Chlorhexidine vs. Povidone for Skin Antisepsis in Tissue Expander-Based Breast Reconstruction: A Propensity Score-Matched Analysis
Abstract
1. Introduction
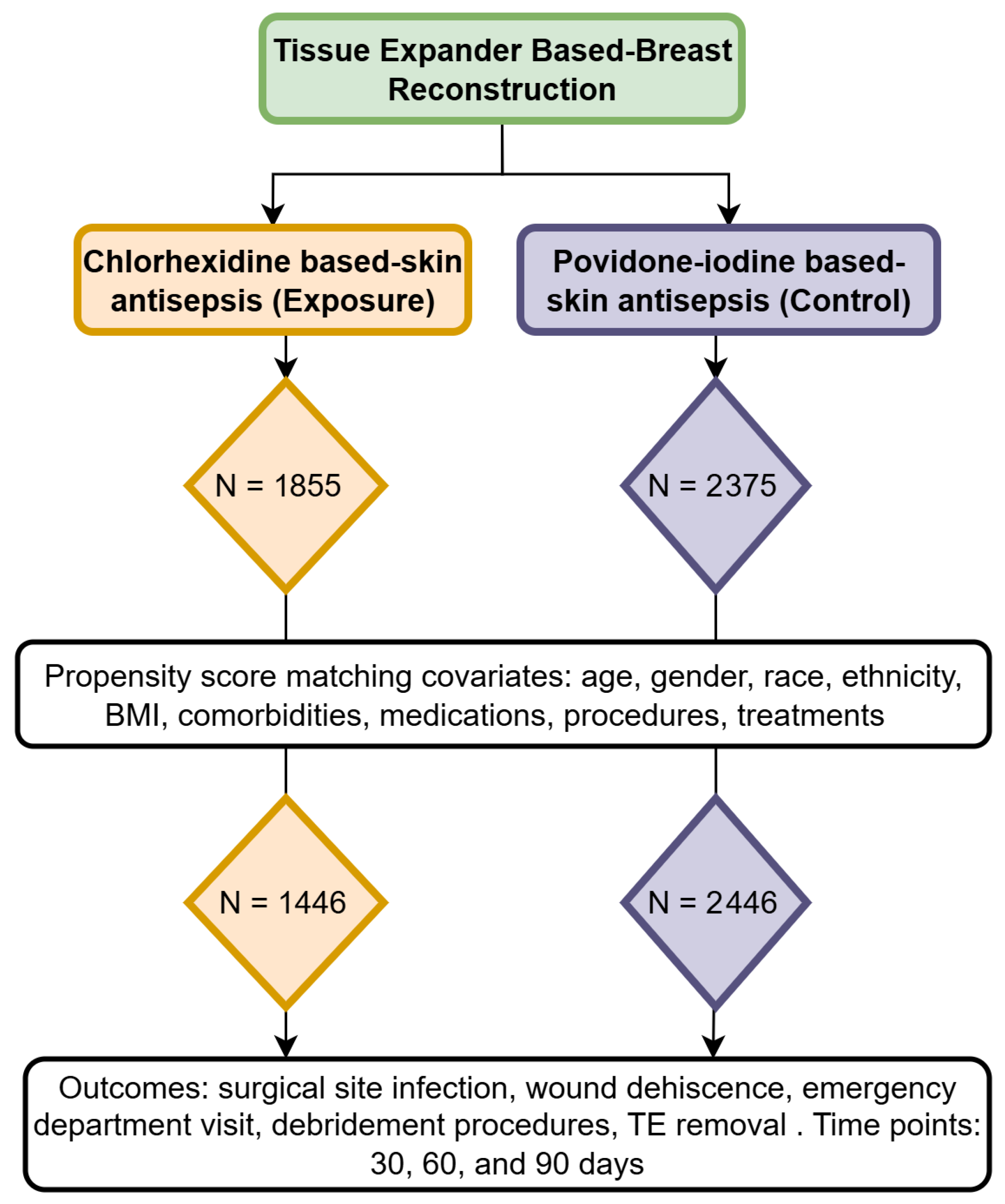
2. Materials and Methods
2.1. Cohorts
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TE | Tissue expander |
| SSI | Surgical site infection |
| CPT | Current procedural terminology |
| ICD | International classification of diseases |
| CI | Confidence interval |
| RR | Risk ratio |
| OR | Odds ratio |
Appendix A
| Procedure/Diagnosis | Code |
|---|---|
| Chlorhexidine Cohort | |
| Must have | |
| Tissue expander-based breast reconstruction | CPT: 19357 |
| AND (same day) | |
| Chlorhexidine | RxNorm: 2358 |
| AND cannot have (same day) | |
| Povidone-iodine | RxNorm: 8611 |
| Povidone cohort | |
| Must have | |
| Tissue expander-based breast reconstruction | CPT: 19357 |
| AND (same day) | |
| Povidone-iodine | RxNorm: 8611 |
| AND cannot have (same day) | |
| Chlorhexidine | RxNorm: 2358 |
| Outcomes | |
| Surgical site infection | ICD10: T81.41XA, T81.42XA, T81.43XA, T81.49XA |
| Wound dehiscence | ICD10: T81.30XA |
| Emergency department visit | CPT: 1013711 |
| Debridement | CPT: 1003164 |
| Tissue expander removal | CPT: 11971, ICD10: 0HPU0NZ, 0HPT0NZ, 0HPU3NZ, 0HPT3NZ, |
References
