Predictive Value of Point-of-Care Proenkephalin for Worsening Renal Function and Mortality in Patients Presenting to Emergency Department with Acute Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Routine Clinical and Laboratory Assessment
2.3. POC PENK Analysis
2.4. Echocardiography
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
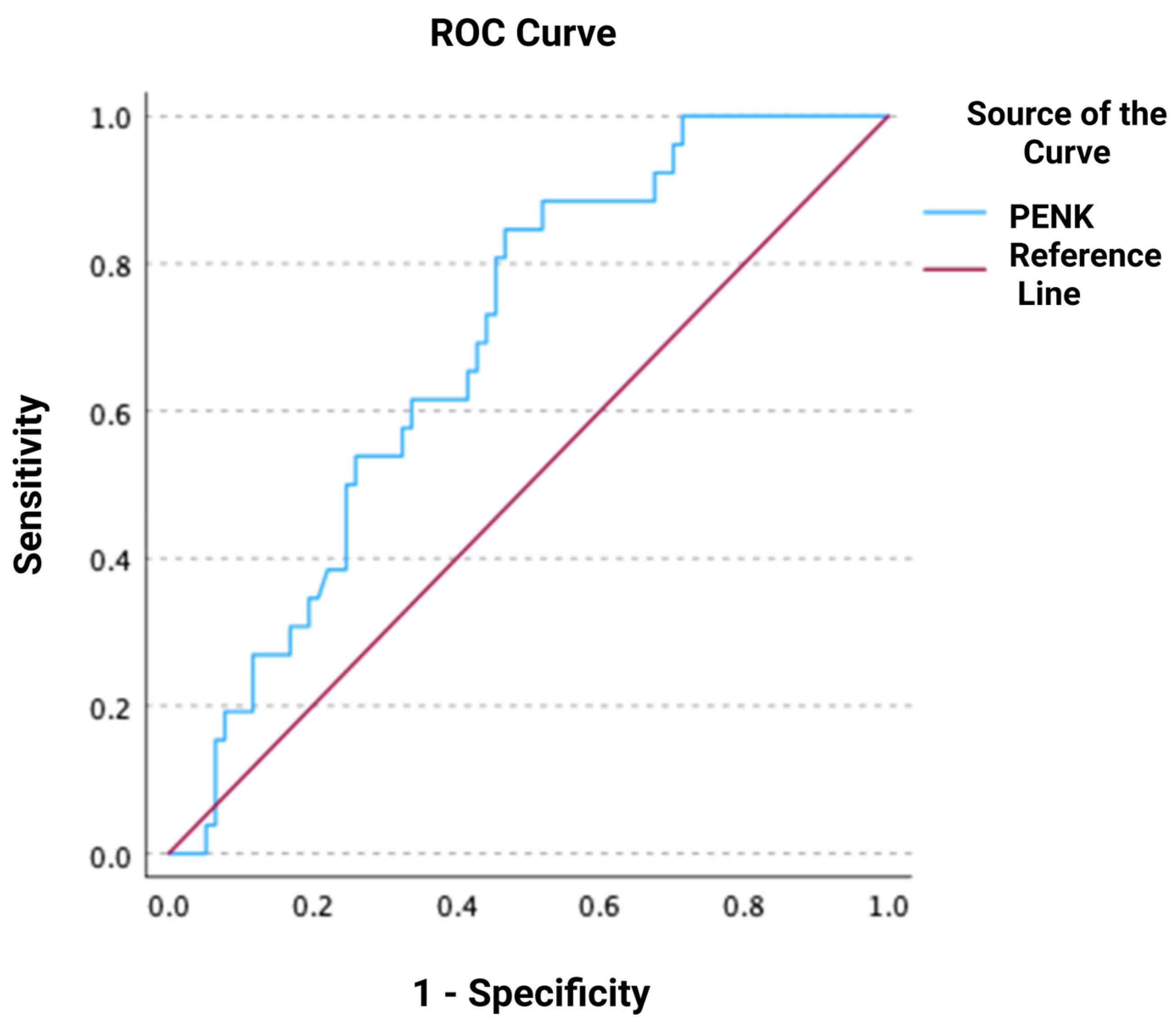
3.2. Association of PENK with Worsening Renal Function
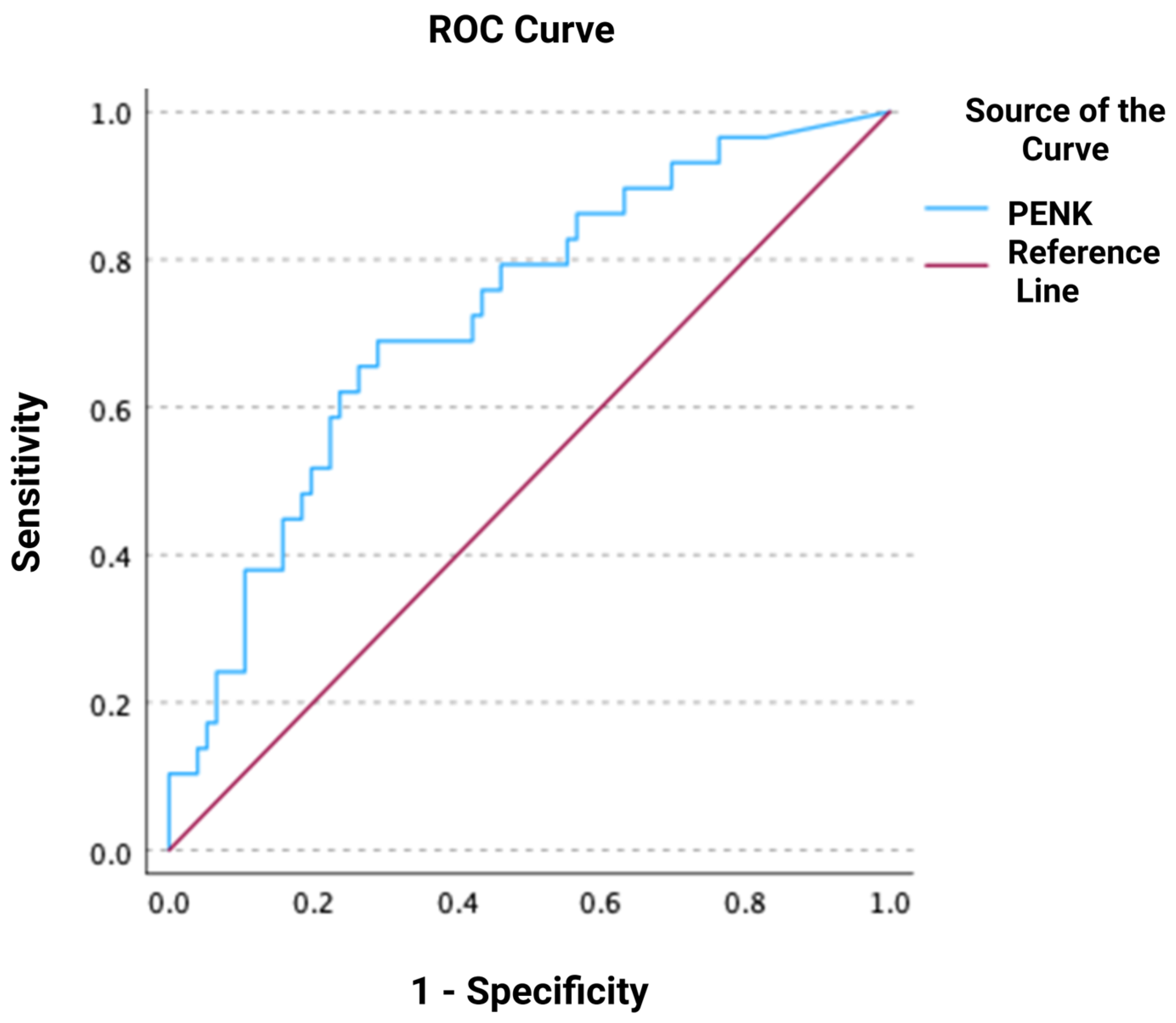
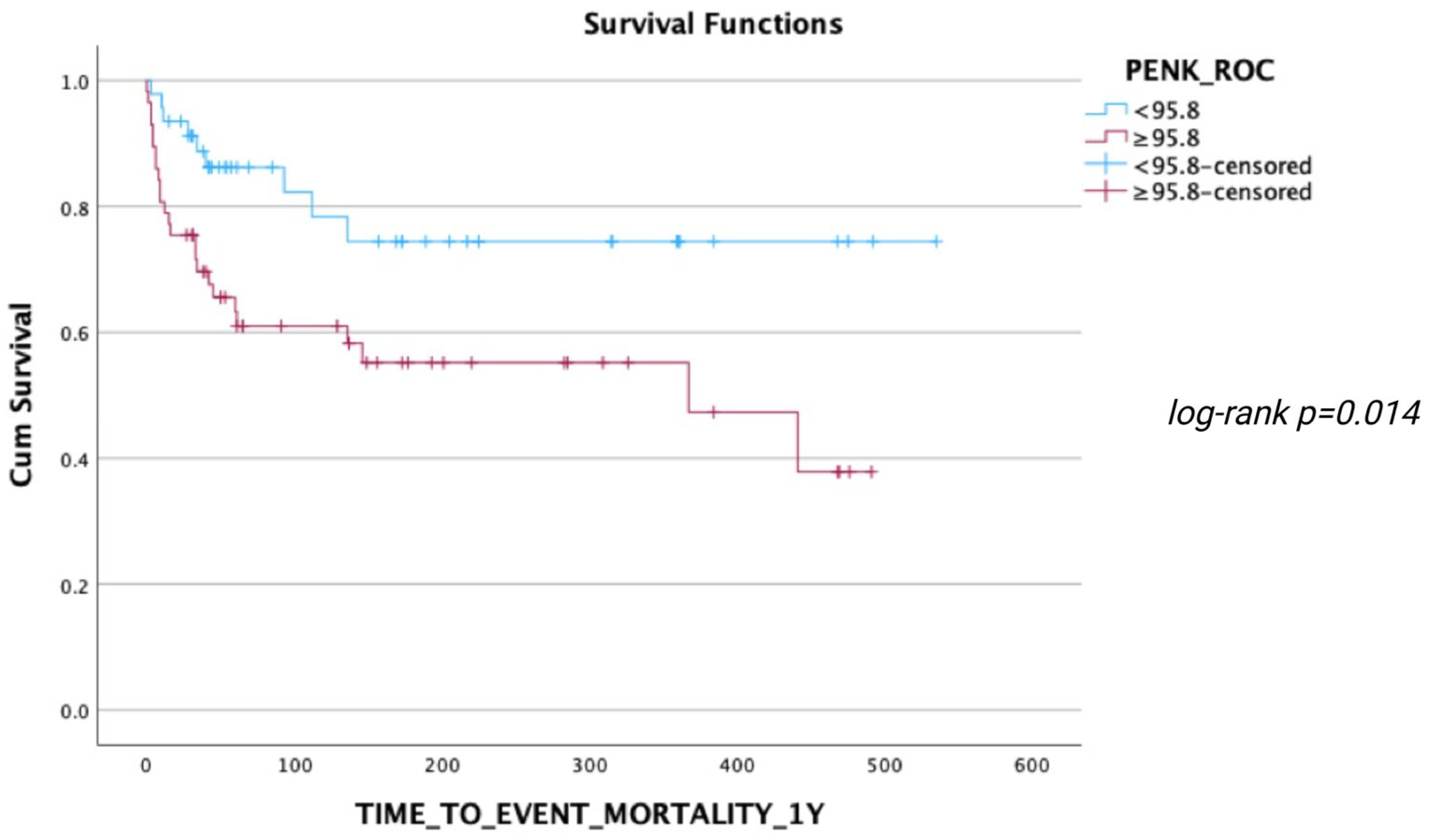
3.3. Association of PENK with Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marenzi, G.; Cosentino, N.; Imparato, L.; Trombara, F.; Leoni, O.; Bortolan, F.; Franchi, M.; Rurali, E.; Poggio, P.; Campodonico, J.; et al. Temporal Trends (2003–2018) of in-Hospital and 30-Day Mortality in Patients Hospitalized with Acute Heart Failure. Int. J. Cardiol. 2025, 419, 132693. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Ryu, K.H. Renal Dysfunction in Acute Heart Failure. Korean Circ. J. 2011, 41, 565. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From Physiology to Clinical Application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.E.; Januzzi, J.L. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef]
- Matsiras, D.; Ventoulis, I.; Verras, C.; Bistola, V.; Bezati, S.; Fyntanidou, B.; Polyzogopoulou, E.; Parissis, J.T. Proenkephalin 119–159 in Heart Failure: From Pathophysiology to Clinical Implications. J. Clin. Med. 2025, 14, 2657. [Google Scholar] [CrossRef]
- Fontana, F.; Bernardi, P.; Pich, E.M.; Capelli, M.; Bortoluzzi, L.; Spampinato, S.; Canossa, M. Relationship between Plasma Atrial Natriuretic Factor and Opioid Peptide Levels in Healthy Subjects and in Patients with Acute Congestive Heart Failure. Eur. Heart J. 1993, 14, 219–225. [Google Scholar] [CrossRef]
