Predictors of Suboptimal Response After Radiofrequency Ablation of Benign Thyroid Nodules
Abstract
1. Introduction
2. Materials and Methods
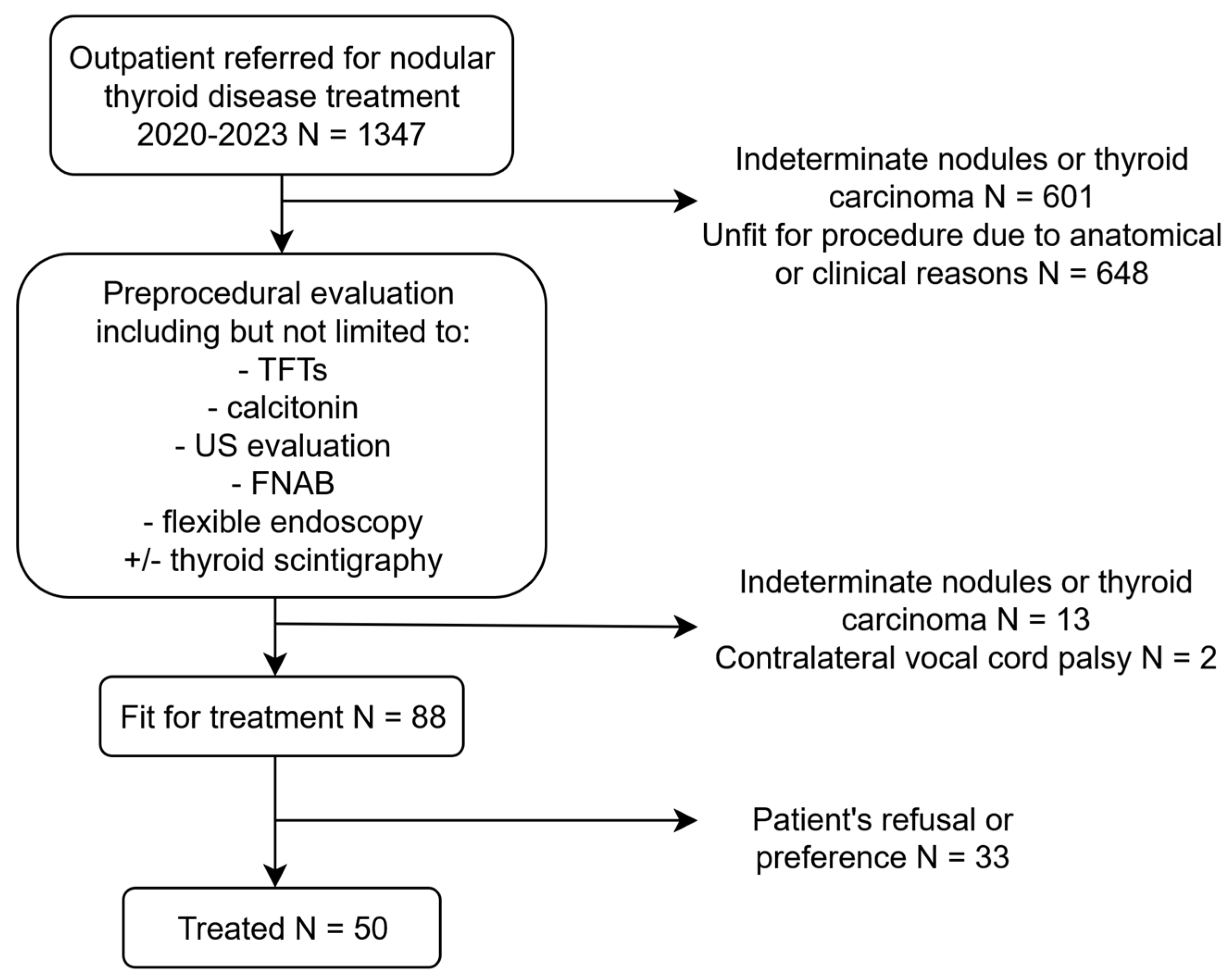
2.1. Study Design and Setting
2.2. Data Collection
2.3. RFA Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durante, C.; Cava, F.; Paciaroni, A.; Filetti, S. Benign thyroid nodules: Diagnostic and therapeutic approach. Recent. Prog. Med. 2008, 99, 263–270. [Google Scholar]
- Yan, L.; Zhang, M.; Li, X.; Li, Y.; Luo, Y. A Nomogram to Predict Regrowth After Ultrasound-Guided Radiofrequency Ablation for Benign Thyroid Nodules. Front. Endocrinol. 2022, 12, 774228. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Torlontano, M. Nonsurgical Approaches to the Management of Thyroid Nodules. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 384–394. [Google Scholar] [CrossRef]
- Javadov, M.; Karatay, E.; Ugurlu, M.U. Clinical and Functional Results of Radiofrequency Ablation and Microwave Ablation in Patients with Benign Thyroid Nodules. Saudi Med. J. 2021, 42, 838–846. [Google Scholar] [CrossRef]
- He, L.; Zhao, W.; Xia, Z.; Su, A.; Li, Z.; Zhu, J. Comparative Efficacy of Different Ultrasound-Guided Ablation for the Treatment of Benign Thyroid Nodules: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2021, 16, e0243864. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Luo, S.-D.; Chiang, P.-L.; Lin, A.-N.; Wang, C.-K.; Chou, C.-K.; Chi, S.-Y.; Chen, M.-H.; Lin, W.-C. Factors Influencing a Favorable Outcome for RFA of Huge Benign Thyroid Nodules: Preliminary Results and Short-Term Evaluation. Int. J. Endocrinol. 