- Viola, G.M.; Selber, J.C.; Crosby, M.; Raad, I.I.; Butler, C.E.; Villa, M.T.; Kronowitz, S.J.; Clemens, M.W.; Garvey, P.; Yang, W.; et al. Salvaging the Infected Breast Tissue Expander: A Standardized Multidisciplinary Approach. Plast. Reconstr. Surg. -Glob. Open 2016, 4, e732. [Google Scholar] [CrossRef]
- Kraenzlin, F.S.; Saunders, H.; Aliu, O.; Cooney, D.; Rosson, G.D.; Sacks, J.M.; Broderick, K.; Manahan, M.A. Classification of breast tissue expander infections: Back to the basics. J. Surg. Oncol. 2019, 120, 142–147. [Google Scholar] [CrossRef]
- Cheadle, W.G. Risk Factors for Surgical Site Infection. Surg. Infect. 2006, 7, s7–s11. [Google Scholar] [CrossRef]
- Spruce, L. Reducing the Risk of Surgical Site Infection with Effective Preoperative Patient Skin Antisepsis. AORN J. 2020, 112, 82–83. [Google Scholar] [CrossRef]
- Boyce, J.M. Best products for skin antisepsis. Am. J. Infect. Control 2023, 51, A58–A63. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Barbara, J. Chlorhexidine–mechanism of action and its application to dentistry. J. Stomatol. 2017, 70, 405–417. [Google Scholar]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection; World Health Organization: Geneva, Switzerland, 2016. Available online: https://pubmed.ncbi.nlm.nih.gov/27929621/ (accessed on 26 May 2025).
