 17 (4.7%) patients, in the LA and RA groups, respectively. Mortality occurred in one patient in the LA group (0.2%) [23,24,25,26,27,28,29].
Meta-Analysis
Before-surgery age (5 studies, 607 patients; MD 0.42, 95 percent CI −2.90 to 3.73; p = 0.0001; I2 = 83%, random-effect model), BMI (5 studies, 607 patients; MD −0.24, 95 percent CI −1.44 to 0.96; p < 0.00001; I2 = 89%, random-effect model), and lesion size (5 studies, 607 patients; MD −0.66, 95 percent CI −1.18 to 0.13; p < 0.00001; I2 = 88%, random-effect model) were statistically significantly different between the LA and RA groups (Figure 2A–F).
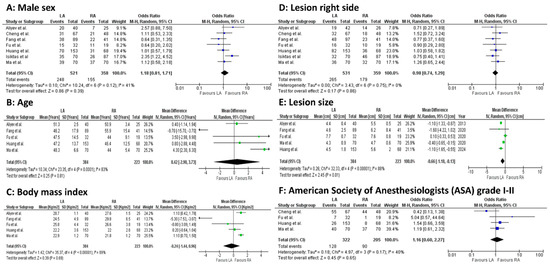
Figure 2.
Meta-analysis of preoperative outcomes. (A): Male sex. (B): Age. (C): Body mass index. (D): Lesion right side. (E): Lesion size. (F): American Society of Anesthesiologists (ASA) grade I–II [23,24,25,26,27,28,29].
Intraoperatively, statistically significant differences occurred for EBL (4 studies, 386 patients; MD 29.52, 95 percent CI 4.19 to 54.84; p < 0.00001; I2 = 90%, random-effect model; CoE: Low) and operative time (4 studies, 386 patients; MD 3.85, 95 percent CI −16.11 to 23.80; p < 0.00001; I2 = 91%, random-effect model; CoE: Low), both in favor of the LA group (Figure 3A–F).
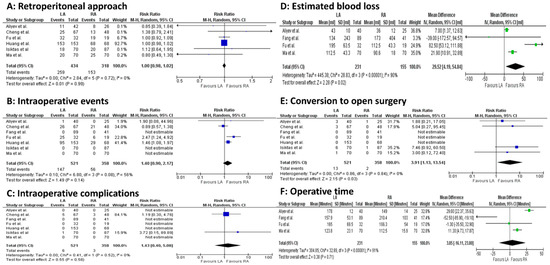
Figure 3.
Meta-analysis of intraoperative outcomes. (A): Retroperitoneal approach. (B): Intraoperative events. (C): Intraoperative complications. (D): Estimated blood loss. (E): Conversion to open surgery. (F): Operative time [23,24,25,26,27,28,29].
Among intraoperative cardiovascular variables, a statistically significant difference occurred for the lowest systolic blood pressure (4 studies, 556 patients; MD −1.09, 95 percent CI −2.35 to 0.18; p < 0.00001; I2 = 99%, random-effect model; CoE: Low) in the LA group (Figure 4A–C).
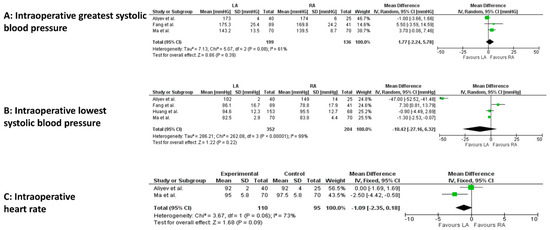
Figure 4.
Meta-analysis of intraoperative cardiovascular variables. (A): Intraoperative greatest systolic blood pressure. (B): Intraoperative lowest systolic blood pressure. (C): Intraoperative heart rate [23,24,26,29].
Postoperatively, LOS was significantly shorter in the RA group (4 studies, 386 patients; MD 0.42, 95 percent CI 0.09 to 0.74; p < 0.0001; I2 = 88%, random-effect model; CoE: Low) (Figure 5A–C).