2023, 2023, 9021903. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.H.; Sinclair, C.F.; Lang, B.; Spiezia, S.; Yu, M.; Ha, E.J.; Na, D.G.; Offi, C.; Patel, K.N.; Baek, J.H. A Comprehensive Review of Interventional Ablation Techniques for the Management of Thyroid Nodules and Metastatic Lymph Nodes. Surgery 2022, 171, 920–931. [Google Scholar] [CrossRef]
- Bernardi, S.; Stacul, F.; Michelli, A.; Giudici, F.; Zuolo, G.; de Manzini, N.; Dobrinja, C.; Zanconati, F.; Fabris, B. 12-Month Efficacy of a Single Radiofrequency Ablation on Autonomously Functioning Thyroid Nodules. Endocrine 2017, 57, 402–408. [Google Scholar] [CrossRef]
- Sim, J.S.; Baek, J.H.; Lee, J.; Cho, W.; Jung, S.I. Radiofrequency Ablation of Benign Thyroid Nodules: Depicting Early Sign of Regrowth by Calculating Vital Volume. Int. J. Hyperth. 2017, 33, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.M.; You, J.Y.; Kim, H.Y.; Chai, Y.J.; Kim, H.K.; Dionigi, G.; Tufano, R.P. Successful Radiofrequency Ablation Strategies for Benign Thyroid Nodules. Endocrine 2019, 64, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.; Yar Mohammad, A.; Khan, S. The Sensitivity of TIRADS Scoring on Ultrasonography in the Management of Thyroid Nodules. Pak. J. Med. Sci. 2023, 39, 870–874. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Ha, E.J.; Baek, J.H.; Lee, J.H. Moving-Shot versus Fixed Electrode Techniques for Radiofrequency Ablation: Comparison in an Ex-Vivo Bovine Liver Tissue Model. Korean J. Radiol. 2014, 15, 836–843. [Google Scholar] [CrossRef]