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, 13. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Mimoz, O.; Lucet, J.-C.; Kerforne, T.; Pascal, J.; Souweine, B.; Goudet, V.; Mercat, A.; Bouadma, L.; Lasocki, S.; Alfandari, S.; et al. Skin antisepsis with chlorhexidine–alcohol versus povidone iodine–alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): An open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 2015, 386, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O.; Wall, M.J.J.; Itani, K.M.; Otterson, M.F.; Webb, A.L.; Carrick, M.M.; Miller, H.J.; Awad, S.S.; Crosby, C.T.; Mosier, M.C.; et al. Chlorhexidine–Alcohol versus Povidone–Iodine for Surgical-Site Antisepsis. N. Engl. J. Med. 2010, 362, 18–26. [Google Scholar] [CrossRef]
- Widmer, A.F.; Atkinson, A.; Kuster, S.P.; Wolfensberger, A.; Klimke, S.; Sommerstein, R.; Eckstein, F.S.; Schoenhoff, F.; Beldi, G.; Gutschow, C.A.; et al. Povidone Iodine vs Chlorhexidine Gluconate in Alcohol for Preoperative Skin Antisepsis. JAMA 2024, 332, 541–549. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef]
- Nguyen, T.-L.; Collins, G.S.; Spence, J.; Daurès, J.-P.; Devereaux, P.J.; Landais, P.; Le Manach, Y. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 2017, 17, 78. [Google Scholar] [CrossRef]
- Levin, I.; Amer-Alshiek, J.; Avni, A.; Lessing, J.B.; Satel, A.; Almog, B. Chlorhexidine and Alcohol Versus Povidone-Iodine for Antisepsis in Gynecological Surgery. J. Women’s Health 2011, 20, 321–324. [Google Scholar] [CrossRef]
- Cruz, M.R.B.; Amaral, D.C.; Gonçalves, O.R.; Cyrino, L.G.; Nascimento, L.M.; Barroso, F.V.C.; Louzada, R.N.; Rassi, T.N.d.O.; Mora-Paez, D.J.; Guedes, J.; et al. Chlorhexidine Compared with Povidone–Iodine in Intravitreal Injection: A Systematic Review and Meta-Analysis. J. Ocul. Pharmacol. Ther. 2025, 41, 162–168. [Google Scholar] [CrossRef]
- Hsieh, H.-H.; Yu, Y.; Chang, C.-J.; Chang, T.-Y. A comparative meta-analysis of povidone–iodine–alcohol vs. chlorhexidine–alcohol for preoperative skin antisepsis in abdominal surgery. Am. J. Surg. 2025, 244, 116318. [Google Scholar] [CrossRef]
- Machinski, E.; da Cruz, V.F.; Conde, R.A.; Filho, A.R.O.; Varone, B.B.; Gobbi, R.G.; Helito, C.P.; Leal, D.P. Chlorhexidine or Povidone-Iodine Solution Irrigation Versus Saline Irrigation for the Prevention of Postoperative Infections in Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2025, 25, S0883–S5403. [Google Scholar] [CrossRef]
- Ademuyiwa, A.O.; Hardy, P.; Runigamugabo, E.; Sodonougbo, P.; Behanzin, H.; Kangni, S.; Agboton, G.; Adagrah, L.A.; Adjei-Acquah, E.; Acquah, A.O.; et al. Reducing surgical site infections in low-income and middle-income countries (FALCON): A pragmatic, multicentre, stratified, randomised controlled trial. Lancet 2021, 398, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Dior, U.P.; Kathurusinghe, S.; Cheng, C.; Reddington, C.; Daley, A.J.; Ang, C.; Healey, M. Effect of Surgical Skin Antisepsis on Surgical Site Infections in Patients Undergoing Gynecological Laparoscopic Surgery. JAMA Surg. 2020, 155, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Lin, J.; Liu, J.; Gong, Y.; Zheng, S.; Mei, L.; Tang, X.; Xie, L.; Li, H.; Zhang, C.; et al. Chlorhexidine gluconate versus povidone-iodine for nasal bacteria decolonization before transsphenoidal surgery in patients with pituitary neuroendocrine tumors: A prospective, randomized, double-blind, noninferiority trial. Int. J. Surg. 2025, 111, 697–705. [Google Scholar] [CrossRef]