- Ernst, A.; Köhrle, J.; Bergmann, A. Proenkephalin A 119–159, a Stable Proenkephalin a Precursor Fragment Identified in Human Circulation. Peptides 2006, 27, 1835–1840. [Google Scholar] [CrossRef]
- Siranart, N.; Laohasurayotin, K.; Phanthong, T.; Sowalertrat, W.; Ariyachaipanich, A.; Chokesuwattanaskul, R. Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 4887. [Google Scholar] [CrossRef]
- Beunders, R.; Struck, J.; Wu, A.H.B.; Zarbock, A.; Di Somma, S.; Mehta, R.L.; Koyner, J.L.; Nadim, M.K.; Maisel, A.S.; Murray, P.T.; et al. Proenkephalin (PENK) as a Novel Biomarker for Kidney Function. J. Appl. Lab. Med. 2017, 2, 400–412. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.; Szczesna, K.; Schulze, A.; Ngo, H.; Doyle, M.; Do, T.; Vu, M.; Nguyen, J.; Löffler, J.; Borshchivska, M.; et al. Proenkephalin A 119–159 (penKid)—A Novel Biomarker and Its Quantification on the Nexus IB10 POC System for Assessing Kidney Function. Clin. Chem. Lab. Med. CCLM 2023, 61, e121–e125. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical Performance of an Immunoassay to Measure Proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. Proenkephalin, Renal Dysfunction, and Prognosis in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 56–69. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Hu, H.-J.; Zhao, X.-J.; Chi, W.-W.; Liu, D.-M.; Wang, Q.; Cui, W. Urine N-Terminal pro-B-Type Natriuretic Peptide and Plasma Proenkephalin Are Promising Biomarkers for Early Diagnosis of Cardiorenal Syndrome Type 1 in Acute Decompensated Heart Failure: A Prospective, Double-Center, Observational Study in Real-World. Ren. Fail. 2022, 44, 1487–1498. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Navarin, S.; Melander, O.; Zoccoli, G.; Hartmann, O.; Bergmann, A.; Struck, J.; Bachus, E.; Di Somma, S.; et al. Bioactive Adrenomedullin, Proenkephalin A and Clinical Outcomes in an Acute Heart Failure Setting. Open Heart 2019, 6, e001048. [Google Scholar] [CrossRef]
- Emmens, J.E.; Ter Maaten, J.M.; Damman, K.; Van Veldhuisen, D.J.; De Boer, R.A.; Struck, J.; Bergmann, A.; Sama, I.E.; Streng, K.W.; Anker, S.D.; et al. Proenkephalin, an Opioid System Surrogate, as a Novel Comprehensive Renal Marker in Heart Failure. Circ. Heart Fail. 2019, 12, e005544. [Google Scholar] [CrossRef]
- Jäntti, T.; Tarvasmäki, T.; Harjola, V.-P.; Pulkki, K.; Turkia, H.; Sabell, T.; Tolppanen, H.; Jurkko, R.; Hongisto, M.; Kataja, A.; et al. Predictive Value of Plasma Proenkephalin and Neutrophil Gelatinase-Associated Lipocalin in Acute Kidney Injury and Mortality in Cardiogenic Shock. Ann. Intensive Care 2021, 11, 25. [Google Scholar] [CrossRef]
- Matsue, Y.; Ter Maaten, J.M.; Struck, J.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Clinical Correlates and Prognostic Value of Proenkephalin in Acute and Chronic Heart Failure. J. Card. Fail. 2017, 23, 231–239. [Google Scholar] [CrossRef]
- Arfsten, H.; Goliasch, G.; Bartko, P.E.; Prausmüller, S.; Spinka, G.; Cho, A.; Novak, J.; Mascherbauer, J.; Haslacher, H.; Strunk, G.; et al. Neprilysin Inhibition Does Not Alter Dynamic of proenkephalin-A 119–159 and Pro-substance P in Heart Failure. ESC Heart Fail. 2021, 8, 2016–2024. [Google Scholar] [CrossRef]
- Grycuk, W.; Jakubowska, Z.; Małyszko, J. Proenkephalin Levels and Its Determinants in Patients with End-Stage Kidney Disease Treated with Hemodialysis and Peritoneal Dialysis. Int. J. Mol. Sci. 2023, 24, 15015. [Google Scholar] [CrossRef]