- Bisceglia, A.; Rossetto, R.; Garberoglio, S.; Franzin, A.; Cerato, A.; Maletta, F.; Papotti, M.G.; Ghigo, E.; Pagano, L.; Maccario, M.; et al. Predictor Analysis in Radiofrequency Ablation of Benign Thyroid Nodules: A Single Center Experience. Front. Endocrinol. 2021, 12, 638880. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, S.J.; Park, S.H.; Seo, M. Radiofrequency Ablation of Benign Thyroid Nodules: Evaluation of the Treatment Efficacy Using Ultrasonography. Ultrason. Seoul Korea 2016, 35, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Rucco, M.; Creanza, A.; Mormile, A.; Limone, P.P.; Garberoglio, R.; Spiezia, S.; Monti, S.; Cugini, C.; El Dalati, G.; et al. Machine Learning Prediction of Radiofrequency Thermal Ablation Efficacy: A New Option to Optimize Thyroid Nodule Selection. Eur. Thyroid J. 2020, 9, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Lee, J.H.; Ha, E.J.; Sung, J.Y.; Kim, J.K.; Baek, J.H. Radiofrequency Ablation of Benign Non-Functioning Thyroid Nodules: 4-Year Follow-up Results for 111 Patients. Eur. Radiol. 2013, 23, 1044–1049. [Google Scholar] [CrossRef]
- Yan, L.; Luo, Y.; Xie, F.; Zhang, M.; Xiao, J. Residual Vital Ratio: Predicting Regrowth after Radiofrequency Ablation for Benign Thyroid Nodules. Int. J. Hyperth. 2020, 37, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-K.; Xu, H.-X.; Lu, F.; Sun, L.-P.; He, Y.-P.; Guo, L.-H.; Li, X.-L.; Bo, X.-W.; Yue, W.-W. Factors Associated with Initial Incomplete Ablation for Benign Thyroid Nodules after Radiofrequency Ablation: First Results of CEUS Evaluation. Clin. Hemorheol. Microcirc. 