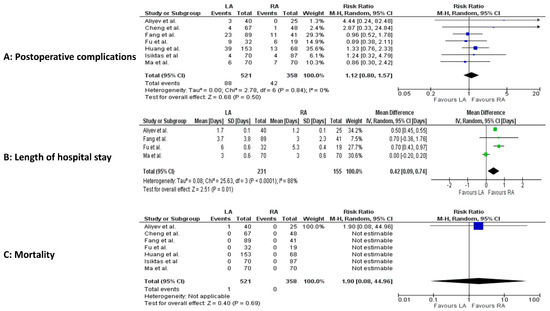
Figure 5.
Meta-analysis of postoperative results. (A): Postoperative complications. (B): Postoperative length of hospital stay. (C): Postoperative mortality [23,24,25,26,27,28,29].
Figure 6 and Figure 7 report the assessment of perioperative evidence according to the GRADE method of the included articles.
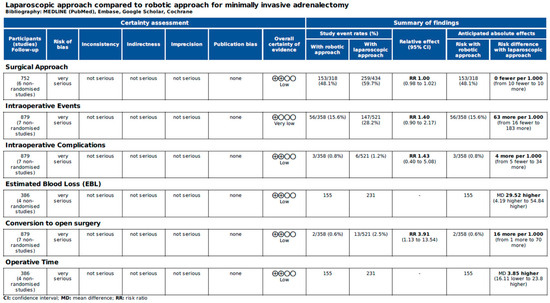
Figure 6.
Assessment of intraoperative evidence of the included articles according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [22].
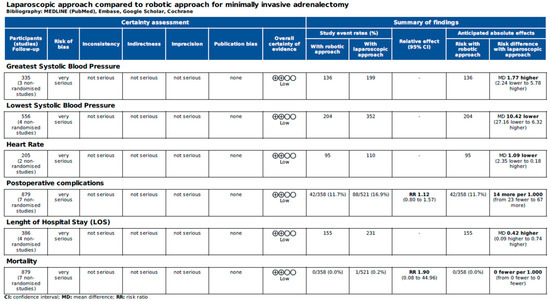
Figure 7.
Assessment of cardiovascular variables and postoperative evidence of the included articles according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [22].
4. Discussion
Adrenalectomy can be technically demanding, particularly in the presence of large tumors, due to the retroperitoneal location of the adrenal glands and their close relationship with major vascular structures and vital abdominal organs [5,30,31]. Since its initial description by Gagner et al. in 1992 [32], the laparoscopic approach (LA) has progressively gained acceptance and is now considered the gold standard for the surgical management of most adrenal tumors [6,32]. LA has demonstrated a favorable safety profile with a low incidence of complications. Nonetheless, certain scenarios—such as large adrenal masses, the need for lymph node dissection, or surgery in obese patients—may present significant technical challenges [30,31,32,33,34]. To address the inherent limitations of laparoscopy, including two-dimensional visualization, limited instrument articulation, surgeon discomfort, and a steep learning curve, robotic-assisted surgery has been introduced. The robotic platform is being increasingly adopted in adrenal surgery, although current evidence remains limited, and large-scale comparative studies evaluating perioperative safety and short-term outcomes relative to laparoscopy are still lacking [13].
This debate in the literature is also open for what concerns the treatment of a severe such as PHEO in which surgical resection remains the therapeutic gold standard [5,6]. Therefore, we conducted a meta-analysis of the existing literature and drew objective conclusions on this topic.
In our meta-analysis, before surgery, patients in the LA group and the RA group had statistically significant differences in terms of age, BMI, and lesion size. As far as BMI is concerned, minimally invasive surgery is an improvement factor in surgical practice compared to the traditional open approach [35]. In this case, the meta-analysis shows a greater predominance of the choice of the laparoscopic approach in patients with a higher BMI, and this depends on the type of minimally invasive approach chosen with a greater prevalence of the retroperitoneal laparoscopic approach for patients with a higher BMI. The impact of obesity on outcomes after adrenalectomy for pheochromocytoma is unclear. A recent study evaluating outcomes after minimally invasive and open adrenalectomy for pheochromocytoma in obese patients, reports that obesity does not increase complications, but it does increase LOS, and the retroperitoneal approach may uniquely benefit patients with obesity [35].