- Charehbili, A.; Swijnenburg, R.-J.; van de Velde, C.; Bremer, J.v.D.; van Gijn, W. A Retrospective Analysis of Surgical Site Infections after Chlorhexidine–Alcohol versus Iodine–Alcohol for Pre-Operative Antisepsis. Surg. Infect. 2014, 15, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Noorani, A.; Rabey, N.; Walsh, S.R.; Davies, R.J. Systematic review and meta-analysis of preoperative antisepsis with chlorhexidine versus povidone–iodine in clean-contaminated surgery. Br. J. Surg. 2010, 97, 1614–1620. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.W.; Guo, B.; Xu, C.C. Preoperative Antisepsis with Chlorhexidine Versus Povidone-Iodine for the Prevention of Surgical Site Infection: A Systematic Review and Meta-analysis. World J. Surg. 2020, 44, 1412–1424. [Google Scholar] [CrossRef]
- Privitera, G.P.; Costa, A.L.; Brusaferro, S.; Chirletti, P.; Crosasso, P.; Massimetti, G.; Nespoli, A.; Petrosillo, N.; Pittiruti, M.; Scoppettuolo, G.; et al. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: A systematic review and meta-analysis. Am. J. Infect. Control 2017, 45, 180–189. [Google Scholar] [CrossRef]
- Guenezan, J.; Marjanovic, N.; Drugeon, B.; O Neill, R.; Liuu, E.; Roblot, F.; Palazzo, P.; Bironneau, V.; Prevost, F.; Paul, J.; et al. Chlorhexidine plus alcohol versus povidone iodine plus alcohol, combined or not with innovative devices, for prevention of short-term peripheral venous catheter infection and failure (CLEAN 3 study): An investigator-initiated, open-label, single centre, randomised-controlled, two-by-two factorial trial. Lancet Infect. Dis. 2021, 21, 1038–1048. [Google Scholar] [CrossRef]
- Wade, R.G.; Bourke, G.; Wormald, J.C.R.; Totty, J.P.; Stanley, G.H.M.; Lewandowski, A.; Rakhra, S.S.; Gardiner, M.D. Chlorhexidine versus povidone–iodine skin antisepsis before upper limb surgery (CIPHUR): An international multicentre prospective cohort study. BJS Open 2021, 5, zrab117. [Google Scholar] [CrossRef] [PubMed]
- Beber, S.A.B.; Sanborn, R.M.B.; Miller, P.E.; Kasser, J.R.; Waters, P.M.; Watkins, C.J.; Shore, B.J.M. Jumping on the Bandwagon: Comparing the Efficacy of Chlorhexidine Versus Povidone-Iodine Preoperative Skin Antiseptic in Preventing Surgical Site Infections Following Pediatric Orthopaedic Surgery. J. Pediatr. Orthop. 2022, 42, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Dörfel, D.; Maiwald, M.; Daeschlein, G.; Müller, G.; Hudek, R.; Assadian, O.; Kampf, G.; Kohlmann, T.; Harnoss, J.C.; Kramer, A. Comparison of the antimicrobial efficacy of povidone-iodine-alcohol versus chlorhexidine-alcohol for surgical skin preparation on the aerobic and anaerobic skin flora of the shoulder region. Antimicrob. Resist. Infect. Control 2021, 10, 17. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. The Hospital Infection Control Practices Advisory Committee Guideline for Prevention of Surgical Site Infection, 1999. Infect. Control Hosp. Epidemiol. 1999, 20, 247–280. [Google Scholar] [CrossRef]