2017, 65, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, L.; Wei, Y.; Zhao, Z.; Wu, J.; Yu, M. Influence Factors and Nomogram for Volume Reduction Rate in Benign Thyroid Nodule after Thermal Ablation. Int. J. Hyperth. 2023, 40, 2220562. [Google Scholar] [CrossRef]
- Negro, R.; Salem, T.M.; Greco, G. Laser Ablation Is More Effective for Spongiform than Solid Thyroid Nodules. A 4-Year Retrospective Follow-up Study. Int. J. Hyperth. 2016, 32, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, M.; Garino, F.; Alberto, M.; Garberoglio, R.; Rossetto, R.; Bonelli, N.; Spiezia, S.; De Santis, M.; Monti, S.; Deiana, M.G.; et al. Radiofrequency Ablation for Benign Thyroid Nodules According to Different Ultrasound Features: An Italian Multicentre Prospective Study. Eur. J. Endocrinol. 2019, 180, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, M.; Trimboli, P.; Garino, F.; Mormile, A.; Magliona, G.; Ramunni, M.J.; Giovanella, L.; Limone, P.P. Long-Term Efficacy of a Single Session of RFA for Benign Thyroid Nodules: A Longitudinal 5-Year Observational Study. J. Clin. Endocrinol. Metab. 2019, 104, 3751–3756. [Google Scholar] [CrossRef] [PubMed]

| Variable | N (%); Median [IQR] | |
|---|---|---|
| AGE | 51 (46–60) | |
| GENDER | Female | 37 (74.00%) |
| Male | 13 (26.00%) | |
| WEIGHT (KG) | 67.0 (60–78) | |
| HEIGHT (M) | 1.65 (1.61–1.70) | |
| BMI | 25.28 (22.41–26.84) | |
| ACR-TIRADS | 1 | 11 (23.90%) |
| 2 | 12 (26.10%) | |
| 3 | 18 (39.10%) | |
| 4 | 5 (10.90%) | |
| Missing | 4 | |
| AP (CM) | 2.50 (1.90–2.96) | |
