Flexible Bronchoscopy and Non-Small-Cell Lung Cancer Staging: A Narrative Review of Modern Techniques for Optimized Clinical Decision-Making
Abstract
1. Introduction
2. Methodology and Search Strategy
3. Flexible Bronchoscopy and Adjunct Techniques: The Role of BAL and Bronchial Brushing in NSCLC Evaluation
4. Modern NSCLC Diagnostic Methodology
5. Emerging Technologies: AI and Bronchoscopic Innovation
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| FB | Flexible bronchoscopy |
| EBUS-TBNA | Endobronchial ultrasound with fine-needle aspiration |
| PET-CT | Positron emission tomography |
| LC | Lung cancer |
| FDG | Fluorodeoxyglucose |
| AFB | Autofluorescence bronchoscopy |
| NBI | Narrow-band imaging |
| BAL | Bronchoalveolar lavage |
| WLB | White light bronchoscopy |
| ACCP | American College of Chest Physicians |
| EGFR | Epidermal growth factor |
| ALK | Receptor anaplastic lymphoma kinase |
| PD-L1 | Programmed death-ligand 1 |
| ROSE | Rapid on-site evaluation |
| AI | Artificial intelligence |
| ENB | Electromagnetic navigation bronchoscopy |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 5, 33588. [Google Scholar] [CrossRef]
- Olteanu, G.E.; Vigdorovits, A.; Barna, R.A.; Mazilu, L.; Manolache, V.; Preoteasa, V.; Curcean, S.; Roman, A.; Motas, N.; Dediu, M.; et al. Lung Cancer in Romania. J. Thorac. Oncol. 2024, 19, 1492–1503. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Apple, J.; DerSarkissian, M.; Shah, A.; Chang, R.; Chen, Y.; He, X.; Chun, J. Economic burden of early-stage non-small-cell lung cancer: An assessment of healthcare resource utilization and medical costs. J. Comp. Eff. Res. 2023, 12, e230107. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef]
- Shinagawa, N. A review of existing and new methods of bronchoscopic diagnosis of lung cancer. Respir. Investig. 2019, 57, 3–8. [Google Scholar] [CrossRef]
- Iftikhar, I.H.; Musani, A.I. Narrow-Band Imaging Bronchoscopy in the Detection of Premalignant Airway Lesions: A Meta -Analysis of Diagnostic Test Accuracy. Ther. Adv. Respir. Dis. 2015, 9, 123–133. [Google Scholar] [CrossRef]
- Argentieri, G.; Valsecchi, C.; Petrella, F.; Jungblut, L.; Frauenfelder, T.; Del Grande, F.; Rizzo, S. Implementation of the 9th TNM for lung cancer: Practical insights for radiologists. Eur. Radiol. 2025, 35, 4395–4402. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J. The relevance of bronchoalveolar lavage fluid analysis for lung cancer patients. Expert. Rev. Respir. Med. 2020, 14, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.R.; Ha, D.M.; Schwarz, M.I.; Chan, E.D. Bronchoalveolar lavage as a diagnostic procedure: A review of known cellular and molecular findings in various lung diseases. J. Thorac. Dis. 2020, 12, 4991–5019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, D.; Li, S.; Ren, J.; Huang, W.; Liu, D.; Wang, W. Bronchoalveolar lavage fluid assessment facilitates precision medicine for lung cancer. Cancer Biol. Med. 2023, 21, 230–251. [Google Scholar] [CrossRef]