The tumor size was larger in the RA group. This could be the inherent selection bias due to the operative advantages of RA [11,12,36,37,38]. Most of the studies included in this article reported large PHEOs, since the mean tumor size was larger than 4 cm or even 8 cm [23,24,25,26,27,28,29]. Large PHEOs might be related to intense vascularization, adhesion with surrounding tissues, including the inferior vena cava on the right side, and higher cancer risk, which could significantly increase the difficulty of dissection [31,39,40,41]. In general, the size does not constitute a criterion of exclusion for minimally invasive surgery because it constitutes only a predictive factor of malignancy [31,39,40,41]. Obviously, the rules of adequate and accurate manipulation to avoid the rupture of the adrenal capsule must always be followed, especially in the case of pheochromocytomas [30,39,42].
Regarding the perioperative outcomes, in the present analysis, we found a higher rate of intraoperative events (28.2% versus 15.6%), intraoperative complication (1.2% versus 0.8%), conversion to open surgery (2.5% versus 0.6%) and postoperative complications (16.9% versus 11.7%) in the LA group in comparison to the RA group. Moreover, EBL, operative time, and LOS were in favor of RA [23,24,25,26,27,28,29].
Another important aspect to consider is that pheochromocytomas are often catecholamine-secreting tumors. Intraoperatively, hemodynamic crisis is the most challenging factor during the manipulation of the tumor [43,44,45]. Due to the lack of tactile feedback, several studies were concerned about the risk of bleeding, cardiovascular accidents, and intraoperative hypertensive crisis during the dissection of PHEO [45]. Our study found similar perioperative hemodynamic outcomes between the two groups with the exception of a higher lowest systolic blood pressure. However, this could be related to the preoperative α- and β blockers, which could significantly improve perioperative hemodynamic stability [45].
This systematic review and meta-analysis study has several limitations such as the small number of articles included and, as a consequence, the small number of patients for each group, which restricts the statistical power and generalizability of the findings. Furthermore, it is not possible to carry out a sub-analysis based on lesion side, BMI, and surgical approach which could add value to the analysis, due to data reported in the included studies. Moreover, the retrospective nature of most of the included studies introduces inherent biases such as selection and confounding, which have been acknowledged in our risk of bias assessment. The assessment of the risk of bias (Table 1) and the further GRADE assessment of the individual studies (Figure 6 and Figure 7) confirm what has been said. The high degree of heterogeneity observed in the meta-analysis complicates the pooling of results and the interpretation of outcomes, as well as the lack of adequate follow-up for the analysis of long-term oncological results, the lack of a description of centers included as high or low volume, which could influence the results, and the lack of data regarding learning curve and cost analysis. However, despite these limitations, the present study provides the wider systematic review reported in the literature until now, detailing the results of laparoscopic and robotic approaches for the treatment of pheochromocytoma and comparing them, suggesting the potential advantages of the robotic approach.
5. Conclusions
Both laparoscopic adrenalectomy (LA) and robotic adrenalectomy (RA) have been shown to be safe and feasible options for the surgical management of pheochromocytoma. According to the existing literature, the robotic approach may offer certain advantages over laparoscopy in selected parameters, including reduced estimated blood loss, shorter operative times, decreased length of hospital stay, and potentially improved outcomes in the treatment of large pheochromocytomas. However, due to the limited number of available studies—most of which are retrospective and observational in nature—and the significant heterogeneity in patient populations and methodologies, high-quality prospective randomized controlled trials are still needed to establish definitive evidence on the superiority of one technique over the other.
Author Contributions
All authors contributed equally to this work. A.G. took care of the scientific project and the creation of the database for the selection of the articles together with C.B. A.B. took care of the statistical part and the scientific supervision of all the work together with C.B., P.P. and S.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors certify that there is no actual or potential conflict of interest concerning this article, and they state that there are no financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated—including relevant commercial or other sources of funding for the individual author(s) or the associated department(s) or organization(s), personal relationships, or direct academic competition.