| TV (CM) | 3.20 (2.70–3.91) | |
| SAG (CM) | 4.20 (3.34–4.75) | |
| VOLUME (CM3) | 17.85 (10.27–27.69) | |
| PROCEDURE TIME (S) | 329 (236–447) | |
| MAXIMUM POWER (W) | 55 (55–65) | |
| DIAGNOSIS | MNG | 22 (44.00%) |
| Previously ablated nodule | 1 (2.00%) | |
| Toxic nodule | 2 (4.00%) | |
| Single thyroid nodule | 25 (50.00%) | |
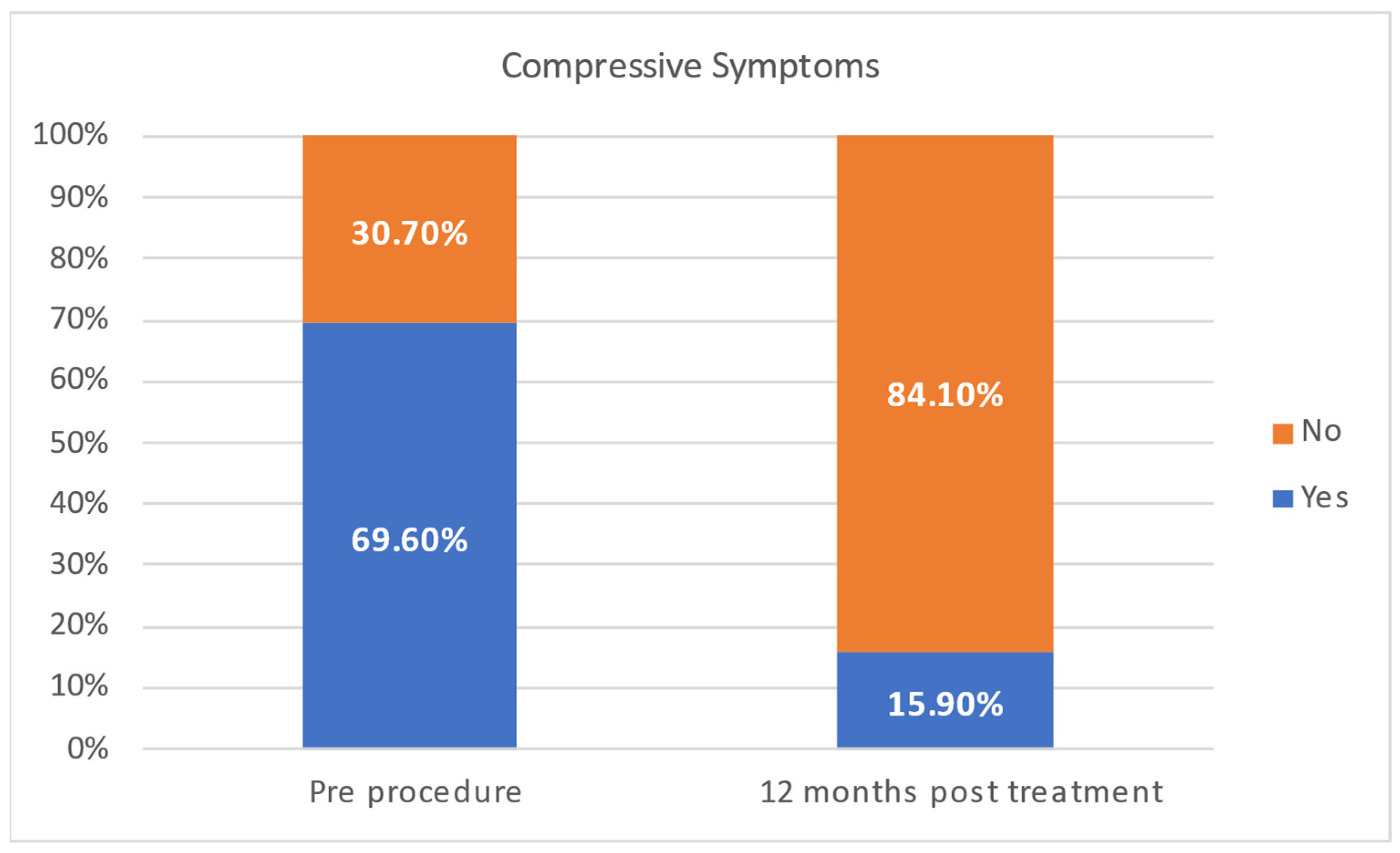
| COMPRESSIVE SYMPTOMS | Yes | 25 (50.00%) |
| No | 15 (30.00%) | |
| missing | 10 (25.00%) | |
| TRACHEAL DEVIATION | Yes | 11 (22.00%) |
| No | 39 (78.00%) | |
| VRR (%) | 1 month | 32.60 (26.16–47.42) |
| 6 months | 54.67 (40.70–68.94) | |
| 1 year | 58.24 (48.01–71.88) |
| Group A | Group B | ||
|---|---|---|---|
| N (%), Median [IQR] | N (%), Median [IQR] | p-Value | |
| Age | 50 (42–59) | 55 (46–64) | 0.18 |
| Gender | 0.04 | ||
| Female | 21 (87.50%) | 16 (61.50%) | |
| Male | 3 (12.50%) | 10 (38.50%) | |
| Weight (kg) | 62.50 (59.00–75.00) | 69.50 (60.00–84.00) | 0.19 |
| Height (m) | 1.65 (1.62–1.70) | 1.66 (1.61–1.70) | 0.78 |
| BMI | 24.61 (21.77–26.71) | 25.77 (23.37–29.32) | 0.09 |
| ACR-TIRADS | 0.56 | ||
| 1 | 5 (22.70%) | 6 (25.00%) | |
| 2 | 7 (31.80%) | 5 (20.80%) | |
| 3 | 8 (36.40%) | 10 (41.70%) | |
| 4 | 2 (9.10%) | 3 (12.50%) | |
| Missing | 4 | ||
| AP (cm) | 2.10 (1.85–2.90) | 2.76 (2.35–3.10) | 0.02 |
| TV (cm) | 2.86 (2.29–3.32) | 3.84 (2.98–4.21) | 0.003 |
| SAG (cm) | 3.60 (3.12–4.50) | 4.40 (4.13–4.92) | 0.01 |
| Volume (mL) | 12.05 (5.71–19.10) | 25.08 (5.30–33.00) | 0.003 |
| Procedure time (s) | 319 (242–402) | 352 (220–467) | 0.46 |