- Eslami, A.R.D. Antimicrobial Assay of Chlorhexidine-Wetted Textile Napkins for Surgical Site Disinfection in Ocular Surgery. Int. J. Clin. Med. 2013, 04, 577–581. [Google Scholar] [CrossRef]
- Kataria, K.; Bagdia, A.; Srivastava, A. Are Breast Surgical Operations Clean or Clean Contaminated? Indian J. Surg. 2015, 77, 1360–1362. [Google Scholar] [CrossRef]
- Oleck, N.C.; Gu, C.; Phillips, B.T.M. Defining Mastectomy Skin Flap Necrosis: A Systematic Review of the Literature and a Call for Standardization. Plast. Reconstr. Surg. 2022, 149, 858e–866e. [Google Scholar] [CrossRef] [PubMed]
- Bolton, L. Surgical Site Infection in Cancer Patients. Wounds A Compend. Clin. Res. Pract. 2021, 33, 260–262. [Google Scholar] [CrossRef]
- Calderwood, M.S.; Anderson, D.J.; Bratzler, D.W.; Dellinger, E.P.; Garcia-Houchins, S.; Maragakis, L.L.; Nyquist, A.C.; Perkins, K.M.; Preas, M.A.; Saiman, L.; et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 695–720. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; Wärnberg, F.; Kovacs, A.; Bagge, R.O. Impact of Surgical Care Bundle on Surgical Site Infection after Non-Reconstructive Breast Cancer Surgery: A Single-Centre Retrospective Comparative Cohort Study. Cancers 2023, 15, 919. [Google Scholar] [CrossRef]
- Campbell, M.M.; Turi, J.; Collier, S.; English, C.; Sistla, V.; Smith, M.J.; Moorthy, G.; Seidelman, J.; Smith, B.A.; Lewis, S.S.; et al. Implementation of Bundled Interventions to Reduce Surgical Site Infections in Pediatric Patients Undergoing Cardiothoracic Surgery: A Quality Improvement Project. AORN J. 2025, 121, 127–139. [Google Scholar] [CrossRef]
- Hekman, K.E.; Michel, E.; Blay, E.; Helenowski, I.B.; Hoel, A.W. Evidence-Based Bundled Quality Improvement Intervention for Reducing Surgical Site Infection in Lower Extremity Vascular Bypass Procedures. J. Am. Coll. Surg. 2019, 228, 44–53. [Google Scholar] [CrossRef]
- Guo, X.M.; Runge, M.; Miller, D.; Aaby, D.; Milad, M. A bundled intervention lowers surgical site infection in hysterectomy for benign and malignant indications. Int. J. Gynecol. Obstet. 2020, 150, 392–397. [Google Scholar] [CrossRef]
- Arroyo-Garcia, N.; Badia, J.M.; Vázquez, A.; Pera, M.; Parés, D.; Limón, E.; Almendral, A.; Piriz, M.; Díez, C.; Fraccalvieri, D.; et al. An interventional nationwide surveillance program lowers postoperative infection rates in elective colorectal surgery. A cohort study (2008–2019). Int. J. Surg. 2022, 102, 106611. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, R.-G.; Helgiu, A.; Cimpean, A.-M.; Ichim, C.; Todor, S.B.; Iliescu-Glaja, M.; Bodea, I.C.; Crainiceanu, Z.P. Assessing Fat Grafting in Breast Surgery: A Narrative Review of Evaluation Techniques. J. Clin. Med. 2024, 13, 7209. [Google Scholar] [CrossRef] [PubMed]

| Covariate | Chlorhexidine Cohort (n = 1855) | Povidone Cohort (n = 1855) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 51.6 (11.2) | 50.6 (11.5) | 0.007 |
| Gender, n (%) | |||
| Female | 1855 (100.0) | 2375 (100.0) | |
| Male | 0 (0.0) | 0 (0.0) | |
| Race, n (%) | |||