| All Patients (n = 107) | PENK Tertile 1 (<50–76.4) (n = 36) | PENK Tertile 2 (76.5–166.5) (n = 36) | PENK Tertile 3 (166.6 - >500) (n = 35) | p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 72 (13) | 68 (12) | 71 (15) | 78 (9) | 0.003 |
| Men, n (%) | 62 (58%) | 23 (64%) | 21 (58%) | 18 (51%) | 0.567 |
| AHF phenotype, n (%) | <0.001 | ||||
| Pulmonary edema | 26 (24%) | 8 (22%) | 11 (30%) | 7 (20%) | |
| Acutely decompensated chronic HF | 66 (62%) | 27 (75%) | 19 (53%) | 20 (57%) | |
| Cardiogenic shock | 10 (9%) | 1 (3%) | 1 (3%) | 8 (23%) | |
| Right HF | 5 (5%) | 0 | 5 (14%) | 0 | |
| Comorbidities, n (%) | |||||
| Arterial hypertension | 52 (49%) | 14 (39%) | 23 (64%) | 15 (44%) | 0.082 |
| Atrial fibrillation | 47 (44%) | 16 (44%) | 17 (47%) | 14 (41%) | 0.878 |
| Coronary artery disease | 37 (35%) | 15 (42%) | 9 (25%) | 13 (38%) | 0.295 |
| Prior MI | 27 (25%) | 10 (28%) | 7 (19%) | 10 (29%) | 0.586 |
| Permanent pacemaker | 12 (11%) | 2 (6%) | 6 (17%) | 4 (12%) | 0.329 |
| COPD | 23 (22%) | 8 (22%) | 8 (23%) | 7 (21%) | 0.973 |
| Thyroid disease | 26 (24%) | 6 (17%) | 9 (25%) | 11 (32%) | 0.312 |
| Diabetes mellitus | 50 (47%) | 20 (56%) | 14 (39%) | 16 (47%) | 0.367 |
| Chronic kidney disease | 15 (14%) | 3 (8%) | 3 (8%) | 9 (27%) | 0.044 |
| Stroke | 12 (11%) | 4 (11%) | 5 (14%) | 3 (9%) | 0.799 |
| Dyslipidemia | 36 (34%) | 12 (33%) | 16 (44%) | 8 (24%) | 0.181 |
| Prior medications, n (%) | |||||
| ACEi | 16 (15%) | 5 (14%) | 4 (11%) | 7 (21%) | 0.497 |
| ARB | 27 (25%) | 12 (34%) | 6 (17%) | 9 (27%) | 0.233 |
| ARNI | 11 (10%) | 2 (6%) | 5 (14%) | 4 (12%) | 0.503 |
| Beta-blockers | 61 (57%) | 20 (57%) | 17 (47%) | 24 (73%) | 0.097 |