| Maximum power (W) | 55 (55–63) | 55 (55–65) | 0.67 |
| Diagnosis | 0.733 | ||
| MNG | 11 (45.80%) | 11 (42.30%) | |
| Previously ablated nodule | 1 (4.20%) | - | |
| Toxic nodule | 1 (4.20%) | 1 (3.80%) | |
| Single thyroid nodule | 11 (45.80%) | 14 (53.80%) | |
| Compressive symptoms | 0.283 | ||
| No | 7 (30.70%) | 8 (47.10%) | |
| Yes | 16 (69.60%) | 9 (52.90%) | |
| Tracheal deviation | 0.119 | ||
| No | 21 (87.50%) | 18 (69.20%) | |
| Yes | 3 (12.50%) | 8 (30.80%) | |
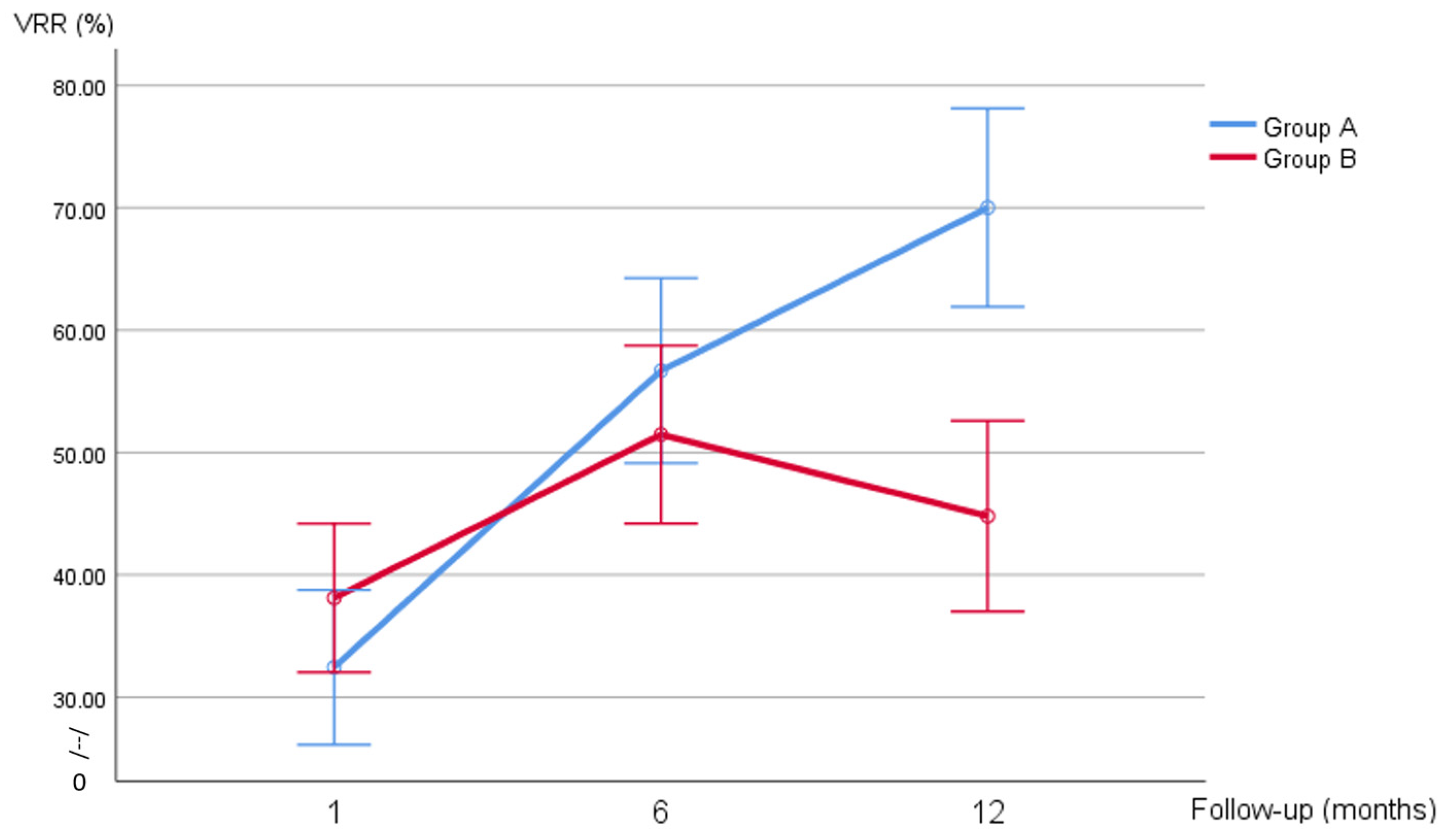
| VRR (%) | |||
| 1 month | 31.44 (21.40–38.43) | 37.65 (26.98–47.53) | 0.151 |
| 6 months | 57.10 (41.56–73.47) | 51.71 (39.42–61.54) | |
| 1 year | 71.81 (59.18–80.71) | 49.58 (33.79–57.46) | |
| Composition | 0.75 | ||
| Mixed, mainly cystic | 0 (0.00%) | 1 (4.20%) | |
| Mixed, mainly solid | 7 (31.8%) | 9 (37.50%) | |
| Solid | 10 (45.50%) | 9 (37.50%) | |
| Spongiform | 5 (22.70%) | 5 (20.80%) | |
| Missing | 4 | ||
| Margins | 0.98 | ||
| Ill-defined | 12 (54.50%) | 13 (54.20%) | |
| Smooth | 10 (45.50%) | 11 (45.80%) | |
| Missing | 4 | ||
| Calcifications | 0.34 | ||
| Absent | 21 (95.50%) | 20 (100.00%) | |
| Macrocalcifications | 1 (4.50%) | 0 (0.00%) | |
| Missing | 8 | ||
| Vascularization | 0.43 | ||
| Absent | 1 (4.50%) | 0 (0.00%) | |
| Mixed | 16 (72.70%) | 15 (65.20%) | |
| Perinodular | 5 (22.70%) | 8 (34.80%) | |
| Missing | 5 | ||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
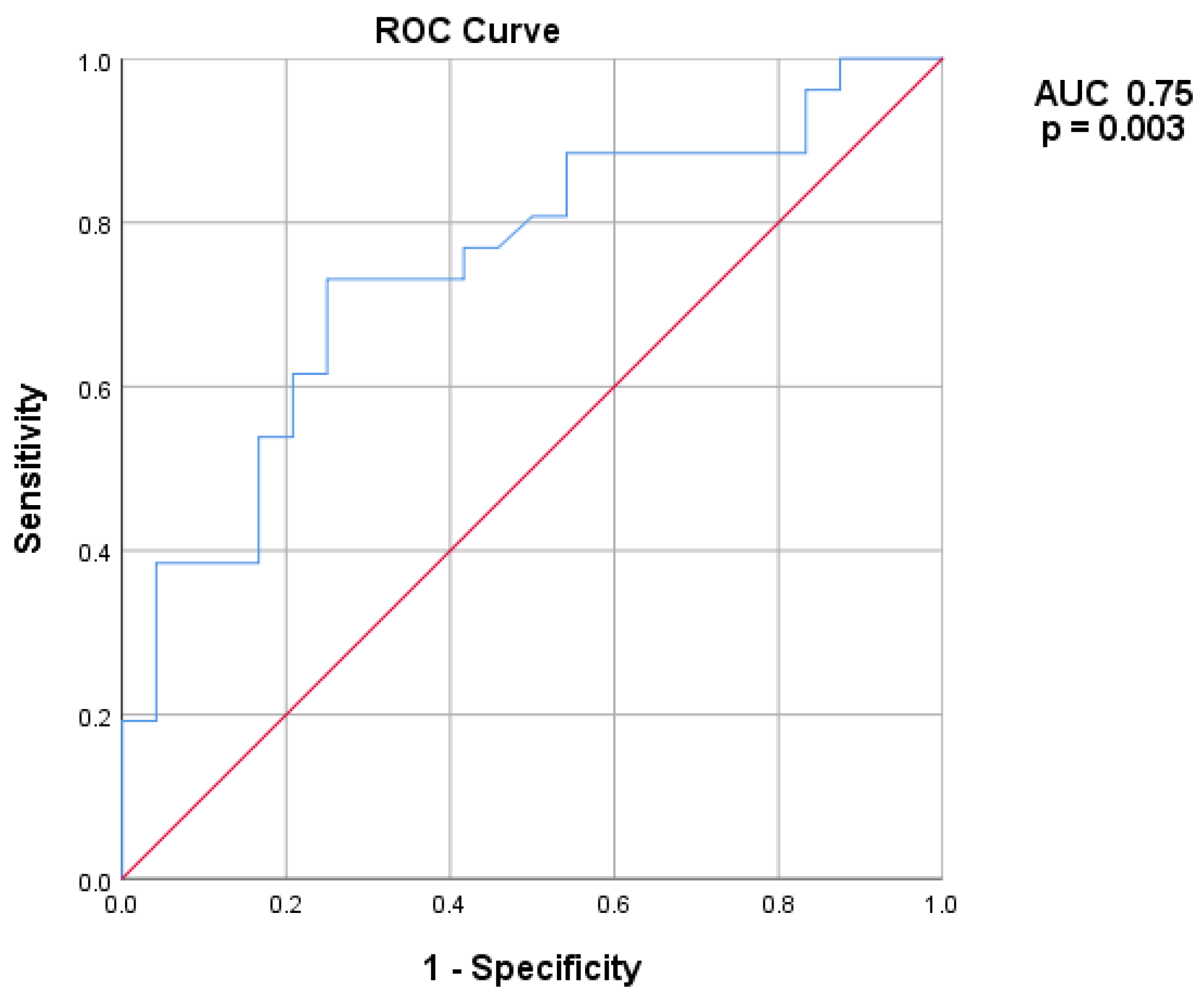
| TIRADS > 2 | 1.418 | 0.444/4.531 | 0.555 | 6.525 | 1.073/39.674 | 0.042 |