| White | 1454 (78.4) | 1693 (71.3) | <0.001 |
| Black or African American | 210 (11.3) | 293 (12.3) | 0.311 |
| American Indian or Alaskan Native | 10 (0.5) | 10 (0.4) | 0.579 |
| Native Hawaiian or Pacific Islander | 10 (0.5) | 18 (0.8) | 0.384 |
| Asian | 55 (3.0) | 109 (4.6) | 0.007 |
| Other race | 52 (2.8) | 97 (4.1) | 0.025 |
| Unknown race | 75 (4.0) | 161 (6.8) | <0.001 |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 1699 (91.6) | 1724 (72.6) | <0.001 |
| Hispanic or Latino | 98 (5.3) | 156 (6.6) | 0.081 |
| Unknown ethnicity | 58 (3.1) | 495 (20.8) | <0.001 |
| Body mass index, n (%) | |||
| Underweight | 66 (3.6) | 69 (2.9) | 0.231 |
| Normal weight | 647 (34.9) | 1020 (42.9) | <0.001 |
| Overweight | 688 (37.1) | 1179 (49.6) | <0.001 |
| Obesity I | 505 (27.2) | 829 (34.9) | <0.001 |
| Obesity II | 279 (15.0) | 461 (19.4) | <0.001 |
| Obesity III | 126 (6.8) | 227 (9.6) | 0.001 |
| Comorbidities, n (%) | |||
| Tobacco use | 49 (2.6) | 79 (3.3) | 0.197 |
| Diabetes | 123 (6.6) | 222 (9.3) | 0.001 |
| Hyperlipidemia | 332 (17.9) | 382 (16.1) | 0.118 |
| Hypertensive disease | 557 (30.0) | 744 (31.3) | 0.364 |
| Immune disorders | 58 (3.1) | 52 (2.2) | 0.057 |
| HIV infection | 10 (0.5) | 10 (0.4) | 0.579 |
| Acute and chronic kidney disease | 79 (4.3) | 81 (3.4) | 0.151 |
| Malignant neoplasm of the breast | 1673 (90.2) | 2006 (84.5) | <0.001 |
| Procedures, n (%) | |||
| Breast biopsy | 28 (1.5) | 27 (1.1) | 0.288 |
| Biologic implant placement | 1509 (81.3) | 1784 (75.1) | <0.001 |
| Medication, n (%) | |||
| Immunosuppressant | 76 (4.1) | 78 (3.3) | 0.161 |
| Corticosteroid | 1733 (93.4) | 2280 (96.0) | <0.001 |
| Other treatments, n (%) | |||
| Radiation | 93 (5.0) | 112 (4.7) | 0.655 |
| Chemotherapy | 639 (34.4) | 913 (38.4) | 0.007 |
| Covariate | Chlorhexidine Cohort (n = 1446) | Povidone Cohort (n = 1446) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 51.3 (11.4) | 50.9 (11.4) | 0.405 |
| Gender, n (%) | |||
| Female | 1446 (100.0) | 1446 (100.0) | |
| Male | 0 (0.0) | 0 (0.0) | |
| Race, n (%) | |||
| White | 1114 (77.0) | 1115 (77.1) | 0.965 |
| Black or African American | 164 (11.3) | 154 (10.7) | 0.552 |
| American Indian or Alaskan Native | 10 (0.7) | 10 (0.7) | 1.000 |
| Native Hawaiian or Pacific Islander | 10 (0.7) | 10 (0.7) | 1.000 |
| Asian | 53 (3.7) | 59 (4.1) | 0.563 |
| Other race | 52 (3.6) | 60 (4.1) | 0.441 |
| Unknown race | 54 (3.7) | 50 (3.5) | 0.690 |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 1298 (89.8) | 1292 (89.3) | 0.715 |
| Hispanic or Latino | 90 (6.2) | 95 (6.6) | 0.704 |
| Unknown ethnicity | 58 (4.0) | 59 (4.1) | 0.925 |
| Body mass index, n (%) | |||
| Underweight | 48 (3.3) | 50 (3.5) | 0.837 |
| Normal weight | 606 (41.9) | 608 (42.0) | 0.940 |
| Overweight | 657 (45.4) | 657 (45.4) | 1.000 |
| Obesity I | 469 (32.4) | 477 (33.0) | 0.751 |
| Obesity II | 255 (17.6) | 261 (18.0) | 0.771 |
| Obesity III | 119 (8.2) | 123 (8.5) | 0.788 |
| Comorbidities, n (%) | |||
| Tobacco use | 43 (3.0) | 51 (3.5) | 0.402 |
| Diabetes | 115 (8.0) | 120 (8.3) | 0.734 |
| Hyperlipidemia | 258 (17.8) | 269 (18.6) | 0.596 |
| Hypertensive disease | 464 (32.1) | 452 (31.3) | 0.631 |