References
- Omura, M.; Saito, J.; Yamaguchi, K.; Kakuta, Y.; Nishikawa, T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens. Res. 2004, 27, 193–202. [Google Scholar] [CrossRef]
- Zelinka, T.; Eisenhofer, G.; Pacak, K. Pheochromocytoma as a catecholamine producing tumor: Implications for clinical practice. Stress 2007, 10, 195–203. [Google Scholar] [CrossRef]
- Patel, D.; Phay, J.E.; Yen, T.W.F.; Dickson, P.V.; Wang, T.S.; Garcia, R.; Yang, A.D.; Solórzano, C.C.; Kim, L.T. Update on Pheochromocytoma and Paraganglioma from the SSO Endocrine/Head and Neck Disease-Site Work Group. Part 1 of 2: Advances in Pathogenesis and Diagnosis of Pheochromocytoma and Paraganglioma. Ann. Surg. Oncol. 2020, 27, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- De Crea, C.; Pennestri, F.; Voloudakis, N.; Sessa, L.; Procopio, P.F.; Gallucci, P.; Bellantone, R.; Raffaelli, M. Robot-assisted vs laparoscopic lateral transabdominal adrenalectomy: A propensity score matching analysis. Surg. Endosc. 2022, 36, 8619–8629. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Nissen, N.N.; Bannykh, S.I. Cardiac complications as initial manifestation of pheochromocytoma: Frequency, outcome, and predictors. Endocr. Pract. 2012, 18, 483–492. [Google Scholar] [CrossRef]
- Bihain, F.; Klein, M.; Nomine-Criqui, C.; Brunaud, L. Robotic adrenalectomy in patients with pheochromocytoma: A systematic review. Gland Surg. 2020, 9, 844–848. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Chang, X.; Han, Z. Laparoscopic adrenalectomy (LA) vs open adrenalectomy (OA) for pheochromocytoma (PHEO): A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Q.; Wang, S.Y.; Chen, Q.; Liu, Y.T.; Li, Z.L.; Sun, T. Laparoscopic versus open surgery for pheochromocytoma: A meta-analysis. BMC Surg. 2020, 20, 167. [Google Scholar] [CrossRef]
- Mihai, I.; Boicean, A.; Teodoru, C.A.; Grigore, N.; Iancu, G.M.; Dura, H.; Bratu, D.G.; Roman, M.D.; Mohor, C.I.; Todor, S.B.; et al. Laparoscopic Adrenalectomy: Tailoring Approaches for the Optimal Resection of Adrenal Tumors. Diagnostics 2023, 13, 3351. [Google Scholar] [CrossRef]
- Inaishi, T.; Kikumori, T.; Takeuchi, D.; Ishihara, H.; Miyajima, N.; Shibata, M.; Takano, Y.; Nakanishi, K.; Noda, S.; Kodera, Y. Obesity Does Not Affect Peri- and Postoperative Outcomes of Transabdominal Laparoscopic Adrenalectomy. Nagoya J. Med. Sci. 2018, 80, 21. [Google Scholar]
- Wang, L.; Zeng, W.; Wu, Y.; Gong, Z. Comparison of clinical efficacy and safety between robotic-assisted and laparoscopic adrenalectomy for pheochromocytoma: A systematic review and meta-analysis. J. Robot. Surg. 2024, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Li, J.; Peng, L.; Yang, X.; Xu, Y.; Li, X.; Li, Y.; Zhang, Z.; Wu, J. Comparison of Perioperative Outcomes of Robotic-Assisted vs Laparoscopic Adrenalectomy for Pheochromocytoma: A Meta-Analysis. Front. Oncol. 2021, 11, 724287. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Minervini, A.; Tellini, R.; Ji, C.; Bergamini, C.; Giordano, A.; Lu, Q.; Chen, W.; Zhang, F.; Ji, H.; et al. Perioperative outcomes of robotic and laparoscopic adrenalectomy: A large international multicenter experience. Surg. Endosc. 2021, 35, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, K.Y.; Kim, J.K.; Yoon, D.S. The first laparoscopic resection of extra-adrenal pheochromocytoma using the da Vinci robotic system. J. Laparoendosc. Adv. Surg. Tech. A 2009, 19, 63–65. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 1 March 2025).