| MRA | 33 (31%) | 7 (20%) | 13 (36%) | 13 (39%) | 0.179 |
| SGLT2i | 11 (10%) | 6 (17%) | 3 (9%) | 2 (7%) | 0.327 |
| Statins | 47 (44%) | 17 (49%) | 15 (43%) | 15 (46%) | 0.891 |
| Any diuretic | 61 (57%) | 17 (49%) | 19 (53%) | 25 (76%) | 0.051 |
| Antiplatelets | 34 (32%) | 11 (31%) | 13 (36%) | 10 (30%) | 0.860 |
| Anticoagulants | 51 (48%) | 17 (49%) | 17 (47%) | 17 (52%) | 0.936 |
| ECG on admission, n (%) | 0.422 | ||||
| Sinus rhythm | 58 (54%) | 20 (56%) | 20 (56%) | 18 (51%) | |
| Atrial fibrillation/flutter | 40 (37%) | 15 (42%) | 11 (31%) | 14 (40%) | |
| Paced rhythm | 8 (8%) | 1 (3%) | 5 (14%) | 2 (6%) | |
| Ventricular tachycardia | 1 (1%) | 0 | 0 | 1 (3%) | |
| Vital signs on admission | |||||
| Heart rate (bpm), mean (SD) | 100 (30) | 104 (27) | 95 (29) | 100 (34) | 0.407 |
| SBP (mmHg), mean (SD) | 137 (40) | 144 (35) | 144 (41) | 122 (40) | 0.037 |
| DBP (mmHg), mean (SD) | 77 (20) | 84 (18) | 78 (19) | 70 (20) | 0.012 |
| Focused echocardiography on admission | |||||
| LVEF (%), median [IQR] | 37 [25–50] | 30 [26–40] | 40 [21–50] | 40 [25–50] | 0.683 |
| LVEF categories, n (%) | 0.564 | ||||
| ≤40 | 52 (49) | 22 (61) | 14 (42) | 16 (49) | |
| 41–49 | 21 (20) | 6 (17) | 7 (21) | 8 (24) | |
| >50 | 29 (27) | 8 (22) | 12 (36) | 9 (27) | |
| PASP (mmHg), median [IQR] | 45 [30–55] | 40 [30–50] | 45 [33–55] | 40 [30–55] | 0.825 |
| Right-ventricular diameter (mm), median [IQR] | 34 [31–40] | 33 [32–41] | 34 [31–39] | 35 [32–40] | 0.736 |
| IVC diameter | 0.342 | ||||
| <15 mm, n (%) | 7 (7%) | 0 | 4 (13%) | 3 (11%) | |
| 15–25 mm, n (%) | 44 (41%) | 17 (53%) | 13 (43%) | 14 (50%) | |
| >25 mm, n (%) | 39 (36%) | 15 (47%) | 13 (43%) | 11 (39%) | |
| Laboratory tests, median [IQR] | |||||