- Kalkanis, A.; Papadopoulos, D.; Testelmans, D.; Kopitopoulou, A.; Boeykens, E.; Wauters, E. Bronchoalveolar Lavage Fluid-Isolated Biomarkers for the Diagnostic and Prognostic Assessment of Lung Cancer. Diagnostics 2022, 12, 2949. [Google Scholar] [CrossRef]
- Nguyen-Dang, K.; Bui-Thi, H.D.; Duong-Minh, N.; Pham-Quang, T.; Nguyen-Ho, L.; Lam-Quoc, D.; Dang-Vu, T.; Tran-Ngoc, N.; Nguyen-Thi, P.; Le-Thuong, V. The Role and Associated Factors of Liquid-Based Cytology of Bronchoalveolar Lavage Fluid in Lung Cancer Diagnosis: A Prospective Study. Cureus 2023, 15, e48483. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, K.B.; Aggarwal, R. Yield of different bronchoscopic techniques in diagnosis of lung cancer. Int. J. Res. Med. Sci. 2017, 5, 4098–4103. [Google Scholar] [CrossRef]
- Liam, C.K.; Pang, Y.K.; Poosparajah, S. Diagnostic yield of flexible bronchoscopic procedures in lung cancer patients according to tumour location. Singapore Med. J. 2007, 48, 625–631. [Google Scholar]
- Biciuşcă, V.; Popescu, I.A.S.; Traşcă, D.M.; Olteanu, M.; Stan, I.S.; Durand, P.; Camen, G.C.; Bălteanu, M.A.; Cazacu, I.M.; Demetrian, A.D.; et al. Diagnosis of lung cancer by flexible fiberoptic bronchoscopy: A descriptive study. Rom. J. Morphol. Embryol. 2022, 63, 369–381. [Google Scholar] [CrossRef]
- Gupta, R.C.; Purohit, S.D.; Sharma, M.P.; Bhardwaj, S. Primary bronchogenic carcinoma: Clinical profile of 279 cases from mid-west Rajasthan. Indian J. Chest Dis. Allied. Sci. 1998, 40, 109–116. [Google Scholar]
- Thippanna, G.; Venu, K.; Gopalakrishnaiah, V.; Reddy, P.N.; Charan, B.G. A profile of lung cancer patients in Hyderabad. J. Indian Med. Assoc. 1999, 97, 357–359. [Google Scholar] [PubMed]
- Kashyap, S.; Mohapatra, P.; Singh, R. Pattern of primary lung cancer among Bidi smokers in North-Western Himalayan region of India. Lung Cancer 2003, 41, S111. [Google Scholar] [CrossRef]
- Bernasconi, M.; Koegelenberg, C.F.N.; Koutsokera, A.; Ogna, A.; Casutt, A.; Nicod, L.; Lovis, A. Iatrogenic bleeding during flexible bronchoscopy: Risk factors, prophylactic measures and management. ERJ Open Res. 2017, 21, 00084-2016. [Google Scholar] [CrossRef] [PubMed]
- Ishak, M.; Chakraborty, D.; Kassirian, S.; Dhaliwal, I.; Mitchell, M.A. Risk of iatrogenic pneumothorax based on location of transbronchial biopsy: A retrospective cohort study. BMC Res. Notes 2023, 16, 14. [Google Scholar] [CrossRef]