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal literature search for systematic reviews in surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45 Pt A, 139–145. [Google Scholar] [CrossRef]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2024. Available online: https://gradepro.org (accessed on 1 March 2025).
- Aliyev, S.; Karabulut, K.; Agcaoglu, O.; Wolf, K.; Mitchell, J.; Siperstein, A.; Berber, E. Robotic versus laparoscopic adrenalectomy for pheochromocytoma. Ann. Surg. Oncol. 2013, 20, 4190–4194. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, Y.; Zhuo, R.; Dai, J.; Fang, C.; Wang, C.; Zhao, J.; He, W.; Zhu, Y.; Xu, D.; et al. Surgical outcomes of a randomized controlled trial compared robotic versus laparoscopic adrenalectomy for pheochromocytoma. Eur. J. Surg. Oncol. 2020, 46 Pt A, 1843–1847. [Google Scholar] [CrossRef]
- Fu, S.Q.; Zhuang, C.S.; Yang, X.R.; Xie, W.J.; Gong, B.B.; Liu, Y.F.; Liu, J.; Sun, T.; Ma, M. Comparison of robot-assisted retroperitoneal laparoscopic adrenalectomy versus retroperitoneal laparoscopic adrenalectomy for large pheochromocytoma: A single-centre retrospective study. BMC Surg. 2020, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.M.; Rosen, J.; Saidian, A.; Bae, S.; Tanno, F.Y.; Chambo, J.L.; Bloom, J.; Gordetsky, J.; Srougi, V.; Phillips, J.; et al. Perioperative outcomes of laparoscopic, robotic, and open approaches to pheochromocytoma. J. Robot. Surg. 2020, 14, 849–854. [Google Scholar] [CrossRef]
- Isiktas, G.; Nazli Avci, S.; Ergun, O.; Krishnamurthy, V.; Jin, J.; Siperstein, A.; Berber, E. Laparoscopic versus robotic adrenalectomy in pheochromocytoma patients. J. Surg. Oncol. 2022, 126, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, Y. Comparison of Perioperative Outcomes Between Laparoscopic and Robot-Assisted Adrenalectomy for Large Pheochromocytoma (≥5 cm): A Retrospective Study. Cancer Manag. Res. 2023, 15, 1207–1216. [Google Scholar] [CrossRef]
- Huang, H.; Sun, T.; Liu, Z. Robot-assisted versus laparoscopic pheochromocytoma resection and construction of a nomogram to predict perioperative hemodynamic instability. Eur. J. Surg. Oncol. 2024, 50, 107986. [Google Scholar] [CrossRef]
- Giordano, A.; Alemanno, G.; Bergamini, C.; Valeri, A.; Prosperi, P. Laparoscopic adrenalectomy for giant adrenal tumours: Technical considerations and surgical outcome. J. Minimal Access Surg. 2021, 17, 76–80. [Google Scholar] [CrossRef]
- Öz, B.; Cücük, Ö.; Gök, M.; Akcan, A.; Sözüer, E. Laparoscopic transperitoneal adrenalectomy for adrenal tumours of 6 cm or greater: A single-centre experience. J. Minimal Access Surg. 2024, 20, 47–54. [Google Scholar] [CrossRef]
- Gagner, M.; Lacroix, A.; Bolté, E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N. Engl. J. Med. 1992, 327, 1033. [Google Scholar] [CrossRef]
- Giordano, A.; Feroci, F.; Podda, M.; Botteri, E.; Ortenzi, M.; Montori, G.; Guerrieri, M.; Vettoretto, N.; Agresta, F.; Bergamini, C. Minimally invasive versus open adrenalectomy for adrenocortical carcinoma: The keys surgical factors influencing the outcomes-a collective overview. Langenbecks Arch. Surg. 2023, 408, 256. [Google Scholar] [CrossRef]