| White blood cells, ×103/μL | 10.2 [7.9–13.0] | 10.3 [8.4–12.4] | 9.7 [6.6–13.4] | 11.4 [8.3–13.1] | 0.338 |
| Hemoglobin, g/dL | 12.5 [10.4–13.8] | 12.8 [10.3–14.8] | 12.8 [11.4–13.8] | 11.7 [10.0–13.7] | 0.180 |
| Platelets, ×103/μL | 257 [202–315] | 269 [210–320] | 250 [210–293] | 244 [199–353] | 0.666 |
| BUN, mg/dL | 60 [38–96] | 41 [32–58] | 50 [37–70] | 109 [71–155] | <0.001 |
| Glucose, mg/dL | 134 [103–185] | 143 [96–198] | 112 [97–151] | 164 [125–219] | 0.001 |
| Serum creatinine, mg/dL | 1.1 [0.9–1.7] | 1.0 [0.8–1.2] | 1.1 [0.8–1.5] | 1.9 [1.5–2.4] | <0.001 |
| eGFR, ml/min/1.73m2 | 62 [37–87] | 81 [65–98] | 68 [49–87] | 32 [24–45] | <0.001 |
| Serum sodium, mEq/L | 137 [133–139] | 138 [135–141] | 139 [135–140] | 134 [128–137] | 0.001 |
| Serum potassium, mEq/L | 4.6 [4.1–5.2] | 4.5 [4.1–5.0] | 4.5 [4.0–4.9] | 5.2 [4.3–6.1] | 0.006 |
| AST, U/L | 24 [19–50] | 22 [18–33] | 25 [17–41] | 31 [22–74] | 0.050 |
| ALT, U/L | 19 [13–37] | 19 [13–29] | 17 [9–34] | 20 [16–60] | 0.127 |
| CRP, mg/dL | 15 [8–39] | 10.1 [3.7–28.4] | 21.8 [8.8–33.6] | 28.6 [8.7–104.0] | 0.081 |
| hs-cTnT, pg/mL | 39 [23–85] | 29 [17–59] | 30 [22–83] | 71 [39–142] | <0.001 |
| NT-proBNP, pg/mL | 4684 [2607–12,049] | 2932 [1992–4507] | 6070 [2965–11,552] | 9235 [3382–26,104] | 0.001 |
| PENK, pmol/L | 111 [60–193] | NA | NA | NA | |
| Blood gas analysis, median [IQR] | |||||
| pH | 7.41 [7.33–7.45] | 7.42 [7.39–7.45] | 7.42 [7.34–7.47] | 7.38 [7.29–7.43] | 0.031 |
| pCO2, mmHg | 39 [32–45] | 38 [32–44] | 38 [32–46] | 40 [33–46] | 0.829 |
| HCO3, mEq/L | 25 [21–28] | 25 [22–29] | 25 [21–28] | 23 [19–29] | 0.564 |
| Lactate, mmol/L | 1.7 [1.3–2.7] | 1.6 [1.3–2.1] | 1.5 [1.1–2.7] | 2.0 [1.3–4.8] | 0.251 |
| Hypoxemia, n (%) | 70 (65%) | 27 (82%) | 20 (65%) | 23 (68%) | 0.258 |