- Shimizu, T.; Okachi, S.; Imai, N.; Hase, T.; Morise, M.; Hashimoto, N.; Sato, M.; Hasegawa, Y. Risk factors for pulmonary infection after diagnostic bronchoscopy in patients with lung cancer. Nagoya J. Med. Sci. 2020, 82, 69–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elhidsi, M.; Zaini, J.; Rachmadi, L.; Asmarinah, A.; Kekalih, A.; Soeroso, N.; Rasmin, M. Clinical and Bronchoscopy Assessment in Diagnosing the Histopathology Type of Primary Central Lung Tumors. Open Respir. Med. J. 2024, 18, e18743064318977. [Google Scholar] [CrossRef]
- Yarmus, L.B.; Akulian, J.; Lechtzin, N.; Shah, A.; Yasin, F.; Kamdar, B.; Ernst, A.; Ost, D.E. Comparison of 21-Gauge and 22-Gauge Aspiration Needle in Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: Results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013, 143, 1036–1043. [Google Scholar] [CrossRef]
- Ahn, J.H. An update on the role of bronchoscopy in the diagnosis of pulmonary disease. Yeungnam Univ. J. Med. 2020, 37, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Lewis, S.Z.; Diekemper, R.; Addrizzo-Harris, D.; Alberts, W.M. Executive Summary: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, 7S–37S. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lema, L.; Fernández-Villar, A.; Ruano-Ravina, A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: A systematic review. Eur. Respir. J. 2009, 33, 1156–1164. [Google Scholar] [CrossRef]
- Asano, F.; Aoe, M.; Ohsaki, Y.; Okada, Y.; Sasada, S.; Sato, S.; Suzuki, E.; Senba, H.; Fujino, S.; Ohmori, K. Deaths and complications associated with respiratory endoscopy: A survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2012, 17, 478–485. [Google Scholar] [CrossRef]
- Tournoy, K.G.; Annema, J.T.; Krasnik, M.; Herth, F.J.; van Meerbeeck, J.P. Endoscopic and endobronchial ultrasonography according to the proposed lymph node map definition in the seventh edition of the tumor, node, metastasis classification for lung cancer. J. Thorac. Oncol. 2009, 4, 1576–1584. [Google Scholar] [CrossRef]
- Folch, E.; Santacruz, J.F.; Machuzak, M.S.; Gildea, T.R.; Majid, A. Safety and Efficacy of EBUS-Guided TBNA Through the Pulmonary Artery: A Preliminary Report. Chest 2011, 140, 600. [Google Scholar] [CrossRef]
- Casal, R.F.; Staerkel, G.A.; Ost, D.; Almeida, F.A.; Uzbeck, M.H.; Eapen, G.A.; Jimenez, C.A.; Nogueras-Gonzalez, G.M.; Sarkiss, M.; Morice, R.C. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest 2012, 142, 568–573. [Google Scholar] [CrossRef]