| BMI | 1.181 | 1.006/1.386 | 0.042 | 1.391 | 1.003/1.930 | 0.048 |
| Baseline volume (cm3) | 1.081 | 1.021/1.145 | 0.008 | 1.108 | 1.025/1.198 | 0.010 |
| Gender | 4.375 | 1.032/18.556 | 0.045 | 1.560 | 0.198/12.317 | 0.673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, G.; Medas, F.; Gobbo, G.; Rossi, L.; Lazzari, G.; Serbusca, D.; Morelli, E.; Cappellacci, F.; Puccini, M.; Materazzi, G.; et al. Predictors of Suboptimal Response After Radiofrequency Ablation of Benign Thyroid Nodules. J. Clin. Med. 2025, 14, 5719. https://doi.org/10.3390/jcm14165719
Di Filippo G, Medas F, Gobbo G, Rossi L, Lazzari G, Serbusca D, Morelli E, Cappellacci F, Puccini M, Materazzi G, et al. Predictors of Suboptimal Response After Radiofrequency Ablation of Benign Thyroid Nodules. Journal of Clinical Medicine. 2025; 14(16):5719. https://doi.org/10.3390/jcm14165719
Chicago/Turabian StyleDi Filippo, Giacomo, Fabio Medas, Giulia Gobbo, Leonardo Rossi, Giovanni Lazzari, Dorin Serbusca, Eleonora Morelli, Federico Cappellacci, Marco Puccini, Gabriele Materazzi, and et al. 2025. "Predictors of Suboptimal Response After Radiofrequency Ablation of Benign Thyroid Nodules" Journal of Clinical Medicine 14, no. 16: 5719. https://doi.org/10.3390/jcm14165719
APA StyleDi Filippo, G., Medas, F., Gobbo, G., Rossi, L., Lazzari, G., Serbusca, D., Morelli, E., Cappellacci, F., Puccini, M., Materazzi, G., & Canu, G. L. (2025). Predictors of Suboptimal Response After Radiofrequency Ablation of Benign Thyroid Nodules. Journal of Clinical Medicine, 14(16), 5719. https://doi.org/10.3390/jcm14165719