- Ortenzi, M.; Balla, A.; Ghiselli, R.; Vergari, R.; Silecchia, G.; Guerrieri, E.; Maria Paganini, A.; Guerrieri, M. Minimally invasive approach to the adrenal gland in obese patients with Cushing’s syndrome. Minim. Invasive Ther. Allied Technol. 2019, 28, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, K.; Parente, A.; Wang, Y.; Wang, N.; Wang, Z.; Śledziński, M.; Hellmann, A.; Raffaelli, M.; Pennestrì, F.; Sywak, M.; et al. Outcomes for Patients with Obesity Undergoing Adrenalectomy for Pheochromocytoma: An International Multicenter Analysis. Ann. Surg. Oncol. 2025, 32, 1709–1720. [Google Scholar] [CrossRef]
- Du, L.; Yang, Z.; Qi, J.; Wang, Y. Robotic adrenalectomy versus laparoscopic adrenalectomy for pheochromocytoma: A systematic review and meta-analysis. Wideochir. Inne Tech. Maloinwazyjne 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Balla, A.; Corallino, D.; Ortenzi, M.; Palmieri, L.; Meoli, F.; Guerrieri, M.; Paganini, A.M. Cancer risk in adrenalectomy: Are adrenal lesions equal or more than 4 cm a contraindication for laparoscopy? Surg. Endosc. 2022, 36, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Alzelfawi, L.; Almajed, E.; Alhindawi, Z.; AlDosari, L.; Alhumaidan, A.; Alharthi, B. Feasibility of laparoscopic adrenalectomy in adrenal masses greater than 5 centimeters: A systematic review and meta-analysis. Gland Surg. 2024, 13, 952–968. [Google Scholar] [CrossRef]
- Alberici, L.; Paganini, A.M.; Ricci, C.; Balla, A.; Ballarini, Z.; Ortenzi, M.; Casole, G.; Quaresima, S.; Di Dalmazi, G.; Ursi, P.; et al. Development and validation of a preoperative “difficulty score” for laparoscopic transabdominal adrenalectomy: A multicenter retrospective study. Surg. Endosc. 2022, 36, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, K.; Qing, J.; Chen, J.; Li, C.; Zhou, Y. Hyper-realistic rendering-assisted laparoscopic adrenalectomy for giant adrenal tumors: A pilot study. World J. Urol. 2024, 42, 550. [Google Scholar] [CrossRef]
- Gaillard, M.; Razafinimanana, M.; Challine, A.; Araujo, R.L.C.; Libé, R.; Sibony, M.; Barat, M.; Bertherat, J.; Dousset, B.; Fuks, D.; et al. Laparoscopic or Open Adrenalectomy for Stage I–II Adrenocortical Carcinoma: A Retrospective Study. J. Clin. Med. 2023, 12, 3698. [Google Scholar] [CrossRef]
- Paganini, A.M.; Balla, A.; Guerrieri, M.; Lezoche, G.; Campagnacci, R.; D’Ambrosio, G.; Quaresima, S.; Antonica, M.V.; Lezoche, E. Laparoscopic transperitoneal anterior adrenalectomy in pheochromocytoma: Experience in 62 patients. Surg. Endosc. 2014, 28, 2683–2689. [Google Scholar] [CrossRef]
- Chai, Y.J.; Yu, H.W.; Song, R.Y.; Kim, S.J.; Choi, J.Y.; Lee, K.E. Lateral transperitoneal adrenalectomy versus posterior retroperitoneoscopic adrenalectomy for benign adrenal gland disease: Randomized controlled trial at a single tertiary medical centre. Ann. Surg. 2019, 269, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tai, Y.; Shang, J. Progress in treatment and follow-up of pheochromocytoma. Eur. J. Surg. Oncol. 2025, 51, 110144. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, T.A.A.M.; Elias, A.A.; Adam, A.A.M.; Gadallah, M.A.; Ahmed, S.M.A.; Khyrallh, A.; Alsayed, M.H.; Awad, E.T.K.; Ibrahim, E.A.; Labib, M.F.; et al. Early readmission after adrenalectomy for pheochromocytoma. A retrospective study. Langenbecks Arch. Surg. 2025, 410, 154. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).