- Fujiwara, T.; Yasufuku, K.; Nakajima, T.; Chiyo, M.; Yoshida, S.; Suzuki, M.; Shibuya, K.; Hiroshima, K.; Nakatani, Y.; Yoshino, I. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: A standard endobronchial ultrasound image classification system. Chest 2010, 138, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Satterwhite, L.G.; Berkowitz, D.M.; Parks, C.S.; Bechara, R.I. Central intranodal vessels to predict cytology during endobronchial ultrasound transbronchial needle aspiration. J. Bronchol. Interv. Pulmonol. 2011, 18, 322–328. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, G.K.; Lee, H.S.; Kim, M.S.; Lee, J.M.; Kim, H.Y.; Nam, B.H.; Zo, J.I.; Hwangbo, B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: How many aspirations per target lymph node station? Chest 2008, 134, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Leiro-Fernández, V.; Fernández-Villar, A. Mediastinal Staging for Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 496–505. [Google Scholar] [CrossRef]
- Yasufuku, K.; Pierre, A.; Darling, G.; de Perrot, M.; Waddell, T.; Johnston, M.; da Cunha Santos, G.; Geddie, W.; Boerner, S.; Le, L.W.; et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J. Thorac. Cardiovasc. Surg. 2011, 142, 1393–1400.e1. [Google Scholar] [CrossRef]
- Chao, F.; Zhang, H. PET/CT in the staging of the non-small-cell lung cancer. J. Biomed. Biotechnol. 2012, 2012, 783739. [Google Scholar] [CrossRef]
- Volpi, S.; Ali, J.M.; Tasker, A.; Peryt, A.; Aresu, G.; Coonar, A.S. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann. Transl. Med. 2018, 6, 95. [Google Scholar] [CrossRef]
- Zhu, J.; Pan, F.; Cai, H.; Pan, L.; Li, Y.; Li, L.; Li, Y.; Wu, X.; Fan, H. Positron emission tomography imaging of lung cancer: An overview of alternative positron emission tomography tracers beyond F18 fluorodeoxyglucose. Front. Med. 2022, 9, 945602. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Niu, R.; Gao, J.; Shi, Y.; Shao, X.; Wang, Y.; Shao, X. PET/CT radiomics and deep learning in the diagnosis of benign and malignant pulmonary nodules: Progress and challenges. Front. Oncol. 2024, 14, 1491762. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.S.; Mashkoor, S.; Shafiq, S.; Amber, F.; Kaleemi, R.; Nausheen, S.; Kumari, P.; Hashmi, A.A. Diagnostic Accuracy of FDG PET-CT in Lymph Nodal Staging of Lung Cancer. Cureus 2025, 17, e77880. [Google Scholar] [CrossRef]
- Matache, R.S.; Stanciu-Gavan, C.; Pantile, D.; Iordache, A.M.; Bejgăneanu, A.O.; Șerboiu, C.S.; Nemes, A.F. Clinical and Paraclinical Characteristics of Endobronchial Pulmonary Squamous Cell Carcinoma—A Brief Review. Diagnostics 2023, 13, 3318. [Google Scholar] [CrossRef] [PubMed]
- Farsad, M. FDG PET/CT in the Staging of Lung Cancer. Curr. Radiopharm. 2020, 13, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, H.; Tian, Q.; Chen, J.; Yang, B.; Liu, H. BronchoCopilot: AI in Autonomous Bronchoscopy–Reinforcement Learning Platform for Real-Time Bronchoscope Guidance. arXiv 2024, arXiv:2403.01483. [Google Scholar]