| Oxygen therapy at ED, n (%) | |||||
| NIMV | 19 (18%) | 6 (17%) | 6 (17%) | 7 (20%) | 0.914 |
| IMV | 3 (3%) | 0 | 0 | 3 (9%) | 0.042 |
| Length of stay (days), median [IQR] | 7 [5–11] | 7 [5–9] | 7 [6–12] | 8 [5–15] | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsiras, D.; Polyzogopoulou, E.; Ventoulis, I.; Bistola, V.; Verras, C.; Ikonomidis, I.; Parissis, J. Predictive Value of Point-of-Care Proenkephalin for Worsening Renal Function and Mortality in Patients Presenting to Emergency Department with Acute Heart Failure. J. Clin. Med. 2025, 14, 5730. https://doi.org/10.3390/jcm14165730
Matsiras D, Polyzogopoulou E, Ventoulis I, Bistola V, Verras C, Ikonomidis I, Parissis J. Predictive Value of Point-of-Care Proenkephalin for Worsening Renal Function and Mortality in Patients Presenting to Emergency Department with Acute Heart Failure. Journal of Clinical Medicine. 2025; 14(16):5730. https://doi.org/10.3390/jcm14165730
Chicago/Turabian StyleMatsiras, Dionysis, Effie Polyzogopoulou, Ioannis Ventoulis, Vasiliki Bistola, Christos Verras, Ignatios Ikonomidis, and John Parissis. 2025. "Predictive Value of Point-of-Care Proenkephalin for Worsening Renal Function and Mortality in Patients Presenting to Emergency Department with Acute Heart Failure" Journal of Clinical Medicine 14, no. 16: 5730. https://doi.org/10.3390/jcm14165730
APA StyleMatsiras, D., Polyzogopoulou, E., Ventoulis, I., Bistola, V., Verras, C., Ikonomidis, I., & Parissis, J. (2025). Predictive Value of Point-of-Care Proenkephalin for Worsening Renal Function and Mortality in Patients Presenting to Emergency Department with Acute Heart Failure. Journal of Clinical Medicine, 14(16), 5730. https://doi.org/10.3390/jcm14165730