| Immune disorders | 29 (2.0) | 30 (2.1) | 0.895 |
| HIV infection | 10 (0.7) | 10 (0.7) | 1.000 |
| Acute and chronic kidney disease | 56 (3.9) | 58 (4.0) | 0.848 |
| Malignant neoplasm of the breast | 1273 (88.0) | 1269 (87.8) | 0.820 |
| Procedures, n (%) | |||
| Breast biopsy | 14 (1.0) | 18 (1.2) | 0.477 |
| Biologic implant placement | 1155 (79.9) | 1171 (81.0) | 0.453 |
| Medication, n (%) | |||
| Immunosuppressant | 51 (3.5) | 56 (3.9) | 0.622 |
| Corticosteroid | 1392 (96.3) | 1380 (95.4) | 0.263 |
| Other treatments, n (%) | |||
| Radiation | 76 (5.3) | 76 (5.3) | 1.000 |
| Chemotherapy | 522 (36.1) | 526 (36.4) | 0.877 |
| Time/Outcome | Exposure (Chlorhexidine), n | Control (Povidone), n | Risk Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| 30 days | |||||
| Surgical site infection | 13 | 21 | 0.62 | 0.31–1.23 | 0.168 |
| Wound dehiscence | 10 | 10 | 1.00 | 0.42–2.40 | 1.000 |
| Emergency department visit | 87 | 92 | 0.95 | 0.71–1.26 | 0.700 |
| Debridement | 17 | 24 | 0.71 | 0.38–1.31 | 0.271 |
| TE removal | 51 | 61 | 0.84 | 0.58–1.20 | 0.335 |
| 60 days | |||||
| Surgical site infection | 29 | 35 | 0.83 | 0.51–1.35 | 0.448 |
| Wound dehiscence | 10 | 11 | 0.91 | 0.39–2.13 | 0.827 |
| Emergency department visit | 117 | 132 | 0.89 | 0.70–1.12 | 0.320 |
| Debridement | 29 | 34 | 0.85 | 0.52–1.39 | 0.524 |
| TE removal | 109 | 125 | 0.87 | 0.68–1.12 | 0.275 |
| 90 days | |||||
| Surgical site infection | 32 | 43 | 0.74 | 0.47–1.17 | 0.198 |
| Wound dehiscence | 10 | 13 | 0.77 | 0.34–1.75 | 0.530 |
| Emergency department visit | 137 | 158 | 0.87 | 0.70–1.08 | 0.197 |
| Debridement | 36 | 40 | 0.90 | 0.58–1.40 | 0.642 |
| TE removal | 155 | 167 | 0.93 | 0.76–1.14 | 0.478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posso, A.N.; Mustoe, A.; Neira, M.; Tobin, M.; Yamin, M.; Raquepo, T.; Escobar-Domingo, M.J.; Karinja, S.J.; Lee, B.T. Chlorhexidine vs. Povidone for Skin Antisepsis in Tissue Expander-Based Breast Reconstruction: A Propensity Score-Matched Analysis. J. Clin. Med. 2025, 14, 5734. https://doi.org/10.3390/jcm14165734
Posso AN, Mustoe A, Neira M, Tobin M, Yamin M, Raquepo T, Escobar-Domingo MJ, Karinja SJ, Lee BT. Chlorhexidine vs. Povidone for Skin Antisepsis in Tissue Expander-Based Breast Reconstruction: A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2025; 14(16):5734. https://doi.org/10.3390/jcm14165734
Chicago/Turabian StylePosso, Agustin N., Audrey Mustoe, Manuela Neira, Micaela Tobin, Mohammed Yamin, Tricia Raquepo, Maria J. Escobar-Domingo, Sarah J. Karinja, and Bernard T. Lee. 2025. "Chlorhexidine vs. Povidone for Skin Antisepsis in Tissue Expander-Based Breast Reconstruction: A Propensity Score-Matched Analysis" Journal of Clinical Medicine 14, no. 16: 5734. https://doi.org/10.3390/jcm14165734
APA StylePosso, A. N., Mustoe, A., Neira, M., Tobin, M., Yamin, M., Raquepo, T., Escobar-Domingo, M. J., Karinja, S. J., & Lee, B. T. (2025). Chlorhexidine vs. Povidone for Skin Antisepsis in Tissue Expander-Based Breast Reconstruction: A Propensity Score-Matched Analysis. Journal of Clinical Medicine, 14(16), 5734. https://doi.org/10.3390/jcm14165734