- Brownlee, A.R.; Watson, J.J.; Akhmerov, A.; Nammalwar, S.; Chen, Q.; Soukiasian, S.G.; Soukiasian, H.J. Robotic Navigational Bronchoscopy in Real-World Practice: Diagnostic Yield of 87.9%, with Low Complication Rate (5%). J. Thorac. Dis. 2023, 5, 1–6. [Google Scholar] [CrossRef]

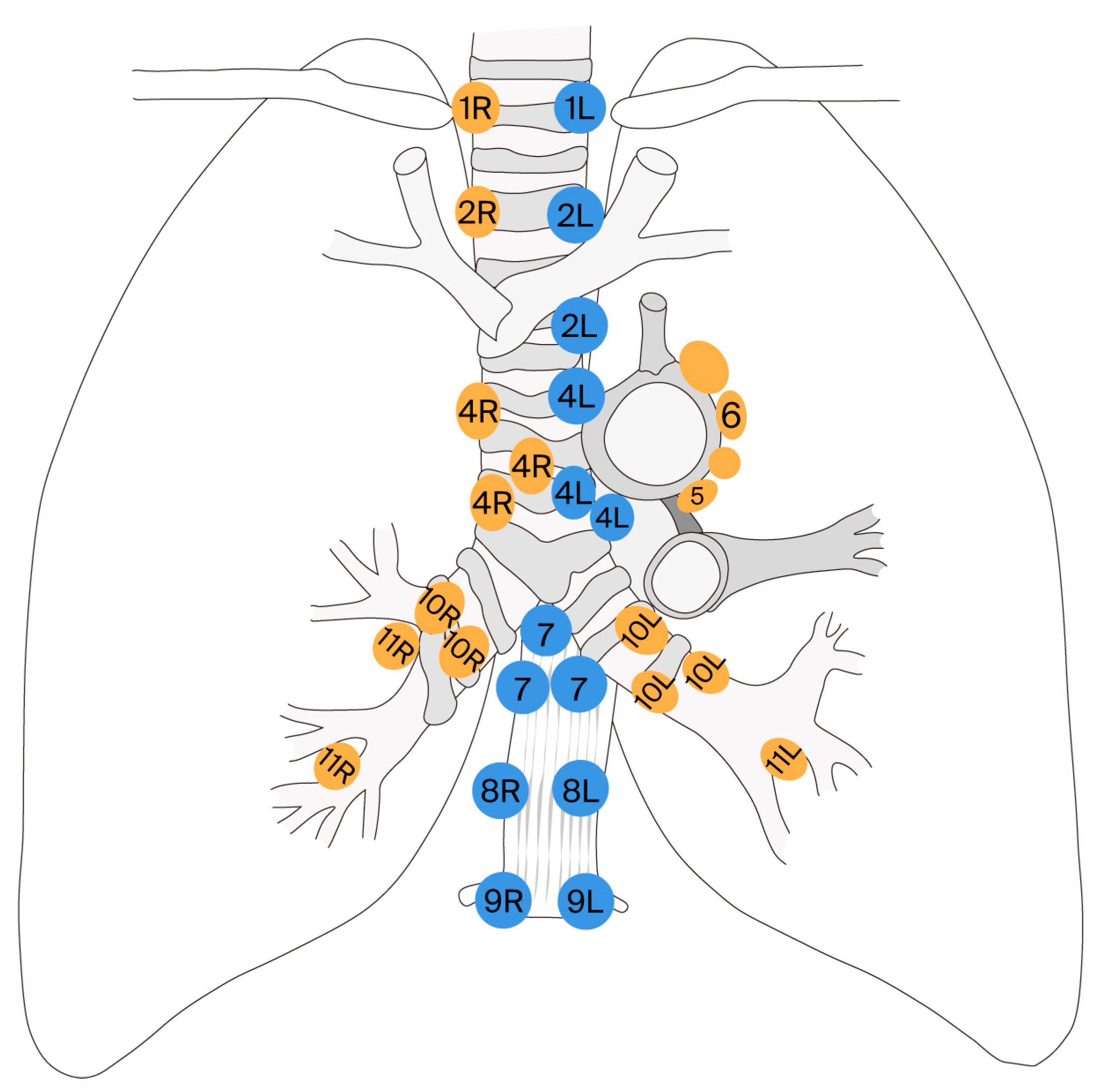
| Lymph Node Station | Name/Location | Accessible via EBUS | Accessible via Mediastinoscopy |
|---|---|---|---|
| 1 | Lower cervical/ supraclavicular lymph nodes | yes | no (generally not accessible) |
| 2R/2L | Upper paratracheal (right/left) | yes | yes |
| 3A | Pre-vascular | yes (difficult) | no |
| 3P | Retrotracheal | yes (very rare) | no |
| 4R/4L | Lower paratracheal (right/left) | yes | yes |
| 5 | Subaortic (aorto-pulmonary) | no | yes (only via extended mediastinoscopy) |
| 6 | Para-aortic/ anterior to pulmonary artery ligament | no | no |
| 7 | Subcarinal | yes | yes |
| 8 | Lower paraesophageal | yes | no |
| 9 | Pulmonary ligament | yes (very rare) | no |
| 10R/10L | Hilar (right/left) | yes | yes (difficult) |
| 11R/11L | Interlobar | yes | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roșu, S.-M.; Mitroi, D.M.; Catană, O.M.; Biciușcă, V.; Stan, S.I.; Mahler, B.; Parliteanu, O.-A.; Mirea, A.A.; Bălteanu, M.A. Flexible Bronchoscopy and Non-Small-Cell Lung Cancer Staging: A Narrative Review of Modern Techniques for Optimized Clinical Decision-Making. J. Clin. Med. 2025, 14, 5773. https://doi.org/10.3390/jcm14165773
Roșu S-M, Mitroi DM, Catană OM, Biciușcă V, Stan SI, Mahler B, Parliteanu O-A, Mirea AA, Bălteanu MA. Flexible Bronchoscopy and Non-Small-Cell Lung Cancer Staging: A Narrative Review of Modern Techniques for Optimized Clinical Decision-Making. Journal of Clinical Medicine. 2025; 14(16):5773. https://doi.org/10.3390/jcm14165773
Chicago/Turabian StyleRoșu, Simona-Maria, Denisa Maria Mitroi, Oana Maria Catană, Viorel Biciușcă, Sorina Ionelia Stan, Beatrice Mahler, Oana-Andreea Parliteanu, Adina Andreea Mirea, and Mara Amalia Bălteanu. 2025. "Flexible Bronchoscopy and Non-Small-Cell Lung Cancer Staging: A Narrative Review of Modern Techniques for Optimized Clinical Decision-Making" Journal of Clinical Medicine 14, no. 16: 5773. https://doi.org/10.3390/jcm14165773
APA StyleRoșu, S.-M., Mitroi, D. M., Catană, O. M., Biciușcă, V., Stan, S. I., Mahler, B., Parliteanu, O.-A., Mirea, A. A., & Bălteanu, M. A. (2025). Flexible Bronchoscopy and Non-Small-Cell Lung Cancer Staging: A Narrative Review of Modern Techniques for Optimized Clinical Decision-Making. Journal of Clinical Medicine, 14(16), 5773. https://doi.org/10.3390/jcm14165773








